Abstract
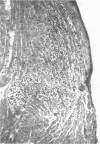
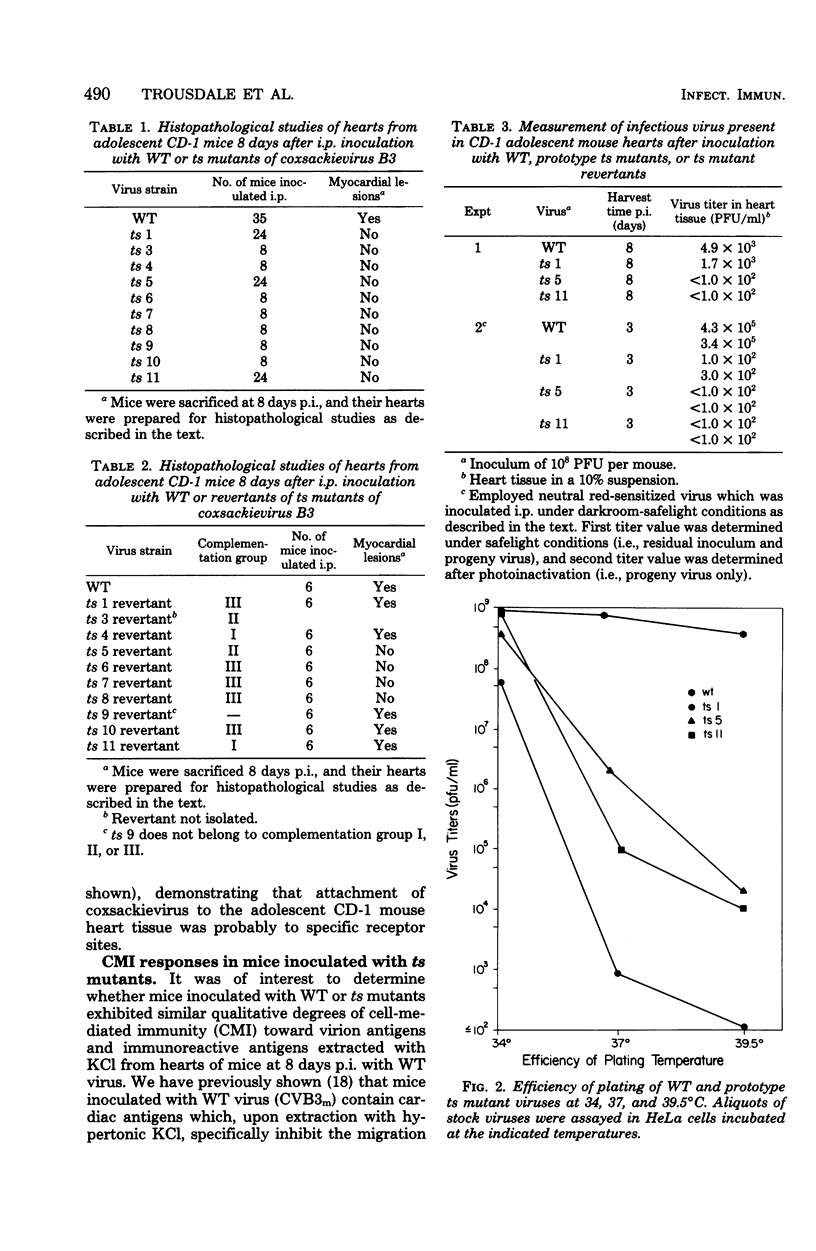
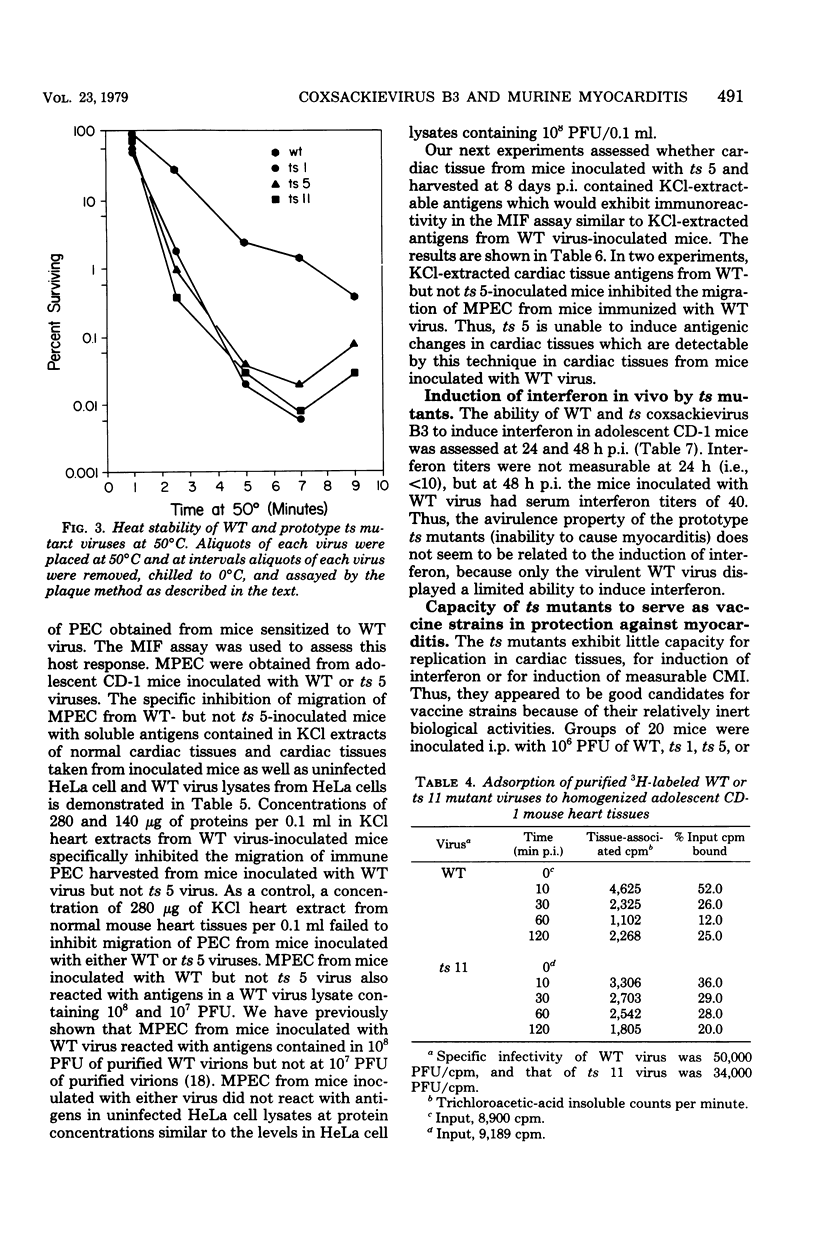
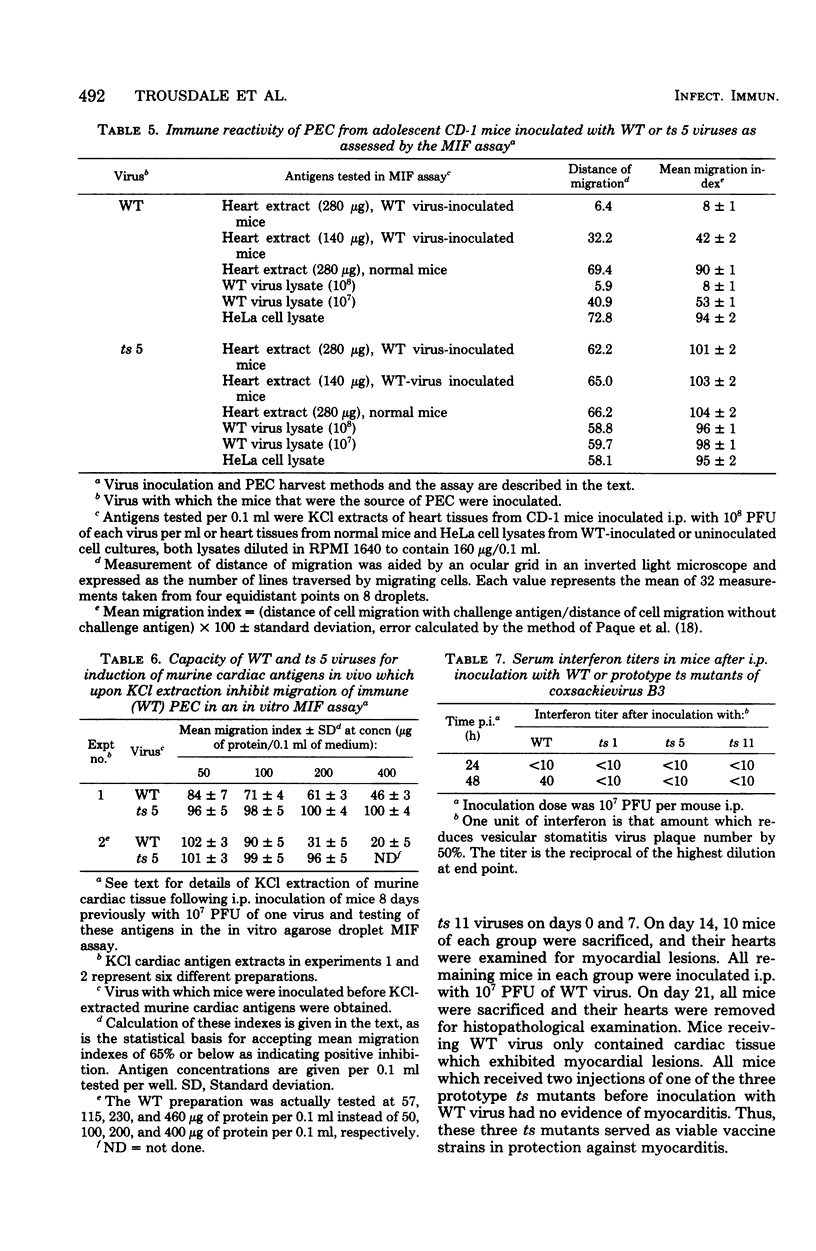
Ten temperature-sensitive (ts) mutants isolated from a myocarditis-inducing wild-type (WT) coxsackievirus B3 parent did not induce myocarditis in adolescent CD-1 mice. An avirulent prototype ts mutant from one of the three complementation groups adsorbed to murine cardiac tissue, as did WT virus. Heart tissues from mice inoculated with WT virus contained 100- to 1,000-fold more virus than heart tissues from mice inoculated with any of the three prototype ts mutants. WT virus exhibited a greater capsid stability and a higher efficiency of replication at 37°C than any of the three prototype ts mutants. All three prototype ts mutants induced less interferon in vivo than WT virus. Cell-mediated immune responses, assessed by the cell migration inhibition assay, were different in mice inoculated with WT virus when compared to ts 5 mutant virus. Peritoneal exudate cells from mice inoculated with WT but not ts 5 virus reacted specifically against antigens in WT virus HeLa cell lysates and antigens extracted with KCl from cardiac tissues of mice inoculated with WT virus. Cardiac tissues of mice inoculated with WT but not ts 5 virus contained KCl-extractable antigens which were able to specifically inhibit the migration of peritoneal exudate cells taken from mice immunized with WT virus. Therefore, ts 5 neither elicited a measurable