Abstract
Hearing and balance rely on the faithful synaptic coding of mechanical input by the auditory and vestibular hair cells of the inner ear. Mechanical deflection of their stereocilia causes the opening of mechanosensitive channels, resulting in hair cell depolarization, which controls the release of glutamate at ribbon-type synapses. Hair cells have a compact shape with strong polarity. Mechanoelectrical transduction and active membrane turnover associated with stereociliar renewal dominate the apical compartment. Transmitter release occurs at several active zones along the basolateral membrane. The astonishing capability of the hair cell ribbon synapse for temporally precise and reliable sensory coding has been the subject of intense investigation over the past few years. This research has been facilitated by the excellent experimental accessibility of the hair cell. For the same reason, the hair cell serves as an important model for studying presynaptic Ca2+ signaling and stimulus-secretion coupling. In addition to common principles, hair cell synapses differ in their anatomical and functional properties among species, among the auditory and vestibular organs, and among hair cell positions within the organ. Here, we briefly review synaptic morphology and connectivity and then focus on stimulus-secretion coupling at hair cell synapses.
Keywords: Hair cell, Ribbon synapse, Cochlear, Vestibular, Inner ear
Introduction
The coding of information with respect to sounds and head movements by the hair cell afferent synapse requires synaptic transmission to be both reliable and temporally precise. Even in the absence of stimulation, the hair cell ribbon-type active zone drives “spontaneous” spiking in the afferent neuron at rates of up to or even beyond 100 Hz (Kiang et al. 1965; Liberman 1982; Brichta and Goldberg 2000). To ensure such remarkable temporal fidelity and high throughput, the hair cell afferent synapse relies on unique molecular and structural specializations, some of which have been elucidated only recently.
Mature afferent hair cell synapses usually display one single synaptic ribbon or synaptic body facing one postsynaptic density (Fig. 1). Ribbons tether a monolayer of synaptic vesicles, with a high packing density (Lenzi et al. 1999). A fraction of these ribbon-associated vesicles “docks” onto the presynaptic membrane. In addition, some docked vesicles are not associated with the ribbon, and some cytosolic vesicles neither touch the presynaptic membrane nor bind to the ribbon. The synaptic vesicles that populate the hair cell active zones comprise only a tiny fraction of the huge number of synaptic vesicles contained in a hair cell (Spicer et al. 1999).
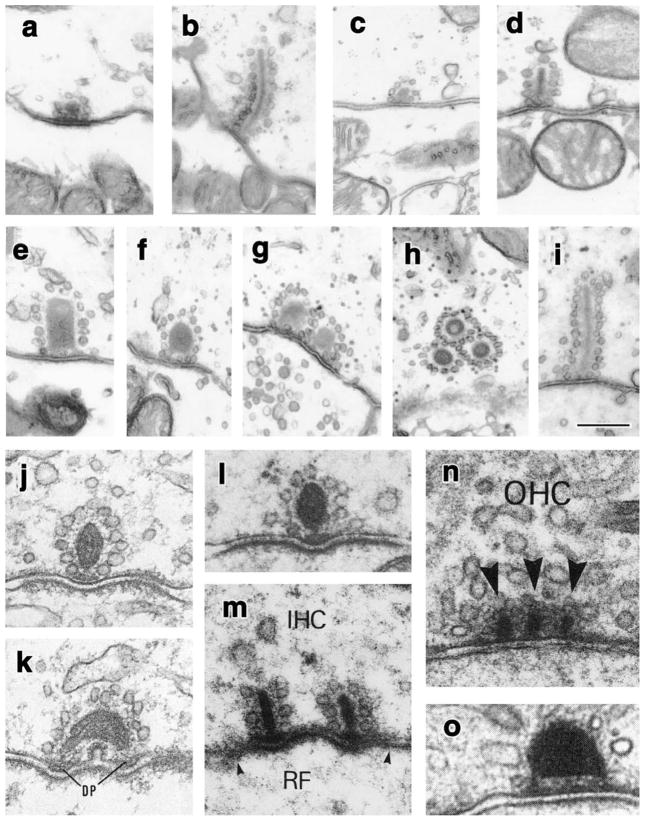
Fig. 1.
Electron micrographs of ribbon synapses from hair cells. a–i Ribbons from type I and type II vestibular hair cells in the adult chinchilla crista ampullaris (from Lysakowski and Goldberg 1997, J. Comp. Neurol. vol. 491, pp. 438–439, modified with permission from John Wiley and Sons, Inc.). Type I hair cells generally have small spherical ribbons, regardless of their location within the sensory epithelium (central, a, b; peripheral, c, d). The elongated ribbon from a central type I hair cell in b is an exception in the chinchilla, although much more common in the monkey (Engstrom et al. 1972). Type II hair cells, particularly those in the central part of the sensory epithelium, where sensitivity is highest, show a large variation in shapes and sizes. They can be large and hollow (e, g), spheroid (f), and elongated and plate-like (i), extending through 10–12 ultrathin sections. They can occur singly (e, f, i) or in clusters (g, h). Ribbons in type II hair cells in the peripheral part of the epithelium tend to be small and spheroid (similar to the peripheral type I hair cell ribbons in c, d). j–o Ribbons from cochlear hair cells in diverse species. Inner hair cells (j–m, IHC) have variable ribbon shapes, whereas outer hair cell (OHC) ribbons tend to be elongated rods (n, o), although the ribbon in o is a less common spheroid ribbon. j, k Middle turn of guinea pig cochlea, inner hair cells (DP dense plaques found presynaptically); modified from Saito (1980) J. Ultrastructural Research, vol. 71, p. 225, with permission from Elsevier. l Cat cochlea, tonotopic location approximately 2.4 kHz, modified from Liberman (1980) Hear. Res., vol. 3, p. 56, with permission from Elsevier. m, n Cat cochlea; inner hair cell (IHC) at 0.5 kHz, and outer hair cell (OHC), respectively, probably from an apical turn, given the multiple ribbons (RF radial fiber afferent, arrows indicate extent of synaptic plaque) arrowheads docked vehicles; modified with permission from Liberman et al. (1990) J. Comp. Neurol. vol. 301, p. 447, with permission from John Wiley and Sons, Inc. (o) Taken from macaque cochlea, outer hair cell, location unknown; from Kimura (1984), modified with permission from Friedmann and Ballantyne (1984) Ultrastructural Altas of the Inner Ear, Taylor and Francis Publishers
To study hair cell transmitter release in vivo and in vitro, microelectrode (e.g., Kiang et al. 1965; Rose et al. 1967; Liberman 1982; Furukawa 1986; Goldberg et al. 1990; Holt et al. 2006), patch-clamp recordings from afferent neurons (Glowatzki and Fuchs 2002; Keen and Hudspeth 2006), patch-clamp measurements of presynaptic exocytic membrane capacitance changes (e.g., Parsons et al. 1994; Moser and Beutner 2000), and fluorescence microscopy of presynaptic membrane turnover (e.g., Griesinger et al. 2005), have been used. These experiments have provided insights into the mechanisms of synaptic coding of auditory and vestibular stimuli, such as stimulus secretion coupling, synaptic vesicle pool dynamics, and the kinetics of transmitter release.
