Abstract
Background
Studies suggest that treatment-resistant hypertension is common and increasing in prevalence among US adults. While hypertension is a risk factor for end-stage renal disease (ESRD), few data are available on the association between treatment-resistant hypertension and ESRD risk.
Study Design
Prospective cohort study.
Setting & Participants
We analyzed data from 9,974 Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study participants treated for hypertension without ESRD at baseline.
Predictor
Treatment-resistant hypertension was defined as uncontrolled blood pressure (BP) with concurrent use of 3 antihypertensive medication classes including a diuretic or use of ≥4 antihypertensive medication classes including a diuretic regardless of BP level.
Outcome
Incident ESRD was identified by linkage of REGARDS Study participants with the US Renal Data System.
Measurements
During a baseline in-home study visit, BP was measured twice and classes of antihypertensive medication being taken were determined by pill bottle inspection.
Results
Over a median follow-up of 6.4 years, there were 152 incident cases of ESRD (110 ESRD cases among 2,147 with treatment-resistant hypertension and 42 ESRD cases among 7,827 without treatment-resistant hypertension). The incidence of ESRD per 1,000 person-years for hypertensive participants with and without treatment-resistant hypertension was 8.86 (95% CI, 7.35–10.68) and 0.88 (95% CI, 0.65–1.19), respectively. After multivariable adjustment, the HR for ESRD comparing hypertensive participants with versus without treatment-resistant hypertension was 6.32 (95% CI, 4.30–9.30). Of the participants who developed incident ESRD during follow-up, 72% had treatment-resistant hypertension at baseline.
Limitations
BP, eGFR, and albuminuria assessed at a single time point.
Conclusions
Individuals with treatment-resistant hypertension are at increased risk for ESRD. Appropriate clinical management strategies are needed to treat treatment-resistant hypertension in order to preserve kidney function in this high-risk group.
Keywords: treatment-resistant hypertension, uncontrolled blood pressure, hypertension, kidney disease, end-stage renal disease, renal failure, antihypertensive medication, kidney disease risk factor
Treatment-resistant hypertension is defined as uncontrolled blood pressure (BP) with concurrent use of 3 or more antihypertensive medication classes, or as use of 4 or more antihypertensive medication classes regardless of BP level. Ideally, one of these antihypertensive medication classes should be a diuretic and every agent should be prescribed at an optimal dosage.1 Based on data from the 2005–2008 National Health and Nutrition Examination Surveys (NHANES), Egan, et al. estimated the prevalence of treatment-resistant hypertension to be 11.8% among hypertensive adults.2
End-stage renal disease (ESRD) is associated with a heavy economic burden and excess risk of mortality.3 Hypertension affects the majority of individuals with chronic kidney disease (CKD) and is a major risk factor for ESRD.4–6 Further, recent studies have reported a high prevalence of treatment-resistant hypertension among individuals with CKD.7–9 Treatment-resistant hypertension has been associated with increased risk of coronary heart disease, stroke, all-cause mortality and, in small clinic-based samples, ESRD. 9,10 However, few data are available on ESRD risk in a large, population-based sample of persons with treatment-resistant hypertension. The goal of the current analysis was to determine whether individuals with treatment-resistant hypertension have an increased risk for ESRD. To do so, we analyzed data from adults participating in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study.
Methods
Study Participants
The REGARDS Study enrolled a population-based sample of black and white US adults aged 45 years or older.11 Between June 2003 and October 2007, 30,239 individuals were enrolled from the 48 contiguous US states and the District of Columbia.11 Participants with hypertension who were taking ≥ 1 class of antihypertensive medication and did not have prevalent ESRD at baseline (n=14,734) formed the base population for the present analysis. Prevalent ESRD was defined by self-report of receipt of dialysis or an ESRD incidence date in the US Renal Data System (USRDS) prior to the REGARDS in-home visit date. Those missing BP, serum creatinine, or albumin-creatinine ratio (ACR) data or information on medications they were taking (n=1,295) were excluded from all analyses. We also excluded participants with uncontrolled BP taking 1 or 2 antihypertensive medication classes (n=3,465) from the main analyses, as we were unable to determine whether these participants had treatment-resistant hypertension. As described below, these participants were included in sensitivity analyses. After these exclusion criteria were applied, data from 9,974 hypertensive participants were included in the main analyses. The REGARDS Study protocol was approved by the institutional review boards governing research in human subjects at the participating centers and all participants provided written consent.
Data Collection
Baseline REGARDS Study data were collected through a computer-assisted telephone interview, an in-home examination conducted in the morning, and self-administered questionnaires. Of relevance to the current analysis, information on the following demographic, behavioral and medical history characteristics was collected during the telephone interview: age, sex, race, region of residence, education, annual household income, smoking status, alcohol consumption, frequency of physical activity, and a history of diabetes, stroke, or myocardial infarction (MI). Medication adherence was assessed using the 4-item Morisky Medication Adherence Scale.