cell-mediated immune response nor induced antigens in cardiac tissues which were immunoreactive with sensitized-(WT virus)-peritoneal exudate cells. Of 9 revertant viruses isolated from the 10 ts mutants, 5 showed covariance in ability to replicate at 39.5°C and capacity for induction of myocarditis. Some revertants exhibited a reduced capsid thermostability compared to WT virus but yet retained the capacity for induction of myocarditis. The data suggest that induction of myocarditis by coxsackievirus B3 variants depends on a combination of several variables, including capsid stability, capacity for replication at 37°C, and expression of the three identified genes. All three prototype ts mutants served as vaccine viruses in preventing myocarditis in adolescent mice subsequently challenged with WT virus. However, all three prototype ts mutants and their revertant variants retained partial to complete lethality in CD-1 neonates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstrand H., Källen B. On the statistical evaluation of the macrophage migration inhibition assay. Scand J Immunol. 1973;2(2):173–187. doi: 10.1111/j.1365-3083.1973.tb02028.x. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Giles T. D. The role of viruses in the production of heart disease. Am J Cardiol. 1972 Feb;29(2):231–240. doi: 10.1016/0002-9149(72)90634-0. [DOI] [PubMed] [Google Scholar]
- CROWTHER D., MELNICK J. L. The incorporation of neutral red and acridine orange into developing poliovirus particles making them photosensitive. Virology. 1961 May;14:11–21. doi: 10.1016/0042-6822(61)90127-1. [DOI] [PubMed] [Google Scholar]