Once the stereocilia are mechanically stimulated, mechanosensitive channels open and mediate the receptor current. The resulting depolarization activates voltage-gated Ca2+ channels, mostly CaV1.3 L-type Ca2+ channels. Ca2+ channels cluster at the active zone (Lewis and Hudspeth 1983; Art and Fettiplace 1987; Roberts et al. 1990; Rodriguez-Contreras and Yamoah 2001; Zenisek et al. 2003; Sidi et al. 2004; Brandt et al. 2005) and are the source of the Ca2+ signal that drives transmitter release (Platzer et al. 2000; Brandt et al. 2003). Two competing hypotheses of stimulus-secretion coupling, viz., the control of synaptic vesicle exocytosis by Ca2+ microdomains (Roberts et al. 1990; Roberts 1994; Tucker and Fettiplace 1995) versus Ca2+ nanodomains (Brandt et al. 2005), have been put forward and will be discussed in this review. Evidence has been provided for the existence of a readily releasable vesicle pool (RRP) that mediates synchronous synaptic transmission. The ribbon stabilizes a large RRP that supports the release of several vesicles within a short time at each active zone (Moser and Beutner 2000; Khimich et al. 2005). Close inspection of the transmitter release permitted by sensitive postsynaptic recordings has indicated that the exocytosis of multiple vesicles can be highly synchronized (Glowatzki and Fuchs 2002; but for an alternative explanation of quantal size variability in vestibular hair cells, see Holt et al. 2006). Loss of the ribbon reduces the RRP and impairs synchronous postsynaptic activation, leading to the conclusion that the ribbon synapse uses the parallel release of several synaptic vesicles to improve the temporal precision of postsynaptic spiking (Khimich et al. 2005). Whereas some hair cells form multiple synaptic contacts with one postsynaptic neuron, each active zone of mammalian auditory inner hair cells (IHCs) drives just one postsynaptic neuron. Thus, in contrast to large central auditory synapses such as the endbulb and the calyx of Held, which achieve highly reliable and temporally precise transmission with many small active zones (for a review, see Schneggenburger and Forsythe 2006, this issue), the ribbon synapse of mammalian auditory hair cells uses a single active zone holding a large RRP, as another structure for the same task.
Short-term synaptic depression, involving partial RRP depletion, most likely contributes to peripheral auditory adaptation (Furukawa and Matsuura 1978; Moser and Beutner 2000; Spassova et al. 2004), which has been implicated in the peripheral processing of complex sound signals (Delgutte 1980). Interestingly, whereas the onset of the postsynaptic response varies greatly across different stimulus intensities applied to goldfish saccular hair cells, the time course of adaptation is invariant (Furukawa and Matsuura 1978). In agreement with the hypothesis that RRP depletion underlies rapid peripheral auditory adaptation, the amplitude of the receptor potential has been found to determine the RRP size, but not the time course of pool depletion to the same extent (Brandt et al. 2005). The putative mechanism underlying this behavior will be discussed later in this review. A detailed discussion of vesicle pools, their dynamics, and potential morphological correlates has been provided recently (Nouvian et al. 2006).
Once released, glutamate binds and activates α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid (AMPA) receptors (Glowatzki and Fuchs 2002) that are clustered in the postsynaptic density (Matsubara et al. 1996). Finally, glutamate is taken up by glutamate transporters (GLAST) of glia-like supporting cells (Furness and Lehre 1997; Furness and Lawton 2003) avoiding glutamate accumulation and hence limiting excitotoxic damage in the case of strong stimulation (Hakuba et al. 2000). The type I vestibular hair cell-calyx synapse isolates the hair cell from the supporting cells.
Progress in understanding the molecular anatomy of hair cell synapses (Safieddine and Wenthold 1999; Eybalin et al. 2002; Khimich et al. 2005) has been slow, mostly because of the limited amount of material (a few thousand hair cells per ear). Although we are far from a detailed description (for a recent review, see Nouvian et al. 2006), we can state that the molecular composition is similar to that of retinal synapses (for a review, see tom Dieck and Brandstätter 2006, this issue). Important differences include the dominance of CaV1.3 channels and the presence of the putative Ca2+ sensor of exocytosis otoferlin (Yasunaga et al. 1999).
Here, we will first review the common features and different aspects of active zone morphology and synaptic connectivity in auditory and vestibular hair cells. We will then discuss the general properties of presynaptic function in hair cells. We will also focus on ion channels and Ca2+ signaling related to transmitter release.
Structure of hair cell ribbon synapses and synaptic connectivity
Specialization of auditory and vestibular hair cell synapses results in substantial anatomical diversification. Hence, few “typical hair cell afferent synapses” exist. Hair cell ribbons vary greatly in size (from ~0.1 to ~0.4 μm) and shape (from plate-like ribbons to spheroid bodies) among the various preparations (Fig. 1, Table 1). Thus, the number of vesicles tethered by a ribbon ranges from 20 to 30 in peripheral vestibular type II hair cells (Lysakowski and Goldberg 1997), from 100 to 200 in mouse cochlear IHCs (Khimich et al. 2005), and from 150 to 300 in central type II vestibular hair cells (Lysakowski and Goldberg 1997) and is ~400 in frog saccular hair cells (Lenzi et al. 1999). The relationship between structural and functional vesicle populations at ribbon-type active zones is still a matter of debate (for a review, see Nouvian et al. 2006). There seems to be an agreement at least for mouse IHCs (Moser and Beutner 2000), turtle hair cells (Schnee et al. 2005), and frog saccular hair cells (Rutherford and Roberts 2006) that the first exhaustible kinetic component (the RRP) corresponds to the docked vesicles (but, for an opposing view, see Edmonds et al. 2004; Spassova et al. 2004).
Table 1.
Synaptic morphology and synaptic connectivity (HC hair cell, Fib fiber, LF low frequency, HF high frequency, Syn synapse, ND not determined). The table summarizes part of the available information obtained in the various preparations. The references are examples from which information was obtained, but which do not represent a complete list. Data were obtained by electron microscopy except for those obtained by immunofluorescence (Khimich et al. 2005)
| Species/Organ | Hair cell | Ribbon: shape, size (nm), and number | Afferent connectivity | References |
|---|---|---|---|---|
| Mammalian cochlea | Inner hair cells | Ellipsoid; 3 axes: 55, 100–200, 200–600; 5–30 | 1 HC: 5–30 Fib; 1 Fib: 1 HC; 1 Fib: 1 Syn | Liberman 1980; Francis et al. 2004; Khimich et al. 2005 |
| Outer hair cell (apex) | Elongate; 100–200; 8–10 | Liberman et al. 1990 | ||
| Avian papilla | Tall hair cells: | Round; 120 (apex), 210 (base); 15 | 1 HC: 1–3 Fib; 1 Fib: 1(−3) HC*; 1 Fib: several Syn | Fischer 1992; Martinez-Dunst et al. 1997 |
| Short hair cells | 0 (HF) −10 (LF) | |||
| Mammalian crista ampullaris | Type I hair cell | Spheroid: 90, Rod: 200–300. ~17 | 1 HC: 1 Fib; 1 Fib: 1–5 HC; 1 Fib: 1–5 Calyces with 15 Syn each | Goldberg et al. 1990 |
| Type II hair cell | Barrel: 200 Elongate 150–300. ~18 | 1 HC: 3–6 Fib; 1 Fib (bouton): −30 HC; 1 Fib (bouton): 50–80 Syn |
Lysakowski and Goldberg 1997 Fernandez et al. 1988 |
|
| Avian crista ampullaris | Type I hair cell type II hair cell |
Spheroid; rod ND | 1 Fib: up to 12 HCs | Lysakowski 1996 |
| Turtle crista ampullaris | Type I hair cell type II hair cell |
Spheroid; ND | 1 Fib: up to 8 HCs | Lysakowski 1996; Brichta and Peterson 1994 |
| Frog crista ampullaris | Type II hair cell | Spheroid; ND | Lysakowski 1996 | |
| Fish crista ampullaris | Type II hair cell | Spheroid 15–25 central; 4–5 peripheral | Wegner 1982; Chang et al. 1992; Lysakowski 1996 |
The way that a hair cell makes synaptic contact with postsynaptic neuron or neurons (synaptic connectivity) varies among auditory and vestibular hair cells and between hair cells of different species. Synaptic connectivity is probably still most uniform in the auditory end organ (Fig. 2, Table 1). Here, we will illustrate this for the mammalian cochlea and the avian basilar papilla. In the mammalian cochlea, a clear functional segregation of the hair cells exists. The IHCs are the genuine sensory cells coding sound information, whereas the outer hair cells (OHCs) have been implicated in the mechanical amplification of sound-induced cochlear vibration. Depending on the species and the position along the tonotopic axis, IHCs contain 5–30 active zones driving a corresponding number of unbranched processes of myelinated spiral ganglion neurons. These type I spiral ganglion neurons (95% of the spiral ganglion neurons) vary in their spontaneous rate and threshold (e.g., Liberman 1982). Synapses of low spontaneous rate fibers, mostly located at the neural (modiolar) side of the IHC, tend to have larger ribbons than those of the high spontaneous rate fibers on the abneural (pillar) side (Merchan-Perez and Liberman 1996; Tsuji and Liberman 1997; Slepecky et al. 2000). OHCs in the apical cochlear turns form ribbon synapses with unmyelinated type II spiral ganglion neurons (Berglund and Ryugo 1987; Liberman et al. 1990). The function of OHC afferent synaptic transmission remains to be clarified.
