Abstract
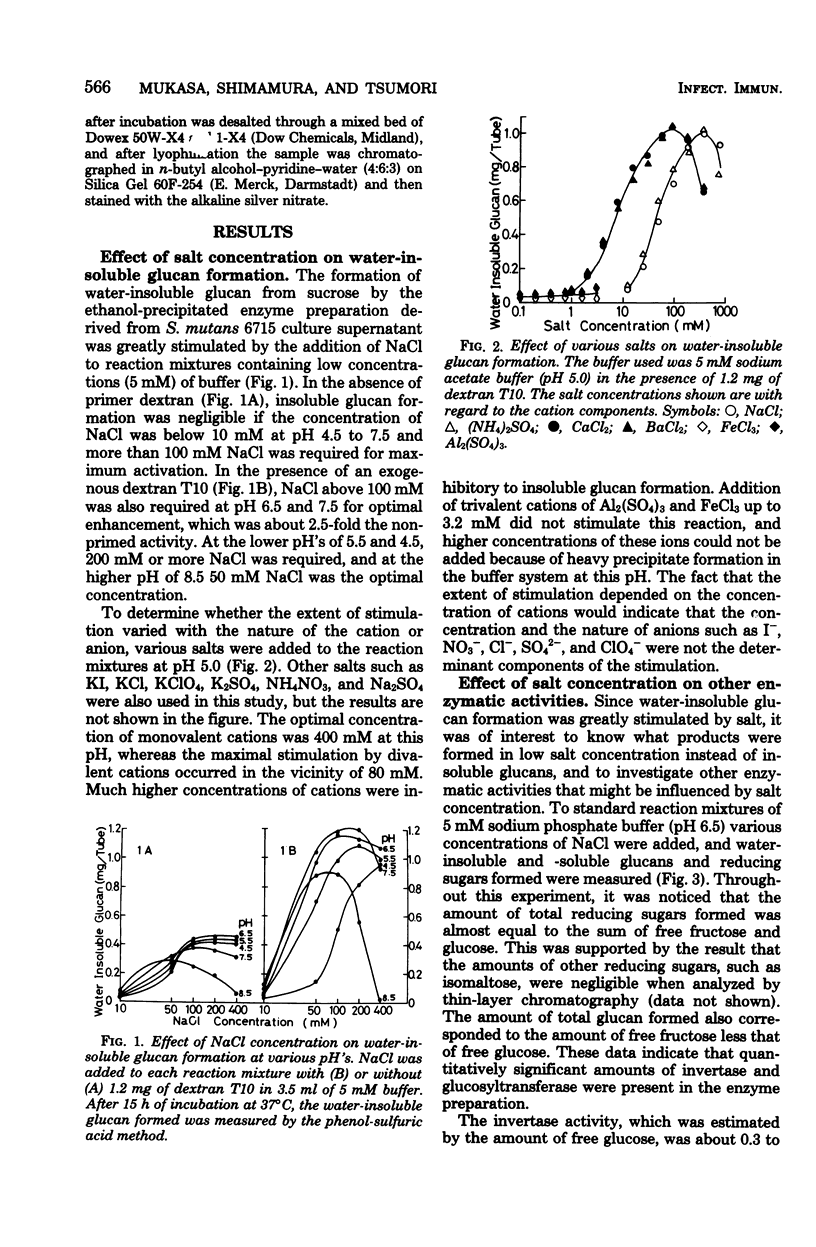
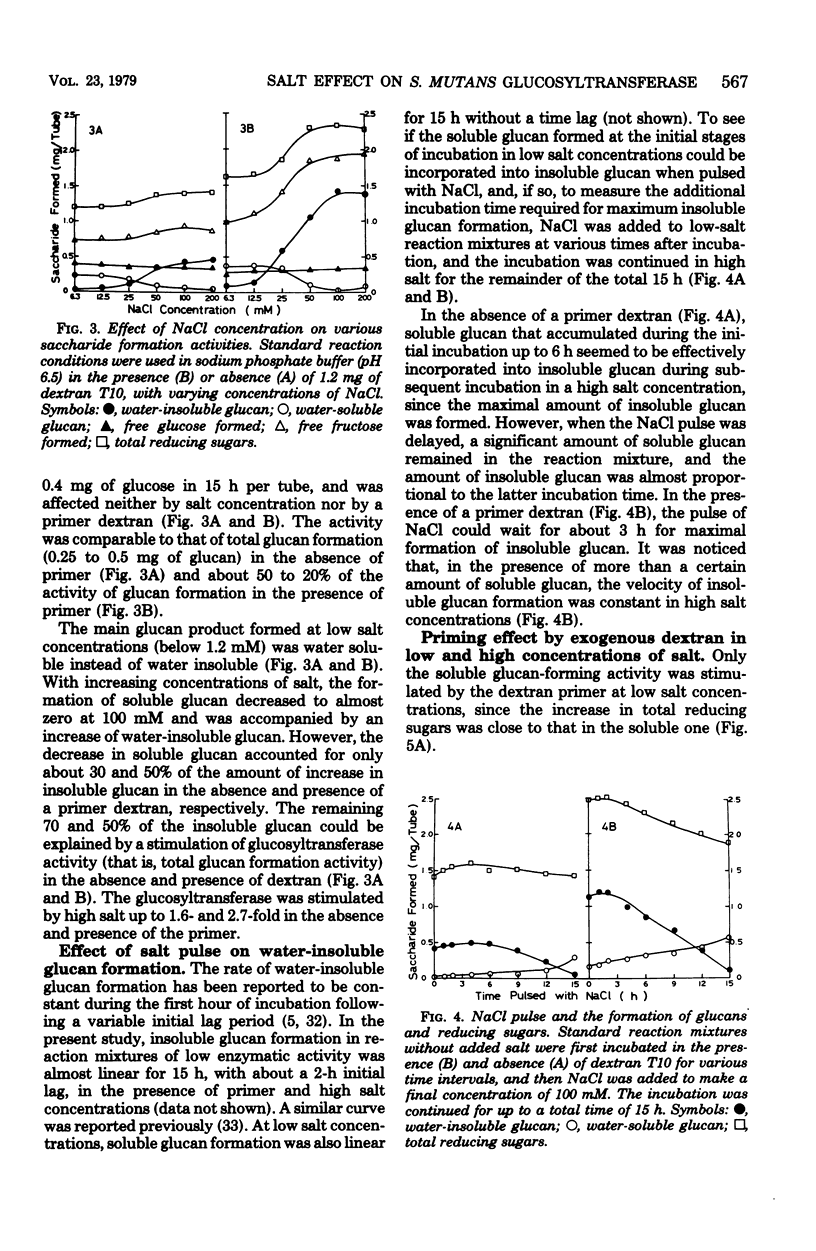
The formation of water-insoluble glucan by extracellular glucosyltransferase from Streptococcus mutans 6715 found to be greatly stimulated by various mono- or divalent cations. An enzyme preparation, obtained by ethanol fractionation, was able to catalyze the formation of water-insoluble glucan from sucrose in the presence of monovalent cations above 100mM or divalent cations above 20 mM at neutral pH. As the concentration of monovalent and divalent cations was reduced to below 10 mM and 1 mM, respectively, the formation of insoluble glucan decreased to a negligible amount. High concentrations of these cations were found to stimulate the formation of insoluble glucan in the following ways: (i) it increased the activity of total glucosyltransferase up to 1.6- and 2.7-fold in the absence and presence of a primer dextran, respectively, and (ii) it changed the formation of soluble glucan to insoluble. It was postulated that one of the essential factors for the formation of insoluble glucan would be to keep more than two water-soluble glucan chains close to enzyme aggregates and that such interaction could be enhanced by the presence of high cation concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Beaman A. J., Wittenberger C. L. Purification, resolution, and interaction of the glucosyltransferases of Streptococcus mutans 6715. Infect Immun. 1977 Oct;18(1):237–246. doi: 10.1128/iai.18.1.237-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN A., WEIL R. M., STERN K. G. On the mechanism of dextran formation; chromatographic studies with C14-labeled sugars. J Biol Chem. 1955 Dec;217(2):977–985. [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Harlander S. K., Schachtele C. F. Streptococcus mutans dextransucrase: stimulation of glucan formation by phosphoglycerides. Infect Immun. 