- DE JAGER H., VAN CREVELD S. Myocarditis in newborns, caused by coxsackie virus; clinical and pathological data. Ann Paediatr. 1956 Jul-Aug;187(1-2):100–118. [PubMed] [Google Scholar]
- Gauntt C. J. Synthesis of ribonucleic acids in KB cells infected with rhinovirus type 14. J Gen Virol. 1973 Nov;21(2):253–267. doi: 10.1099/0022-1317-21-2-253. [DOI] [PubMed] [Google Scholar]
- Gear J. H., Measroch V. Coxsackievirus infections of the newborn. Prog Med Virol. 1973;15:42–62. [PubMed] [Google Scholar]
- HIATT C. W. Photodynamic inactivation of viruses. Trans N Y Acad Sci. 1960 Nov;23:66–78. doi: 10.1111/j.2164-0947.1960.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Helin M., Savola J., Lapinleimu K. Cardiac manifestations during a Coxsackie B5 epidemic. Br Med J. 1968 Jul 13;3(5610):97–99. doi: 10.1136/bmj.3.5610.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAVETT S. N., HEYMANN S., MUNDEL B., PEPLER W. J., LURIE H. I., GEAR J., MEASROCH V., KIRSCH Z. Myocarditis in the new newborn infant; a study of an outbreak associated with Coxsackie group B virus infection in a maternity home in Johannesburg. J Pediatr. 1956 Jan;48(1):1–22. doi: 10.1016/s0022-3476(56)80111-x. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Makinodan T., Santos G. W., Quinn R. P. Immunosuppressive drugs. Pharmacol Rev. 1970 Jun;22(2):189–247. [PubMed] [Google Scholar]