Fig. 2.
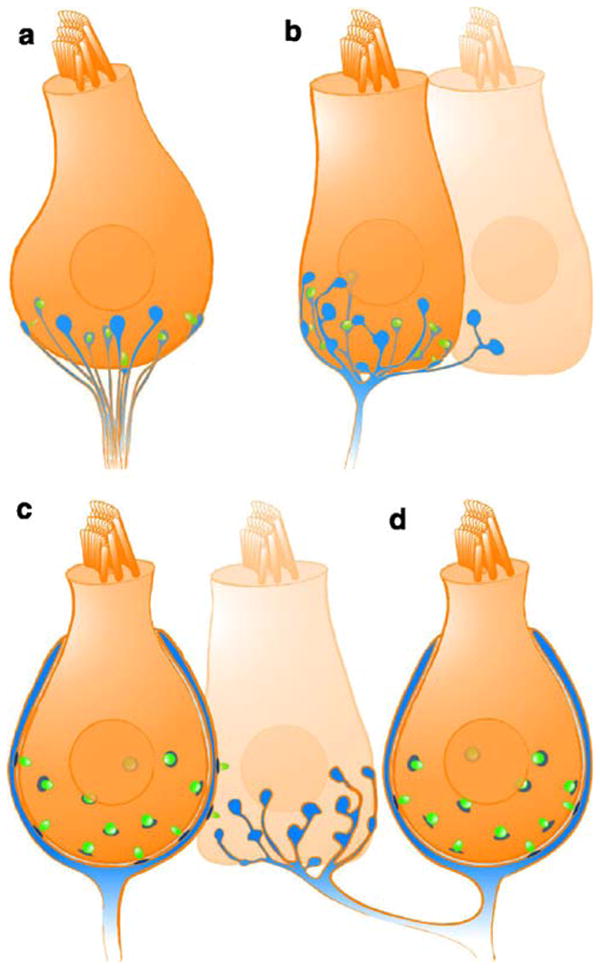
Afferent synaptic connectivity of hair cells. Four different types of afferent connections are found in the auditory and/or vestibular system. For simplification, all other cellular elements (e.g., efferent contacts and supporting cells) have been ignored. a Several unbranched processes, each forming one synapse (blue fiber and postsynaptic density, green synaptic ribbon) with one hair cell, as is typical for mammalian cochlear inner hair cells. b Branched process innervating two hair cells. Each branch collects input from one or more ribbon synapses. c Calyx-type postsynaptic ending enclosing a type I vestibular hair cell and forming several ribbon synapses with the enclosed hair cell in addition to some synapses on its outer surface with a neighboring type II hair cell. Such “outer face” synapses are more common in the central part of the sensory epithelium. d A dimorphic fiber that forms a calyx synapse with a type I hair cell and bouton synapses with type II hair cells
The avian auditory end organ, the basilar papilla, does not show a strict dichotomy of hair cell types. Rather, a continuum ranges from tall hair cells with closest proximity to the neural edge, to the short hair cells near the abneural side of the organ. Likewise, the innervation pattern is not as segregated as in the mammalian cochlea. The number of afferent fibers per tall hair cell is lower than that of IHCs in the cochlea. Most fibers exclusively contact one tall hair cell (Fischer 1992) and collect input from several synapses.
The vestibular system is particularly rich in synaptic specializations (Fig. 2), featuring bouton-, dimorphic-, and calyx-type afferent endings (Fernandez et al. 1988, 1990, 1995; Goldberg et al. 1990). Each vestibular neuron receives input from multiple ribbon synapses of one (or more than one) hair cell in the case of a calyx or dimorphic afferent, or of many hair cells in the case of a bouton or dimorphic afferent (Lysakowski and Goldberg 1997). Calyx-type terminals not only receive ribbon synapse input from the enclosed type I hair cells on their inner face, but can also receive this input from neighboring type II hair cells on their outer face, although whether these outer face synapses have equal weight is unknown. Figure 2 illustrates the spectrum of afferent hair cell connectivity observed in the various preparations.
Convergent input from several synapses of one or multiple hair cells onto an afferent neuron (e.g., vestibular system, avian basilar papilla) is likely to enhance the evoked activity over background. Detection of coincident synaptic inputs by the action-potential-generating element would help to suppress uncorrelated spontaneous synaptic input. On the other hand, the number of statistically independent channels carrying sensory information to second order neurons will be lower than, for example, in the mammalian cochlea with potential consequences for processing in the brainstem. In the vestibular system, with its large variation in synaptic ribbons, afferent fibers, and terminal endings, it would be surprising if these did not permit a wide latitude in coding mechanisms relevant to vestibular function (Goldberg 2000). Future work will be needed to clarify the exact way that the different connectivities relate to coding strategies of auditory and vestibular stimuli.
Ion channels affecting hair cell presynaptic function
Hair cells display a resting membrane conductance based on basolateral potassium channels (KCNQ and erg families) and stereociliar mechanoelectrical transduction channels. Mutation of the gene coding for KCNQ4 causes autosomal dominant hearing impairment DFNA2 in humans (Kubisch et al. 1999). The hearing loss of mouse models of DFNA2 can mostly be explained by the dysfunction/degeneration of mechanically active OHCs (Kharkovets et al. 2006), as had been suggested before in a pharmacological model of DFNA2 (Nouvian et al. 2003). The DFNA2 mouse models show no major change of IHCs synaptic function and no obvious vestibular dysfunction. In addition to KCNQ channels, vestibular type I hair cells express erg channels (Eatock et al. 2002; Wong et al. 2004), which may contribute to a distinctive current found in type I hair cells, IK,L (K.M. Hurley, S. Gaboyard, J.R. Wooltorton, J.L. Garcia, S.D. Price, A. Lysakowski, R.A. Eatock, submitted for publication).