During the in-home examination, two BP measurements were made by trained personnel using standardized protocols. Also, an electrocardiogram was obtained, waist circumference was measured, and blood and urine samples were collected and sent to a central laboratory for analysis. A pill bottle review was conducted to record the names of all prescription and over-the-counter medications participants reported taking during the 2 weeks preceding the in-home study visit. Total and high-density lipoprotein (HDL) cholesterol were measured by colorimetric reflectance spectrophotometry and high sensitivity C-reactive protein was measured using a high-sensitivity particle-enhanced immunonephelometric assay. Serum glucose was measured by colorimetric reflectance spectrophotometry on the Ortho Vitros 950 IRC Clinical Analyzer (Johnson & Johnson Clinical Diagnostics), and diabetes was defined as a fasting serum glucose level ≥ 126 mg/dL, non-fasting serum glucose level ≥ 200 mg/dL, or use of antidiabetes medication. Serum creatinine was measured using an isotope-dilution mass spectrometry (IDMS)-traceable method. Estimated glomerular filtration rate (eGFR) was calculated via the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation12 and categorized as ≥ 60, 45–59, or < 45 mL/min/1.73 m2. Urinary albumin was measured with the BN ProSpec Nephelometer (Dade Behring, Marburg, Germany) and urinary creatinine was measured with a rate-blanked Jaffé procedure, using the Modular-P analyzer (Roche/Hitachi; Indianapolis, IN). The ACR was categorized as < 30 or ≥ 30 mg/g.
Definition of Treatment-Resistant Hypertension
During the in-home examination, BP was measured twice by trained technicians following a standardized protocol using aneroid sphygmomanometers. Participants were asked to sit quietly for 5 minutes with both feet on the floor prior to the BP measurements. Measurements were taken using an appropriately sized cuff, which was inflated to 20 mmHg above the pulse obliteration level and slowly deflated. After a 30-second rest period, this process was repeated on the same arm to obtain the second BP measurement.13 Quality control for BP measurement in the REGARDS Study was monitored by central examination of digit preference and technicians were retrained as necessary.11 The two BP measurements were averaged for analysis. Uncontrolled BP was defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg except for individuals with an ACR ≥ 30 mg/g wherein uncontrolled BP was defined as systolic BP ≥ 130 mmHg and/or diastolic BP ≥ 80 mmHg.14 Medication names recorded during the pill bottle review were coded into generic drug names and subsequently grouped into drug classes. One-pill combinations were classified into multiple medication classes. Each generic drug name was counted in only one class. Drug dose was not recorded. Antihypertensive medication classes were defined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7).15 For the purposes of this study, treatment-resistant hypertension was defined as uncontrolled BP with concurrent use of 3 antihypertensive medication classes including a diuretic or as use of ≥ 4 antihypertensive medication classes including a diuretic regardless of BP level. In the main analyses, the comparison group (i.e. “no treatment-resistant hypertension”) was comprised of participants with controlled BP on 3 or fewer classes of antihypertensive medication. In sensitivity analyses, the comparison group also included participants with uncontrolled BP on 1 or 2 classes of antihypertensive medication.
Definition of ESRD
Incident cases of ESRD were identified through linkage of REGARDS Study participants with the USRDS, which records virtually all incident ESRD cases in the United States. A finder file with unique individual identifiers (name, social security number, and date of birth) was submitted for linkage with the USRDS. Different configurations of full and partial individual identifiers were then sequentially matched. For participants with a partial match to the USRDS, the nonmatching variables were visually inspected to confirm a valid match could not be made. Data from the USRDS included all incident ESRD cases, regardless of treatment modality, through September 30, 2011. For participants not developing ESRD, follow up time ended on their date of death (ascertained through death certificates, National Death Index data, or Social Security Death Index) or, for those who remained alive, September 30, 2011.
Statistical Analysis
Baseline characteristics of REGARDS participants were calculated by treatment-resistant hypertension status. The cumulative incidence of ESRD was calculated using the Kaplan-Meier method for participants with and without treatment-resistant hypertension. Next, using Cox proportional hazards regression models, the crude (unadjusted); age, race-, sex-adjusted; and multivariable-adjusted hazard ratios (HRs) for ESRD associated with treatment-resistant hypertension were calculated for the overall population and for subgroups defined by age, race, sex, history of diabetes, MI, and stroke. Multivariable adjustment included age, race, sex, region of residence, education, income, physical activity, current smoking, alcohol use, statin use, waist circumference, diabetes, total cholesterol, high-density lipoprotein cholesterol, C-reactive protein, and history of MI and stroke.