1978 Feb;19(2):450–456. doi: 10.1128/iai.19.2.450-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L., Shklair I. L. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977 Jan;129(1):351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Inoue M. Cellular adherence, glucosyltransferase adsorption, and glucan synthesis of Streptococcus mutans AHT mutants. Infect Immun. 1978 Feb;19(2):402–410. doi: 10.1128/iai.19.2.402-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Properties of a mutant of Streptococcus mutans altered in glucosyltransferase activity. Infect Immun. 1976 Feb;13(2):345–353. doi: 10.1128/iai.13.2.345-353.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe K. Effect of salts and polyamines on T4 polynucleotide kinase. Biochemistry. 1975 Mar 25;14(6):1225–1229. doi: 10.1021/bi00677a021. [DOI] [PubMed] [Google Scholar]
- Matsuno T., Slade H. D. Composition and properties of a group A streptococcal teichoic acid. J Bacteriol. 1970 Jun;102(3):747–752. doi: 10.1128/jb.102.3.747-752.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Shiota T., Ikeda T., Navia J. M., McGhee J. R. Virulence of Streptococcus mutans: biochemical and pathogenic characteristics of mutant isolates. Proc Soc Exp Biol Med. 1975 Nov;150(2):498–502. doi: 10.3181/00379727-150-39064. [DOI] [PubMed] [Google Scholar]
- Montville T. J., Cooney C. L., Sinskey A. J. Distribution of dextransucrase in Streptococcus mutans and observations on the effect of soluble dextran on dextransucrase activities. Infect Immun. 1977 Dec;18(3):629–635. doi: 10.1128/iai.18.3.629-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J., Cooney C. L., Sinskey A. J. Measurement and synthesis of insoluble and soluble dextran by Streptococcus mutans. J Dent Res. 1977 Aug;56(8):983–989. doi: 10.1177/00220345770560082701. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisizawa T., Imai S., Akada H., Hinoide M., Araya S. Extracellular glucans produced by oral streptococci. Arch Oral Biol. 1976;21(3):207–213. doi: 10.1016/0003-9969(76)90131-x. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Corrigan A. J. The mechanism of dextransucrase action. Activation of dextransucrase from Streptococcus mutans OMZ 176 by dextran and modified dextran and the nonexistence of the primar requirement for the synthesis of dextran. Arch Biochem Biophys. 1977 Oct;183(2):726–731. doi: 10.1016/0003-9861(77)90406-4. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Germaine G. R. Streptococcus mutans dextransucrase: availability of disaggregated enzyme after growth in a chemically defined medium. Infect Immun. 1976 May;13(5):1522–1524. doi: 10.1128/iai.13.5.1522-1524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Staat R. H., Harlander S. K. Dextranases from oral bacteria: inhibition of water-insoluble glucan production and adherence to smooth surfaces by Streptococcus mutans. Infect Immun. 1975 Aug;12(2):309–317. doi: 10.1128/iai.12.2.309-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Gawronski T. H., Schachtele C. F. Detection and preliminary studies on dextranase-producing microorganisms from human dental plaque. Infect Immun. 1973 Dec;8(6):1009–1016. doi: 10.1128/iai.8.6.1009-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Lee T. K., Dunlap R. B. Dihydrofolate reductase from amethopterin-resistant Lactobacillus casei. Effects of pH, salts, temperature, and source of NADPH on enzyme activity and substrate specificity studies. Arch Biochem Biophys. 1977 Jun;181(2):569–579. doi: 10.1016/0003-9861(77)90263-6. [DOI] [PubMed] [Google Scholar]