CaV1.3 Ca2+ channels of hair cells (see below) become activated at the resting potential of the hair cell even in the absence of sensory stimuli (Koschak et al. 2001; Xu and Lipscombe 2001; Brandt et al. 2005; Johnson et al. 2005). Afferent neuron “spontaneous” activity seems to rely entirely on transmitter release from hair cells (Liberman and Kiang 1978; Sewell 1984). Once mechanoelectrical transduction occurs, the apical potassium influx drives receptor potential generation, which further activates voltage-gated K+ and Ca2+ channels. BK (large conductance) currents are activated within hundreds of microseconds and have been implicated in shortening the hair cell membrane time constant required for high temporal precision sound coding, in limiting of the receptor potential, and in electrical frequency tuning (in non-mammalian hair cells). Electrical hair cell tuning to a certain stimulus frequency builds on a intimate interplay of BK and Ca2+ channels, which require close spatial colocalization, most likely at the active zones (Lewis and Hudspeth 1983; Art et al. 1986; Fuchs et al. 1988; Roberts et al. 1990; Tucker and Fettiplace 1995). This has fostered the use of BK channels as a readout of active zone Ca2+ signaling of hair cells (see next section). Surprisingly, BK channels of mammalian cochlear IHCs cluster remotely from the active zones at the apical part of the cell (neck; Pyott et al. 2004; Hafidi et al. 2005) and are largely unaffected by manipulations of Ca2+ channels (Marcotti et al. 2004; Thurm et al. 2005). This finding might be related to the evolution of other efficient tuning mechanisms in mammalian cochlea. Deletion of the Kcnma1 gene in mice has surprisingly little primary impact on hearing as revealed by auditory brainstem responses (Ruttiger et al. 2004), which reflect the sound-evoked synchronized activation of neuronal populations along the auditory pathway. However, single hair cell and single spiral ganglion neuron analysis has revealed defects in the timing of receptor potentials and subsequently of postsynaptic action potential generation (Oliver et al. 2006). Activation of KV-type channels causes a millisecond time course decline of the hair cell receptor potential and may thereby contribute to rapid auditory adaptation (Kros 1996; Moser and Beutner 2000).
Evidence for an essential role of L-type Ca2+ channels for hair cell transmitter release is well documented (Lewis and Hudspeth 1983; Art and Fettiplace 1987; Fuchs et al. 1990; Roberts et al. 1990; Zhang et al. 1999; Moser and Beutner 2000; Platzer et al. 2000; Spassova et al. 2001; Robertson and Paki 2002; Brandt et al. 2003; Dou et al. 2004; Schnee et al. 2005). Two independently generated CaV1.3 knockout mice show complete deafness (lack of auditory brainstem responses) and more than a 90% reduction of hair cell Ca2+ currents (Platzer et al. 2000; Brandt et al. 2003; Michna et al. 2003; Dou et al. 2004), but no obvious balance problems. Interestingly, Ca2+ uncaging, bypassing the defect in Ca2+ influx, elicits robust exocytic capacitance changes (Brandt et al. 2003). This result together with the finding of a largely unchanged molecular synaptic anatomy in young mice (Nemzou et al. 2006) argues that the presynaptic machinery is assembled, despite the lack of Ca2+ influx. The remaining Ca2+ current of cochlear hair cells is at least partially mediated by L-type Ca2+ channels and is insensitive to blockers of N, P/Q, and R-type Ca2+ channels. The Yamoah group has presented single channel evidence for the presence of non-L, possibly N-type, channels in frog saccular hair cells (Rodriguez-Contreras and Yamoah 2001; Rodriguez-Contreras et al. 2002). In frog crista hair cells, R-type Ca2+ channels have been suggested by pharmacological analysis of whole-cell Ca2+ currents (Martini et al. 2000). This, together with normal performance of CaV1.3-deficient mice in screening tests of vestibular function (Dou et al. 2004), has led the authors to conclude that non-L-type channel can mediate transmitter release in vestibular hair cells.
Stimulus-secretion coupling at the hair cell ribbon synapse
Many aspects of presynaptic Ca2+ signaling are inaccessible in small presynaptic boutons of the CNS but can be readily studied and manipulated in hair cells. The classical work of Roberts and Fettiplace and their colleagues on Ca2+ channel number and distribution and on cytosolic Ca2+ buffers has largely contributed to making the hair cell a valuable model system for presynaptic Ca2+ signaling (for previous reviews, see Lenzi and Roberts 1994; Tucker and Fettiplace 1996; Augustine 2001; Augustine et al. 2003). Moreover, the characterization of stimulus-secretion coupling in hair cells is the key to understanding the coding of information regarding sound or vestibular stimuli.
The actual Ca2+ channel release-site topography at a given active zone remains unknown for hair cell ribbon synapses. Do Ca2+ channels cooperate to impose an elevation of the Ca2+ concentration (Ca2+ microdomain), or do individual channels drive “their” vesicles, which are positioned in nanometer proximity (Ca2+ nanodomain)? Figure 3 illustrates both concepts. Roberts and colleagues (1990) have inferred a specific topography of Ca2+ and K+ channels at the synapses of frog saccular hair cells based on the finding of clusters of intramembrane particles in freeze-fracture electron micrographs at presumptive synapses and the numerical correspondence of particle count and physiologically determined numbers of Ca2+ and BK channels. However, numerous particles also exist outside the arrays, and the number and distribution of Ca2+ channels relative to the docked vesicles still awaits quantitative analysis, e.g., by immunoelectron microscopy.
Fig. 3.

Contrasting views of Ca2+ signaling at hair cell active zones. a Modeling of active zone Ca2+ concentration for frog saccular hair cells; from Roberts (1994), reprinted with permission from the Journal of Neuroscience. The model was based on measurements of voltage-gated Ca2+ entry, the accompanying activation of BK current in frog saccular hair cells, and an estimation of cytosolic Ca2+ buffering capacity. Ca2+ microdomains soak the entire active zone with some locally active maxima. b Representation of the inner hair cell active zone seen from the hair cell cytosolic side with the ribbon (medium gray ellipse projection) removed (from Brandt et al. 2005; reprinted with permission from the Journal of Neuroscience). Synaptic vesicles (gray spheres) preferentially dock to the plasma membrane (transparent) opposite the postsynaptic density (light gray). Approximately 80 Ca2+ channels (black dots) were pseudo-randomly scattered at the active zone with a higher density underneath the ribbon (dark gray ellipsoid) than for the rest of the active zone (light gray ellipsoid postsynaptic density). Synaptic vesicles were placed in a more regular array according to electron microscopic findings (c.f. Fig. 1). Domains of elevated [Ca2+] are indicated in black. In the case of weak stimulation, only a few channels open and drive exocytosis of “their” vesicles, whereas an overlap of Ca2+ domains is expected for saturating stimulation
Even if the Ca2+ channel/release site topography at the active zone were known, it remains difficult to predict whether release is controlled by the Ca2+ nanodomain shaped by stochastic gating of one or few Ca2+ channels (as suggested by Brandt et al. 2005) or by a Ca2+ microdomain produced by many channels (Roberts 1994; Tucker and Fettiplace 1995) where the single channel properties are averaged. This difficulty arises because the functional stimulus-secretion coupling further depends on the channel number, distribution, and open probability (set by the stimulus and the maximal open probability), the width of the single channel Ca2+ domain (set by the single channel current and the cytosolic Ca2+ buffers), and the intrinsic Ca2+ sensitivity of exocytosis. Substantial work has been performed in the past to quantify these parameters and will be reviewed here.