We did not adjust for ACR or eGFR in the main analyses, since they may be in the causal pathway between treatment-resistant hypertension and ESRD.16–18 Instead, we calculated multivariable adjusted HRs for ESRD associated with treatment-resistant hypertension stratified by level of baseline ACR (< 30 or ≥ 30 mg/g) and baseline eGFR (≥ 60, 45–59, and <45 mL/min/1.73 m2). To reduce model over-fitting in these stratified analyses, we calculated each participant’s propensity (i.e. predicted probability) for having treatment-resistant hypertension based on variables in the multivariable adjusted model described above. We then adjusted for this propensity in the regression model rather than adjusting for each covariate individually. In sensitivity analyses, we calculated the incidence rates and HRs for ESRD associated with treatment-resistant hypertension (1) limited to participants with perfect medication adherence, defined by appropriate medication-taking behaviors on all 4 Morisky Medication Adherence Scale items, (2) including the 3,465 participants with uncontrolled BP on 1 or 2 classes of antihypertensive medication in the analysis and categorizing this group as not having treatment-resistant hypertension, and (3) including adjustment for ACR and eGFR in a final multivariable model. Also, we calculated incidence rates and HRs for ESRD comparing participants with treatment-resistant hypertension and controlled BP and, separately, with uncontrolled BP to participants without treatment-resistant hypertension. The HRs for ESRD were also calculated for those with treatment-resistant hypertension comparing participants with uncontrolled versus controlled BP. All analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
Participant Characteristics
After excluding participants with uncontrolled BP on 1 or 2 classes of antihypertensive medication, 21.5% of REGARDS Study participants with hypertension had treatment-resistant hypertension. Participants with treatment-resistant hypertension were older on average than their counterparts without treatment-resistant hypertension and more likely to be black, have an annual household income <$20,000, have less than a high school education, use statins, and have diabetes or a history of MI or stroke (Table 1). Those with treatment-resistant hypertension were less likely to be female, to consume alcohol or to participate in physical activity. On average, participants with treatment-resistant hypertension had a larger waist circumference, higher systolic and diastolic BPs and ACR, and lower total cholesterol, HDL cholesterol, and eGFR than those without treatment-resistant hypertension. Details on the number and classes of antihypertensive medications being taken by participants with and without treatment-resistant hypertension are provided in Table S1 (provided as online supplementary material).
Table 1.
Characteristics of REGARDS participants with and without treatment-resistant hypertension.
| No treatment-resistant HTN (n=7,827) |
Treatment-resistant HTN (n=2,147) |
p-value | |
|---|---|---|---|
| Age (y) | 65.4 (8.9) | 67.5 (8.6) | <0.001 |
| Female sex | 58.9 | 50.3 | <0.001 |
| Black race | 45.3 | 59.1 | <0.001 |
| Geographic Region* | 0.1 | ||
| Stroke belt | 35.2 | 34.5 | |
| Stroke buckle | 22.4 | 20.9 | |
| Other | 42.4 | 44.7 | |
| Income <$20,000 | 18.2 | 25.0 | <0.001 |
| < HS education | 13.0 | 19.7 | <0.001 |
| Current smoking | 12.8 | 11.9 | 0.3 |
| Current alcohol use | 34.5 | 31.0 | 0.003 |
| Perfect medication adherence | 70.6 | 67.1 | 0.001 |
| Physical activity | <0.001 | ||
| ≥4 times/wk | 27.8 | 23.9 | |
| 1–3 times/wk | 35.9 | 33.3 | |
| None | 36.3 | 42.8 | |
| Waist circumference(cm) | 97.6 (14.8) | 104.2 (16.2) | <0.001 |
| Statin use | 42.2 | 52.5 | <0.001 |
| Diabetes | 24.7 | 45.6 | <0.001 |
| History of MI | 14.4 | 25.1 | <0.001 |
| History of stroke | 7.5 | 13.4 | <0.001 |
| Total cholesterol (mg/dL) | 186.0 (38.7) | 180.4 (38.8) | <0.001 |
| HDL cholesterol (mg/dL) | 50.9 (15.8) | 48.2 (15.0) | <0.001 |
| C-reactive protein (mg/L) | 2.5 [1.1–5.7] | 2.9 [1.3–6.5] | 0.001 |
| Systolic BP (mmHg) | 122.4 (9.8) | 141.0 (17.7) | <0.001 |
| Diastolic BP (mmHg) | 74.3 (7.7) | 79.8 (11.3) | <0.001 |
| eGFR (mL/min/1.73 m2) | 84.2 (19.5) | 74.8 (23.9) | <0.001 |
| ACR (mg/g) | 7.0 [4.6–12.5] | 17.4 [7.1–74.7] | <0.001 |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Conversion factor for HDL and total cholesterol in mg/dL to mmol/L, ×0.02586.