- Meltzer M. S., Leonard E. J., Rapp H. J., Borsos T. Tumor-specific antigen solubilized by hypertonic potassium chloride. J Natl Cancer Inst. 1971 Sep;47(3):703–709. [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J., Trousdale M. D. Assessment of cell-mediated hypersensitivity against coxsackievirus B3 viral-induced myocarditis utilizing hypertonic salt extracts of cardiac tissue. J Immunol. 1978 May;120(5):1672–1678. [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Effects of immunosuppression on coxsackie B-3 virus infection in mice, and passive protection by circulating antibody. J Gen Virol. 1973 Jun;19(3):339–351. doi: 10.1099/0022-1317-19-3-339. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Kahan B. D. Biological and chemical characterization of human histocompatibility antigens. Fed Proc. 1970 Nov-Dec;29(6):2034–2040. [PubMed] [Google Scholar]
- Stoker D., Kiernat J., Gauntt C. Interferon induction by rhinoviruses and effect of interferon on rhinovirus yields in human cell lines. Proc Soc Exp Biol Med. 1973 May;143(1):23–27. doi: 10.3181/00379727-143-37245. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Gauntt C. J. Isolation of Coxsackievirus B3 temperture-sensitive mutants and their assignment to complementation groups. Biochem Biophys Res Commun. 1976 May 23;76(2):368–375. doi: 10.1016/0006-291x(77)90734-3. [DOI] [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. I. Model and viral specificity1. J Immunol. 1977 Apr;118(4):1159–1164. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]