Number and distribution of Ca2+ channels in hair cells
Evidence arguing for a micrometer-scale clustering of Ca2+ channels at the hair cell ribbon-type active zone comes from loose patch recordings (Roberts et al. 1990), cell-attached recordings (Rodriguez-Contreras and Yamoah 2001), Ca2+ imaging (Issa and Hudspeth 1994; Tucker and Fettiplace 1995; Zenisek et al. 2003), and immunohistochemistry (Sidi et al. 2004; Brandt et al. 2005). Table 2 summarizes the available information on Ca2+ channel number in hair cells and the approximate total and maximal open Ca2+ channel numbers per active zone for the various hair cell types. As pointed out by Martinez-Dunst et al. (1997), hair cell Ca2+ current seems to scale not only with the number of synapses, but also with the size of the active zone. Hair cell Ca2+ channel numbers have been obtained by (1) non-stationary fluctuation analysis, (2) dividing the whole cell Ca2+ current (tail or pulse current) by the single channel current. Approximations of the number of Ca2+ channels per active zone either assume a purely synaptic distribution (Roberts et al. 1990; Tucker and Fettiplace 1995; Martinez-Dunst et al. 1997) or consider a certain extrasynaptic density of Ca2+ channels in hair cells (Brandt et al. 2005).
Table 2.
Number of Ca2+ channel at hair cell synapses. This table summarizes part of the available information obtained in diverse preparations. The analysis is complicated by the low open probability of hair cell L-type Ca2+ channels in the absence of the dihydropyridine agonist of L-type Ca2+ channels, BayK8644. The numbers provided are rough approximations relying on several assumptions (e.g., extrasynaptic Ca2+ channel density) and should be taken with caution (LF low frequency, HF high frequency)
| Species/organ | Hair cell | Total number of channels | Channels per active zone (total/maximal open) | References |
|---|---|---|---|---|
| Frog sacculus | ~1,800 | ~90/~20 | Roberts et al. 1990a, assuming an exclusively synaptic Ca2+ channel localization | |
| Turtle basilar papilla | ~2,240 (HF) ~400 (LF) |
~40 ~20 |
Wu et al. 1995b; Sneary 1988, assuming an exclusively synaptic Ca2+ channel localization | |
| Mammalian cochlea | Inner hair cells | ~1,700 | ~80/~30 | Brandt et al. 2005,a assuming an extrasynaptic channel density of 1 channel/μm2 |
| Avian basilar papilla | Tall hair cells | ~350 (HF) ~200 (LF) |
~15 ~23 |
Martinez-Dunst et al. 1997b, assuming an exclusively synaptic Ca2+ channel localization |
| Short hair cells | ~100 |
Estimates obtained by non-stationary fluctuation analysis on Ca2+ tail currents estimates
Approximations provided by the cited reference based on whole-cell Ca2+ or Ba2+ current measurements and single channel currents
The assumption of a purely synaptic localization has been motivated by the co-variation of Ca2+ current and active zone number (e.g., Martinez-Dunst et al. 1997; Wu et al. 1995; Schnee et al. 2005). However, a substantial, albeit much lower, abundance of L-type Ca2+ channels has been found outside the observed dense clusters (Rodriguez-Contreras and Yamoah 2001) in cell-attached measurements of Ca2+ channel distribution in frog saccular hair cell. Imaging of Ca2+ entry (low affinity dyes and excess non-fluorescent high-affinity exogenous Ca2+ buffers) and of immunostained Ca2+ channels is not capable of detecting extrasynaptic channels, which probably occur at low density. Hence, further experiments, such as immunoelectron microscopy and cell-attached recordings of Ca2+ channel distribution in hair cells with a known relationship to the synapse position, are needed.
Ca2+ buffering in hair cells
Work by the groups of Hudspeth and Roberts and Fettiplace and colleagues has indicated a millimolar concentration of fast mobile Ca2+-binding proteins in frog (Roberts et al. 1990; Edmonds et al. 2000; Heller et al. 2002) and turtle (Tucker and Fettiplace 1995) hair cells (see Table 3). The presence of the mobile proteinaceous Ca2+ buffers calretinin, calbindin, and parvalbumin has now been documented in a variety of cochlear and vestibular hair cells (Edmonds et al. 2000; Heller et al. 2002; Hackney et al. 2003, 2005; Desai et al. 2005a,b). Their relevance for local hair cell Ca2 + signaling has been inferred from recordings of Ca2+-activated large conductance K+ channels (Fettiplace 1992; Roberts 1993; Tucker and Fettiplace 1996; Edmonds et al. 2000), from investigations of the adaptation of mechanoelectrical transduction current (Ricci et al. 1998), from simulations (Roberts 1994), and from capacitance measurements (Moser and Beutner 2000; Spassova et al. 2004). Most likely, they spatiotemporally restrict the presynaptic Ca2+ domains and hence improve the timing of synaptic transmission. Table 3 summarizes currently available information on Ca2+ buffers in hair cells of various species. Not only are the concentrations of the buffers different between the various hair cells, but these proteins also differ in their kinetics of Ca2+ binding. Calbindin and calretinin bind Ca2+ rapidly and are therefore often compared with the fast Ca2+ chelator BAPTA, whereas the two slow Ca2+-binding sites of parvalbumin are mostly considered to be equivalent to two molecules of EGTA. These comparisons are, however, oversimplifications, not taking into consideration distinct properties of each of the four or five binding sites in calbindin D-28k and calretinin, respectively nor cooperativity between binding sites (Schwaller et al. 2002).
Table 3.
Presence and concentrations of calcium-binding proteins in hair cells. The table summarizes part of the available information on calcium binding obtained from various preparations. Hair cells vary in the type and amount of calcium-binding proteins, as determined by the various approaches. The number of binding sites per protein also varies (for additional discussion, see Hackney et al. 2005; Desai et al. 2005b), e.g., mammalian calbindin D-28k has four and calretinin five functional calcium-binding sites, while parvalbumin binds only two calcium ions. The Ca2+ affinity under physiological conditions is rather similar for all three proteins (in the range of 0.3–1.5 μM), while kinetic properties differ considerably: calbindin D-28k and calretinin are considered “fast” buffers, while parvalbumin has properties similar to EGTA (Schwaller et al. 2002) (LF low frequency, HF high frequency, IHC inner hair cell, OHC outer hair cell, + positive, − negative)
| Species/organ | Calbindin-D28k (μM) | Calretinin (μM) | Parvalbumin-α (μM) | Parvalbumin-β (μM) | Parvalbumin–3 (μM) | Ca-binding sites (mM) | Approach | References |
|---|---|---|---|---|---|---|---|---|
| Tall frog saccular | − | 1,200±400 | 6 | Western blot, patch-clamp | Edmonds et al. 2000 | |||
| 700–3000 | Western blot | Heller et al. 2002 | ||||||
| Turtle auditory | 627±151 (HF) | 9±3 (HF) | 256±72 (HF) | 3,0 (HF) | Calibrated immunogold | Hackney et al. 2003 | ||
| 129±95 (LF) | 11±2 (LF) | 223±51 (LF) | 1,0 (LF) | |||||
| 1 | Patch-clamp | Tucker and Fettiplace 1996 | ||||||
| 0.1–0.4 | Patch-clamp | Ricci et al. 1998 | ||||||
| Mature rat cochlear IHC | − | ~35 (HF) | ~160 (HF) | − | 0.49 (HF) | Calibrated immunogold | Hackney et al. 2005 | |
| ~40 (LF) | ~170 (LF) | 0.53 (LF) | ||||||
| Mature rat cochlear OHC | 15±11 (HF) | ~70 (HF) | ~100 (HF) | 2892±120 (HF) | 6.0 (HF) | Calibrated immunogold | Hackney et al. 2005 | |
| 230±38 (LF) | ~40 (LF) | ~290 (LF) | 1954±151 (LF) | 5.4 (LF) | ||||
| Mouse cochlear IHC | + | ++ | ++ | Immunofluorescence | Sendin et al., unpublished | |||
| Submillimolar | Patch-clamp | Moser and Beutner 2000 | ||||||
| Mature rat and mouse type II vestibular hair cells | ++ (70&–80% of type II hair cells) | Immunoperoxidase | Desai et al. 2005a,b |
medium staining
strong staining
The major differences in the amount of buffers in the various hair cells suggest that our understanding of the role of calcium buffers for stimulus-secretion coupling at the hair cell active zone synapse is far from complete. Within the mammalian cochlea, immunohistochemistry and physiology suggest that IHCs contain submillimolar concentrations of Ca2+-binding sites. Surprisingly, OHCs of the cochlea, whose primary function is mechanical amplification, but who have little if any role in sound coding, contain much higher concentrations of Ca2+ buffers (Hackney et al. 2005). A high buffer concentration might be important for stereociliar Ca2+ signaling and hair-bundle-based amplification in these cells (Kennedy et al. 2005). Examination of hair cells from mutant mice lacking the major proteinaceous Ca2+ buffers promises further insights into the role and impact of fast Ca2+ buffering in amplification and synaptic sound coding in the mammalian cochlea.