BP, blood pressure; MI: myocardial infarction; HDL: high-density lipoprotein; eGFR: estimated glomerular filtration rate; ACR: albumin-creatinine ratio; REGARDS, Reasons for Geographic and Racial Differences in Stroke; HTN, hypertension; HS, high school.
Stroke buckle refers to coastal North Carolina, South Carolina, and Georgia. Stroke belt refers to remainder of North Carolina, South Carolina, and Georgia; Alabama, Mississippi, Tennessee, Arkansas, and Louisiana. Other refers to rest of continental United States.
Treatment-Resistant Hypertension and Incident ESRD
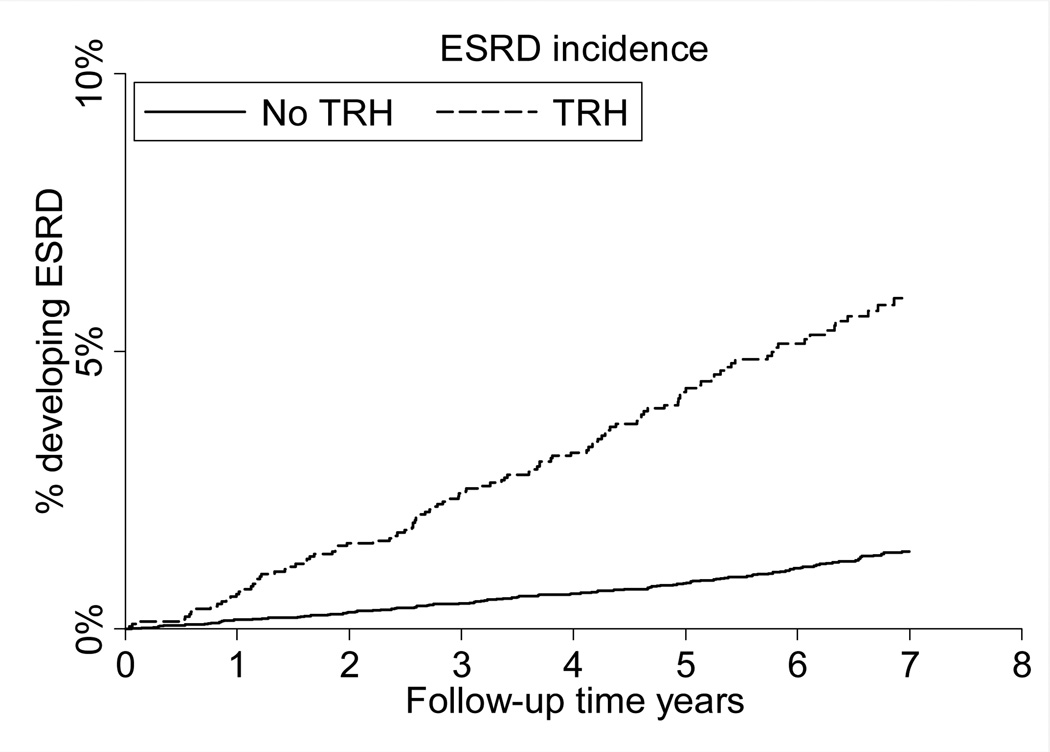
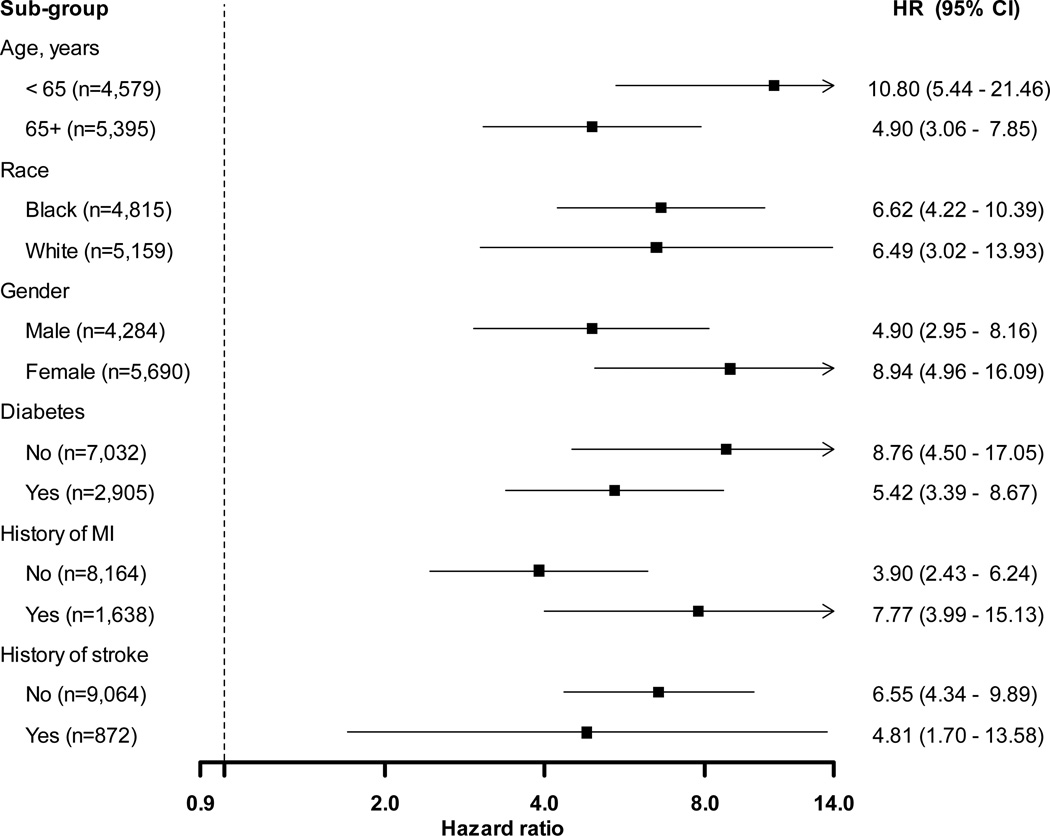
Over a median follow-up of 6.4 (maximum, 8.6) years, 152 participants developed ESRD. Of the 152 cases of incident ESRD, 110 (72%) had treatment-resistant hypertension at the baseline study visit. The cumulative incidence of ESRD was higher for participants with versus without treatment-resistant hypertension (Figure 1). The incidence of ESRD was 8.86 (95% confidence interval [CI], 7.35–10.68) and 0.88 (95% CI, 0.65–1.19) per 1,000 person-years among participants with and without treatment-resistant hypertension, respectively (Table 2). This association was present after age, race, sex and multivariable adjustment and in sub-groups defined by age, race, gender, and a history of diabetes, MI, or stroke (Figure 2). Results were similar when analyses were limited to participants with perfect medication adherence and when participants with uncontrolled BP on 1 or 2 classes of antihypertensive medication were included in the analysis as not having treatment-resistant hypertension; however, treatment-resistant hypertension was not associated with ESRD after multivariable adjustment including ACR and eGFR (HR, 1.39; 95% CI, 0.92–2.11; Table S2).
Figure 1.
Cumulative incidence of end-stage renal disease associated with treatment-resistant hypertension. ESRD: end-stage renal disease
Table 2.
Incidence rates and HRs for ESRD associated with treatment-resistant HTN among REGARDS participants
| No treatment-resistant HTN | Treatment-resistant HTN | |
|---|---|---|
| No. at risk | 7,827 | 2,147 |