Ca2+ sensitivity of release
Transmitter release depends on cooperative binding of more than one incoming Ca2+ ion to the fusion apparatus in most presynaptic cells (Augustine 2001). The cooperativity of incoming Ca2+ ions in regulating release (“apparent” Ca2+ cooperativity) depends on several factors: (1) the binding properties of the Ca2+ sensor of exocytosis (“intrinsic” Ca2+ cooperativity), (2) the topography of the vesicle release site and the Ca2+ channels triggering release at the active zone, and (3) the way that Ca2+ influx is experimentally manipulated. When transmitter release is evoked by depolarization to varying levels, a low Ca2+ cooperativity (close to unity) is observed in presynaptic capacitance measurements (Moser and Beutner 2000; Brandt et al. 2005; Johnson et al. 2005; Schnee et al. 2005) and in paired pre- and postsynaptic recordings (Keen and Hudspeth 2006). This is in sharp contrast to the high intrinsic Ca2+ cooperativity of transmitter release elicited by Ca2+ uncaging (Beutner et al. 2001) in the low micromolar [Ca2+] range (cooperative binding of five ions). In these experiments, the Ca2+-dependent kinetics of exocytosis display saturation at [Ca2+] exceeding 50 μM.
Brandt and colleagues (2005) have compared the effects of changes in open Ca2+ channel number and in single Ca2+ channel current on exocytosis of the RRP in mouse IHCs. A high apparent Ca2+ cooperativity of RRP exocytosis, approaching the intrinsic Ca2+ cooperativity, has been observed during changes of single channel current (changes of extracellular Ca2+) and rapid flicker block (Zn2+) in the range of small Ca2+ currents. On the other hand, near linear changes of RRP exocytosis with the Ca2+ current have been found during pharmacological (dihydropyridine) modulation of Ca2+ channel open probability, mimicking the Ca2+ cooperativity of RRP exocytosis observed upon depolarization to different levels. In contrast to their findings, a Ca2+ microdomain control of exocytosis should have revealed a high Ca2+ cooperativity, irrespective of the way that the Ca2+ influx was manipulated (Augustine et al. 1991; Mintz et al. 1995). Hence, stimulus-secretion coupling in mouse IHCs has been interpreted within the framework of a Ca2+ nanodomain control of synaptic vesicle exocytosis. This hypothesis has further been supported by the small number of open Ca2+ channels per active zone (maximal 30 at saturating stimuli), the low sensitivity to the exogenously added, slow-binding, Ca2+ chelator EGTA (Moser and Beutner 2000), the evidence for nanodomain overlap for strong depolarization (Augustine et al. 1991), and the low Ca2+ affinity of the hair cell Ca2+ sensor (Beutner et al. 2001). The depolarization level has been concluded to determine the number of active channel-release site units, i.e. the RRP, whereas the kinetics of pool depletion are much less dependent on the stimulus (Brandt et al. 2005). This Ca2+ nanodomain control of exocytosis has been postulated to enable the hair cell to be involved in sound coding with high temporal precision even at low sound intensities. The temporal precision of synaptic transmission would be further improved with stronger stimuli, because then more vesicles could be released in parallel, thereby reducing the jitter of postsynaptic spiking.
Concluding remarks
The various hair cell types share common presynaptic properties. These include a high abundance of potassium channels shortening the membrane time constant and the presence of ribbons providing a large RRP for the exocytosis of glutamate driven by Ca2+ influx through CaV1.3 L-type Ca2+ channels. However, they differ extensively in synaptic morphology and connectivity. More research is required to relate the specific molecular anatomy and physiology of a particular hair cell synapse to their specific coding properties.
Acknowledgments
Research at the Moser laboratory was supported by grants from the DFG (SFB406 and CMPB), the European Commission (through the integrated project EuroHear), the Human Frontiers Science Program (HFSP), and the Federal Goverment (through the Bernstein Center for Computational Neuroscience, Göttingen). Research at the Lysakowski laboratory was supported by grants from NIH (R01 DC02521, R01 DC02290, and R01 DC002358) and the American Hearing Research Foundation.
We thank Regis Nouvian, Alexander Meyer and Beat Schwaller for comments on the manuscript, William Roberts for providing figures of his microdomain modeling, Ruth Anne Eatock for discussion on Ca2+ channels in mammalian vestibular hair cells, and Gerhard Hoch and Steven D. Price for excellent technical assistance.
Contributor Information
Tobias Moser, Email: tmoser@gwdg.de, Department of Otolaryngology and Center for Molecular, Physiology of the Brain, University of Göttingen, Robert-Koch-Strasse 40, 37075 Göttingen, Germany.
Andreas Brandt, Department of Otolaryngology and Center for Molecular, Physiology of the Brain, University of Göttingen, Robert-Koch-Strasse 40, 37075 Göttingen, Germany.
Anna Lysakowski, Department of Anatomy & Cell Biology, University of Illinois at Chicago, Chicago IL 60612, USA.