| ESRD events | 42 (0.54) | 110 (5.12) |
| ESRD incidence rate (95% CI) | 0.88 (0.65–1.19) | 8.86 (7.35–10.68) |
| HR (95% CI) for ESRD | ||
| Crude | 1.00 (reference) | 10.06 (7.05–14.36) |
| Age, race, sex-adjusted | 1.00 (reference) | 8.34 (5.82–11.96) |
| Multivariable adjusted1 | 1.00 (reference) | 6.32 (4.30–9.30) |
NOTE: ESRD events are given as number (percentage). Incidence rates are per 1,000 person-years.
CI, confidence interval; ESRD: end-stage renal disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke; HTN, hypertension; HR, hazard ratio
Adjusted for age, race, sex, region of residence, education, income, physical activity, current smoking, alcohol use, statin use, waist circumference, diabetes, total cholesterol, high-density lipoprotein cholesterol, C-reactive protein, history of myocardial infarction, and history of stroke.
Figure 2.
Multivariable adjusted hazard ratios for incident end-stage renal disease associated with treatment-resistant hypertension among Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study participants, in subgroups. Hazard ratios adjusted for age, race, sex, region of residence, education, income, physical activity, current smoking, alcohol use, statin use, waist circumference, diabetes, total cholesterol, high-density lipoprotein cholesterol, c-reactive protein, history of myocardial infarction, and history of stroke.
For both participants with and without treatment-resistant hypertension, ESRD incidence increased as level of ACR increased and eGFR decreased (Table 3). Additionally, within each ACR and eGFR category, the crude ESRD incidence rate was higher among participants with versus without treatment-resistant hypertension. The propensity-adjusted HRs for ESRD associated with treatment-resistant hypertension among those with ACR levels < 30 and ≥ 30 mg/g were 1.55 (95% CI, 0.61–3.94) and 2.54 (95% CI, 1.61–4.00), respectively. The propensity-adjusted HRs for ESRD comparing participants with and without treatment-resistant hypertension were 3.57 (95% CI, 1.58–8.07), 6.53 (95% CI, 1.86–22.96), and 3.22 (95% CI, 2.01–5.15) for participants with eGFR ≥60, 45–59, and <45 mL/min/1.73 m2, respectively.
Table 3.
Incidence rates and HRs for ESRD associated with treatment-resistant HTN among REGARDS study participants, stratified by level of ACR and eGFR
| No treatment-resistant HTN | Treatment-resistant HTN | Adjusted† HR (95% CI) | |||
|---|---|---|---|---|---|
| No. events/No. at risk | IR (95%CI) | No. events/ No. at risk | IR (95% CI) | ||
| ACR | |||||
| < 30 mg/g | 14/7,222 | 0.32 (0.19–0.53) | 9/1,289 | 1.14 (0.59–2.19) | 1.55 (0.61–3.94) |
| ≥ 30 mg/g | 28/605 | 8.20 (5.66–11.88) | 101/858 | 22.28 (18.33–27.08) | 2.54 (1.61–4.00) |
| eGFR | |||||
| ≥ 60 mL/min/1.73 m2 | 12/6,987 | 0.28 (0.16–0.49) | 16/1,549 | 1.70 (1.04–2.78) | 3.57 (1.58–8.07) |
| 45–59 mL/min/1.73 m2 | 3/564 | 0.91 (0.29–2.82) | 15/337 | 8.13 (4.90–13.48) | 6.53 (1.86–22.96) |
| <45 mL/min/1.73m2 | 27/276 | 18.96 (13.00–27.64) | 79/261 | 66.66 (53.47–83.10) | 3.22 (2.01–5.15) |
Abbreviations and definitions: CI, confidence interval; ESRD: end-stage renal disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke; HTN, hypertension; HR, hazard ratio; IR, incidence rate per 1,000 person-year; ACR: albumin-creatinine ratio; eGFR: estimated glomerular filtration rate
Propensity-adjusted for predicted probability of treatment-resistant HTN based on age, race, sex, region of residence, education, income, physical activity, current smoking, alcohol use, statin use, waist circumference, diabetes, total cholesterol, high-density lipoprotein cholesterol, C-reactive protein, history of myocardial infarction, and history of stroke.
ESRD Associated With Controlled and Uncontrolled BP
Characteristics of the study population with treatment-resistant hypertension by BP control are shown in Table S3. Participants with treatment-resistant hypertension and controlled BP and treatment-resistant hypertension with uncontrolled BP each had a higher risk of ESRD when compared to participants without treatment-resistant hypertension (Table 4). After multivariable adjustment and compared to those without treatment-resistant hypertension, the HRs for ESRD were 2.89 (95% CI, 1.52–5.47) and 7.68 (95% CI, 5.18–11.40) for those with treatment-resistant hypertension and controlled and uncontrolled BP, respectively. After multivariable adjustment and compared to those with treatment-resistant hypertension and controlled BP, the HR for ESRD was 2.69 (95% CI, 1.49–4.86) for participants with treatment-resistant hypertension and uncontrolled BP (Table S4).
Table 4.
Incidence rates and HRs for ESRD associated with controlled and uncontrolled BP among REGARDS participants with treatment-resistant HTN
| No treatment-resistant HTN | Treatment-resistant HTN | ||
|---|---|---|---|
| Controlled BP | Uncontrolled BP | ||
| No. at risk | 7,827 | 564 | 1,583 |
| ESRD events | 42 (0.54) | 13 (2.30) | 97 (6.13) |
| ESRD incidence rate (95% CI) | 0.88 (0.65–1.19) | 3.89 (2.26–6.70) | 10.68 (8.75–13.04) |
| HR (95% CI) for ESRD | |||
| Crude | 1.00 (reference) | 4.42 (2.37–8.23) | 12.15 (8.46–17.45) |
| Age, race, sex-adjusted | 1.00 (reference) | 3.83 (2.05–7.15) | 9.94 (6.88–14.36) |
| Multivariable adjusted1 | 1.00 (reference) | 2.89 (1.52–5.47) | 7.68 (5.18–11.40) |
Note: ESRD events are given as number (percentage). Incidence rates are per 1,000 person-years.
ESRD: end-stage renal disease; BP: blood pressure; CI, confidence interval; REGARDS, Reasons for Geographic and Racial Differences in Stroke; HTN, hypertension; HR, hazard ratio
Adjusted for age, race, sex, region of residence, education, income, physical activity, current smoking, alcohol use, statin use, waist circumference, diabetes, total cholesterol, high-density lipoprotein cholesterol, C-reactive protein, history of myocardial infarction, and history of stroke.