References
- Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Crawford AC, Fettiplace R. Electrical resonance and membrane currents in turtle cochlear hair cells. Hear Res. 1986;22:31–36. doi: 10.1016/0378-5955(86)90073-0. [DOI] [PubMed] [Google Scholar]
- Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol. 1987;255:560–570. doi: 10.1002/cne.902550408. [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta AM, Peterson EH. Functional architecture of vestibular primary afferent from the posterior semicircular canal of the turtle, Pseudemys (Trachemys) scripta. J Comp Neurol. 1994;344:481–507. doi: 10.1002/cne.903440402. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Responses to efferent activation and excitatory response-intensity relations of turtle posterior-crista afferents. J Neurophysiol. 2000;83:1224–1242. doi: 10.1152/jn.2000.83.3.1224. [DOI] [PubMed] [Google Scholar]
- Chang JS, Popper AN, Saidel WM. Heterogeneity of sensory hair cells in a fish ear. J Comp Neurol. 1992;324:621–640. doi: 10.1002/cne.903240413. [DOI] [PubMed] [Google Scholar]
- Delgutte B. Representation of speech-like sounds in the discharge patterns of auditorynerve fibers. J Acoust Soc Am. 1980;68:843–857. doi: 10.1121/1.384824. [DOI] [PubMed] [Google Scholar]
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol. 2005;93:267–280. doi: 10.1152/jn.00747.2003. [DOI] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol. 2005;93:251–266. doi: 10.1152/jn.00746.2003. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Brandstätter JH. Ribbon synapses of the retina. Cell Tissue Res. 2006;326(2):339–46. doi: 10.1007/s00441-006-0234-0. [DOI] [PubMed] [Google Scholar]
- Dou H, Vazquez AE, Namkung Y, Chu H, Cardell EL, Nie L, Parson S, Shin HS, Yamoah EN. Null mutation of alpha1D Ca2+ channel gene results in deafness but no vestibular defect in mice. J Assoc Res Otolaryngol. 2004;5:215–226. doi: 10.1007/s10162-003-4020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Hurley KM, Vollrath MA. Mechanoelectrical and voltage-gated ion channels in mammalian vestibular hair cells. Audiol Neurootol. 2002;7:31–35. doi: 10.1159/000046860. [DOI] [PubMed] [Google Scholar]
- Edmonds B, Reyes R, Schwaller B, Roberts WM. Calretinin modifies presynaptic calcium signaling in frog saccular hair cells. Nat Neurosci. 2000;3:786–790. doi: 10.1038/77687. [DOI] [PubMed] [Google Scholar]
- Edmonds BW, Gregory FD, Schweizer FE. Evidence that fast exocytosis can be predominantly mediated by vesicles not docked at active zones in frog saccular hair cells. J Physiol. 2004;560:439–450. doi: 10.1113/jphysiol.2004.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom H, Bergstrom B, Ades HW. Macula utriculi and macula sacculi in the squirrel monkey. Acta Otolaryngol Suppl. 1972;301:75–126. doi: 10.3109/00016487209122691. [DOI] [PubMed] [Google Scholar]
- Eybalin M, Renard N, Aure F, Safieddine S. Cysteine-string protein in inner hair cells of the organ of Corti: synaptic expression and upregulation at the onset of hearing. Eur J Neurosci. 2002;15:1409–1420. doi: 10.1046/j.1460-9568.2002.01978.x. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol. 1988;60:167–181. doi: 10.1152/jn.1988.60.1.167. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:767–780. doi: 10.1152/jn.1990.63.4.767. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Lysakowski A, Goldberg JM. Hair-cell counts and afferent innervation patterns in the cristae ampullares of the squirrel monkey with a comparison to the chinchilla. J Neurophysiol. 1995;73:1253–1269. doi: 10.1152/jn.1995.73.3.1253. [DOI] [PubMed] [Google Scholar]
- Fettiplace R. The role of calcium in hair cell transduction. Soc Gen Physiol Ser. 1992;47:343–356. [PubMed] [Google Scholar]
- Fischer FP. Quantitative analysis of the innervation of the chicken basilar papilla. Hear Res. 1992;61:167–178. doi: 10.1016/0378-5955(92)90048-r. [DOI] [PubMed] [Google Scholar]
- Francis HW, Rivas A, Lehar M, Ryugo DK. Two types of afferent terminals innervate cochlear inner hair cells in C57BL/6J mice. Brain Res. 2004;1016:182–194. doi: 10.1016/j.brainres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Nagai T, Evans MG. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci. 1988;8:2460–2467. doi: 10.1523/JNEUROSCI.08-07-02460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Evans MG, Murrow BW. Calcium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990;429:553–568. doi: 10.1113/jphysiol.1990.sp018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci. 2003;23:11296–11304. doi: 10.1523/JNEUROSCI.23-36-11296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Lehre KP. Immunocytochemical localization of a high-affinity glutamate-aspartate transporter, GLAST, in the rat and guinea-pig cochlea. Eur J Neurosci. 1997;9:1961–1969. doi: 10.1111/j.1460-9568.1997.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Furukawa T. Sound reception and synaptic transmission in goldfish hair cells. Jpn J Physiol. 1986;36:1059–1077. doi: 10.2170/jjphysiol.36.1059. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Matsuura S. Adaptive rundown of excitatory postsynaptic potentials at synapses between hair cells and eight nerve fibres in the goldfish. J Physiol. 1978;276:193–209. doi: 10.1113/jphysiol.1978.sp012228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Lysakowski A, Fernandez C. Morphophysiological and ultrastructural studies in the mammalian cristae ampullares. Hear Res. 1990;49:89–102. doi: 10.1016/0378-5955(90)90097-9. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130:277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005;435:212–215. doi: 10.1038/nature03567. [DOI] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Jones EM, Fettiplace R. The distribution of calcium buffering proteins in the turtle cochlea. J Neurosci. 2003;23:4577–4589. doi: 10.1523/JNEUROSCI.23-11-04577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci. 2005;25:7867–7875. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidi A, Beurg M, Dulon D. Localization and developmental expression of BK channels in mammalian cochlear hair cells. Neuroscience. 2005;130:475–484. doi: 10.1016/j.neuroscience.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Hakuba N, Koga K, Shudou M, Watanabe F, Mitani A, Gyo K. Hearing loss and glutamate efflux in the perilymph following transient hindbrain ischemia in gerbils. J Comp Neurol. 2000;418:217–226. doi: 10.1002/(sici)1096-9861(20000306)418:2<217::aid-cne7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Heller S, Bell AM, Denis CS, Choe Y, Hudspeth AJ. Parvalbumin 3 is an abundant Ca2+ buffer in hair cells. J Assoc Res Otolaryngol. 2002;3:488–498. doi: 10.1007/s10162-002-2050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Xue JT, Brichta AM, Goldberg JM. Transmission between type II hair cells and bouton afferents in the turtle posterior crista. J Neurophysiol. 2006;95:428–452. doi: 10.1152/jn.00447.2005. [DOI] [PubMed] [Google Scholar]
- Issa NP, Hudspeth AJ. Clustering of Ca2+ channels and Ca2+-activated K+ channels at fluorescently labeled presynaptic active zones of hair cells. Proc Natl Acad Sci USA. 1994;91:7578–7582. doi: 10.1073/pnas.91.16.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell’s afferent synapse. Proc Natl Acad Sci USA. 2006;103:5537–5542. doi: 10.1073/pnas.0601103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature. 2005;433:880–883. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006;25:642–652. doi: 10.1038/sj.emboj.7600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kiang NY-S, Watanabe T, Thomas EC, Clark LF. Discharge pattern of single fibers in the cat’s auditory nerve. MIT Press; Cambridge: 1965. [Google Scholar]