Discussion
Using data from a large, population-based sample of black and white adults, we found a strong association between treatment-resistant hypertension and incident ESRD, which persisted after multivariable adjustment. The incidence rate of ESRD among people with treatment-resistant hypertension in this population-based study (8.86 per 1,000 person-years) is over two times higher than USRDS estimates for blacks with diabetes (4.0 per 1,000 population), a population which has historically been considered at especially high risk of ESRD.3 In addition, although 22% of REGARDS participants had treatment-resistant hypertension at baseline, 72% of those who went on to develop ESRD during follow-up had treatment-resistant hypertension at their baseline study visit. These data suggest that treatment-resistant hypertension might be an important marker for increased ESRD risk.
The association between treatment-resistant hypertension and ESRD has been examined in clinic-based studies.7,9 De Nicola and colleagues reported treatment-resistant hypertension to be associated with increased risk of the composite outcome of dialysis, transplantation, or death over a median of 37.6 months of follow-up (HR, 1.85; 95% CI, 1.13–3.03) among 300 patients with CKD.7 More recently, the same group reported an increased risk for cardiovascular events and renal events among 436 clinic patients with treatment-resistant hypertension over 57 months of follow-up (HRs of 1.98 [95% CI, 1.14–3.43] and 2.66 [95% CI, 1.62–4.37], respectively).9 Also, in a study of individuals enrolled in the international Reduction of Atherothrombosis for Continued Health (REACH) registry, treatment-resistant hypertension versus no treatment-resistant hypertension was associated with a multivariable adjusted HR of 1.11 (95% CI, 1.02–1.20) for the composite outcome of cardiovascular death, MI, or stroke.19
In the current study, treatment-resistant hypertension, regardless of BP control, was associated with an increased risk for ESRD. Furthermore, treatment-resistant hypertension with uncontrolled BP was associated with an increased risk for ESRD compared to treatment-resistant hypertension with controlled BP, suggesting that, among individuals with CKD, prevention of treatment-resistant hypertension and achieving BP control among those with treatment-resistant hypertension are important. Achieving BP control is a key challenge in the management of individuals with CKD;20 however, prior studies suggest that, with appropriate interventions, BP control can be achieved and maintained even in populations in which BP control is difficult.21–24 In addition, randomized controlled trials have demonstrated that, among individuals with treatment-resistant hypertension, catheter-based renal denervation can reduce BP in individuals without major adverse effects or changes in kidney function.25,26 However, the optimal BP goal for reducing cardiovascular disease and improving kidney disease outcomes is unclear and whether renal denervation slows the progression of CKD is not known. A small randomized trial demonstrated that reductions in dietary sodium are associated with lower BP among individuals with treatment-resistant hypertension.27 Data from randomized trials are needed to assess the benefits of reducing dietary sodium intake on cardiovascular outcomes among individuals with treatment-resistant hypertension.
The findings of the current study emphasize the need for appropriate clinical management strategies to lower BP among individuals with treatment-resistant hypertension. The American Heart Association scientific statement on diagnosis, evaluation, and treatment of treatment-resistant hypertension recommends diuretics as first-line therapy for persons with hypertension, with the subsequent addition of an ACE inhibitor or angiotensin receptor blocker (ARB) and then a calcium channel blocker, as needed to achieve BP control.1 Furthermore, among individuals with treatment-resistant hypertension, clinical trials indicate that the addition of an aldosterone antagonist lowers systolic BP by 20–25 mm Hg and diastolic BP by 10–15 mm Hg.28,29 However, in the current study, less than 10% of individuals with treatment-resistant hypertension were taking an aldosterone antagonist. Ineffective antihypertensive therapy, including sub-optimal drug combination strategies, constitutes a barrier to BP control. Comparative effectiveness studies are needed to test the effects of different multi-drug medication regimens on BP, cardiovascular and kidney disease outcomes in individuals with treatment-resistant hypertension.
Our study minimizes misclassification of the treatment-resistant hypertension phenotype through the use of a pill-bottle review to identify the number of antihypertensive medication classes being taken, standardized in-home BP measurement, and assessment of medication adherence. Other strengths include the large, population-based sample of blacks and whites and the availability of linkage with the USRDS, which captures virtually all incident ESRD cases in the United States. However, the findings of the current study should be considered in the context of certain limitations. Albuminuria, BP, and eGFR were assessed at a single time point, making misclassification of CKD and treatment-resistant hypertension status possible. An additional limitation is the lack of medication dosing information. Some individuals may have been on an inadequate treatment regimen and thus were not truly treatment-resistant. Ambulatory BP monitoring was not available to rule out white-coat hypertension. Also, we do not have data on potential secondary causes of treatment-resistant hypertension or genetic data to investigate the potential contribution of disease-promoting haplotypes. In the current study, the association between treatment-resistant hypertension and ESRD was attenuated by adjustment for ACR and reduced eGFR, suggesting that these are either confounders or mediators between treatment-resistant hypertension and ESRD risk. Given the strong association between hypertension, reduced eGFR, and albuminuria, it is possible that albuminuria and reduced eGFR might be in the causal pathway between treatment-resistant hypertension and ESRD risk.30 However, with the data currently available in the REGARDS Study, we cannot rule out the possibility that they are confounders.