- Kimura RS. Sensory and accessory epithelia of the cochlea. In: Friedemann I, Ballantyne J, editors. Ultrastructural atlas of the inner ear. Butterworths; London: 1984. pp. 101–132. [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. Alpha 1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Kros CJ. Physiology of mammalian cochlea hair cells. In: Dallos P, et al., editors. The cochlea. Springer; Berlin Heidelberg New York: 1996. pp. 318–385. [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Roberts WM. Calcium signalling in hair cells: multiple roles in a compact cell. Curr Opin Neurobiol. 1994;4:496–502. doi: 10.1016/0959-4388(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RS, Hudspeth AJ. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983;304:538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Single-neuron labeling in cat auditory nerve. Science. 1982;216:1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy [erratum in J Comp Neurol (1991) 304:341] J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Lysakowski A. Synaptic organization of the crista ampullaris in vertebrates. Ann N Y Acad Sci. 1996;781:164–182. doi: 10.1111/j.1749-6632.1996.tb15700.x. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol. 1997;389:419–443. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Kros CJ. Effects of intracellular stores and extracellular Ca(2+) on Ca(2+)-activated K(+) currents in mature mouse inner hair cells. J Physiol. 2004;557:613–633. doi: 10.1113/jphysiol.2003.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Dunst C, Michaels RL, Fuchs PA. Release sites and calcium channels in hair cells of the chick’s cochlea. J Neurosci. 1997;17:9133–9144. doi: 10.1523/JNEUROSCI.17-23-09133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Rossi ML, Rubbini G, Rispoli G. Calcium currents in hair cells isolated from semicircular canals of the frog. Biophys J. 2000;78:1240–1254. doi: 10.1016/S0006-3495(00)76681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Perez A, Liberman MC. Ultrastructural differences among afferent synapses on cochlear hair cells: correlations with spontaneous discharge rate. J Comp Neurol. 1996;371:208–221. doi: 10.1002/(SICI)1096-9861(19960722)371:2<208::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Michna M, Knirsch M, Hoda JC, Muenkner S, Langer P, Platzer J, Striessnig J, Engel J. Cav1.3 (alpha1D) Ca2+ currents in neonatal outer hair cells of mice. J Physiol. 2003;553:747–758. doi: 10.1113/jphysiol.2003.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemzou NRM, Bulankina AV, Khimich D, Giese A, Moser T. Synaptic organization in cochlear inner hair cells deficient for the CaV1.3 (alpha1D) subunit of L-type Ca2+ channels. Neuroscience. 2006;141(4):1849–60. doi: 10.1016/j.neuroscience.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Nouvian R, Ruel J, Wang J, Guitton MJ, Pujol R, Puel JL. Degeneration of sensory outer hair cells following pharmacological blockade of cochlear KCNQ channels in the adult guinea pig. Eur J Neurosci. 2003;17:2553–2562. doi: 10.1046/j.1460-9568.2003.02715.x. [DOI] [PubMed] [Google Scholar]
- Nouvian R, Beutner D, Parsons TD, Moser T. Structure and function of the hair cell ribbon synapse. J Membr Biol. 2006;209:153–165. doi: 10.1007/s00232-005-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Taberner AM, Thurm H, Sausbier M, Arntz C, Ruth P, Fakler B, Liberman MC. The role of BKCa channels in electrical signal encoding in the mammalian auditory periphery. J Neurosci. 2006;26:6181–6189. doi: 10.1523/JNEUROSCI.1047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pyott SJ, Glowatzki E, Trimmer JS, Aldrich RW. Extrasynaptic localization of inactivating calcium-activated potassium channels in mouse inner hair cells. J Neurosci. 2004;24:9469–9474. doi: 10.1523/JNEUROSCI.3162-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci. 1998;18:8261–8277. doi: 10.1523/JNEUROSCI.18-20-08261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM. Spatial calcium buffering in saccular hair cells. Nature. 1993;363:74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994;14:3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Paki B. Role of L-type Ca2+ channels in transmitter release from mammalian inner hair cells. II. Single-neuron activity. J Neurophysiol. 2002;87:2734–2740. doi: 10.1152/jn.2002.87.6.2734. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, Yamoah EN. Direct measurement of single-channel Ca(2+) currents in bullfrog hair cells reveals two distinct channel subtypes. J Physiol. 2001;534:669–689. doi: 10.1111/j.1469-7793.2001.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, Nonner W, Yamoah EN. Ca2+ transport properties and determinants of anomalous mole fraction effects of single voltage-gated Ca2+ channels in hair cells from bullfrog saccule. J Physiol. 2002;538:729–745. doi: 10.1113/jphysiol.2001.013312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967;30:769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Rutherford MA, Roberts WM. Frequency selectivity of synaptic exocytosis in frog saccular hair cells. Proc Natl Acad Sci USA. 2006;103:2898–2903. doi: 10.1073/pnas.0511005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Muller M, Kopschall I, Pfister M, Munkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieddine S, Wenthold RJ. SNARE complex at the ribbon synapses of cochlear hair cells: analysis of synaptic vesicle-and synaptic membrane-associated proteins. Eur J Neurosci. 1999;11:803–812. doi: 10.1046/j.1460-9568.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Saito K. Fine structure of the sensory epithelium of the guinea pig organ of Corti: afferent and efferent synapses of hair cells. J Ultrastruct Res. 1980 May;71(2):222–32. doi: 10.1016/s0022-5320(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Lawton DM, Furness DN, Benke TA, Ricci AJ. Auditory hair cell-afferent fiber synapses are specialized to operate at their best frequencies. Neuron. 2005;47:243–254. doi: 10.1016/j.neuron.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell Tissue Res. 2006 doi: 10.1007/s00441-006-0272-7. this issue. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Meyer M, Schiffmann S. “New” functions for “old” proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1:241–258. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- Sewell WF. The relation between the endocochlear potential and spontaneous activity in auditory nerve fibres of the cat. J Physiol. 1984;347:685–696. doi: 10.1113/jphysiol.1984.sp015090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, Nicolson T. Gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J Neurosci. 2004;24:4213–4223. doi: 10.1523/JNEUROSCI.0223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky NB, Galsky MD, Swartzentruber-Martin H, Savage J. Study of afferent nerve terminals and fibers in gerbil cochlea: distribution by size. Hear Res. 2000;144:124–134. doi: 10.1016/s0378-5955(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Sneary MG. Auditory receptor of the red-eared turtle. II. Afferent and efferent synapses and innervation patterns. J Comp Neurol. 1988;276:588–606. doi: 10.1002/cne.902760411. [DOI] [PubMed] [Google Scholar]
- Spassova M, Eisen MD, Saunders JC, Parsons TD. Chick cochlear hair cell exocytosis mediated by dihydropyridine-sensitive calcium channels. J Physiol. 2001;535:689–696. doi: 10.1111/j.1469-7793.2001.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Avissar M, Furman AC, Crumling MA, Saunders JC, Parsons TD. Evidence that rapid vesicle replenishment of the synaptic ribbon mediates recovery from short-term adaptation at the hair cell afferent synapse. J Assoc Res Otolaryngol. 2004;5:376–390. doi: 10.1007/s10162-004-5003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer SS, Salvi RJ, Schulte BA. Ablation of inner hair cells by carboplatin alters cells in the medial K(+) flow route and disrupts tectorial membrane. Hear Res. 1999;136:139–150. doi: 10.1016/s0378-5955(99)00118-5. [DOI] [PubMed] [Google Scholar]
- Thurm H, Fakler B, Oliver D. Ca2+-independent activation of BKCa channels at negative potentials in mammalian inner hair cells. J Physiol. 2005;569:137–151. doi: 10.1113/jphysiol.2005.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol. 1997;381:188–202. [PubMed] [Google Scholar]
- Tucker T, Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995;15:1323–1335. doi: 10.1016/0896-6273(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Tucker TR, Fettiplace R. Monitoring calcium in turtle hair cells with a calcium-activated potassium channel. J Physiol. 1996;494:613–626. doi: 10.1113/jphysiol.1996.sp021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner N. A qualitative and quantitative study of a sensory epithelium in the inner ear of a fish (Colisa labiosa; Anabantidae) Acta Zoologica. 1982;63:133–146. [Google Scholar]
- Wong WH, Hurley KM, Eatock RA. Differences between the negatively activating potassium conductances of mammalian cochlear and vestibular hair cells. J Assoc Res Otolaryngol. 2004;5:270–284. doi: 10.1007/s10162-004-4051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Art JJ, Goodman MB, Fettiplace R. A kinetic description of the calcium-activated potassium channel and its application to electrical tuning of hair cells. Prog Biophys Mol Biol. 1995;63:131–158. doi: 10.1016/0079-6107(95)00002-5. [DOI] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet. 1999;21:363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Robertson D, Yates G, Everett A. Role of L-type Ca(2+) channels in transmitter release from mammalian inner hair cells. I. Gross sound-evoked potentials. J Neurophysiol. 1999;82:3307–3315. doi: 10.1152/jn.1999.82.6.3307. [DOI] [PubMed] [Google Scholar]