In conclusion, data from the current study demonstrate an increased risk for ESRD among individuals with treatment-resistant hypertension. Additionally, a substantial proportion of participants who developed ESRD had treatment-resistant hypertension at baseline. Strategies are needed to prevent and treat treatment-resistant hypertension in an effort to reduce the incidence of ESRD.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the REGARDS Study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at www.regardsstudy.org.
Support: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Supplementary Material
Table S1: Number of antihypertensive medication classes being taken and percent of participants taking each class.
Table S2: Sensitivity analyses for ESRD associated with treatment-resistant HTN.
Table S3: Characteristics of participants without treatment-resistant HTN and with controlled vs uncontrolled treatment-resistant HTN.
Table S4: HRs for incident ESRD associated with uncontrolled BP in participants with treatment-resistant HTN.
Note: The supplementary material accompanying this article (doi: ________ ) is available at
References
- 1.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008 Jun;51(6):1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011 Aug 30;124(9):1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 Annual Data Report. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 Jan;61(1 Suppl 1):e1–e476. A7. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 4.Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Archives of internal medicine. 2006 Sep 25;166(17):1884–1891. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994) Archives of internal medicine. 2001 May 14;161(9):1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Anderson A, Charleston J, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Mar;55(3):441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Nicola L, Borrelli S, Gabbai FB, et al. Burden of resistant hypertension in hypertensive patients with non-dialysis chronic kidney disease. Kidney & blood pressure research. 2011;34(1):58–67. doi: 10.1159/000322923. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Kader K, Dohar S, Shah N, et al. Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. Journal of hypertension. 2012 May;30(5):960–966. doi: 10.1097/HJH.0b013e328351d08a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Nicola L, Gabbai FB, Agarwal R, et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. Journal of the American College of Cardiology. 2013 Jun 18;61(24):2461–2467. doi: 10.1016/j.jacc.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012 Apr 3;125(13):1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard VJ, Woolson RF, Egan BM, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. Journal of the American Society of Hypertension. 2010 Jan-Feb;4(1):32–41. doi: 10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney International -Supplement. 2012;(2):337–414. [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.Koroshi A. Microalbuminuria, is it so important? Hippokratia. 2007 Jul;11(3):105–107. [PMC free article] [PubMed] [Google Scholar]
- 17.Shimbo D, Muntner P, Mann D, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. 2010 May;55(5):1210–1216. doi: 10.1161/HYPERTENSIONAHA.109.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace SM, McEniery CM, Yasmin, McEniery CM, et al. Isolated systolic hypertension is characterized by increased aortic stiffness and endothelial dysfunction. Hypertension. 2007 Jul;50(1):228–233. doi: 10.1161/HYPERTENSIONAHA.107.089391. [DOI] [PubMed] [Google Scholar]
- 19.Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. European heart journal. 2012 Nov 9; doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
- 20.Khosla N, Kalaitzidis R, Bakris GL. The kidney, hypertension, and remaining challenges. The Medical clinics of North America. 2009 May;93(3):697–715. doi: 10.1016/j.mcna.2009.02.001. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 21.Wright JT, Jr., Agodoa L, Contreras G, et al. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Archives of internal medicine. 2002 Jul 22;162(14):1636–1643. doi: 10.1001/archinte.162.14.1636. [DOI] [PubMed] [Google Scholar]
- 22.ACCORD Study Group. Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. The New England journal of medicine. 2010 Apr 29;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Annals of internal medicine. 2005 Mar 1;142(5):342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 24.Appel LJ, Wright JT, Jr., Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. The New England journal of medicine. 2010 Sep 2;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Symplicity HTN Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011 May;57(5):911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 26.Symplicity HTN Investigators. Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010 Dec 4;376(9756):1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 27.Pimenta E, Gaddam KK, Oparil S, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009 Sep;54(3):475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007 Apr;49(4):839–845. doi: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 29.Pimenta E, Calhoun DA. Resistant hypertension and aldosteronism. Current hypertension reports. 2007 Nov;9(5):353–359. doi: 10.1007/s11906-007-0066-7. [DOI] [PubMed] [Google Scholar]
- 30.Oliveras A, Armario P, Hernandez-Del Rey R, et al. Urinary albumin excretion is associated with true resistant hypertension. Journal of human hypertension. 2010 Jan;24(1):27–33. doi: 10.1038/jhh.2009.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.