Abstract
Lectin-like oxidized LDL (oxLDL) receptor-1 (LOX-1, also known as OLR-1), is a class E scavenger receptor that mediates the uptake of oxLDL by vascular cells. LOX-1 is involved in endothelial dysfunction, monocyte adhesion, the proliferation, migration, and apoptosis of smooth muscle cells, foam cell formation, platelet activation, as well as plaque instability; all of these events are critical in the pathogenesis of atherosclerosis. These LOX-1-dependent biological processes contribute to plaque instability and the ultimate clinical sequelae of plaque rupture and life-threatening tissue ischemia. Administration of anti-LOX-1 antibodies inhibits atherosclerosis by decreasing these cellular events. Over the past decade, multiple drugs including naturally occurring antioxidants, statins, antiinflammatory agents, antihypertensive and antihyperglycemic drugs have been demonstrated to inhibit vascular LOX-1 expression and activity. Therefore, LOX-1 represents an attractive therapeutic target for the treatment of human atherosclerotic diseases. This review aims to integrate the current understanding of LOX-1 signaling, regulation of LOX-1 by vasculoprotective drugs, and the importance of LOX-1 in the pathogenesis of atherosclerosis.
Keywords: Atherosclerosis, Oxidized LDL, LOX-1, Soluble LOX-1, Review
Introduction
Atherosclerosis-related cardiovascular diseases continue to be a major cause of morbidity and mortality in developed and developing countries. Atherosclerosis is a multifactorial disease for which many mechanisms are known, but the cellular and molecular mechanisms precipitating the disease process are not well defined. Atherosclerosis commences with the binding and retention of lipids by modified proteoglycans with hyperelongated glycosaminoglycan chains, followed by a multifactorial inflammatory process [1–4]. Cellular and cytokine-based inflammatory processes represent novel therapeutic targets for the prevention and treatment of atherosclerosis [5].
After the milestone study by Dr. Daniel Steinberg [6], oxidized LDL (oxLDL) received intense interest as it promotes key steps involved in plaque formation and destabilization. The rapid, unregulated uptake of oxLDL by vascular scavenger receptors (SR) is crucial for the transformation of monocyte-derived macrophages to foam cells in atherosclerotic lesions [7]. Lectin-like oxLDL receptor-1 (LOX-1 or OLR-1) is a multiligand SR originally identified as the primary receptor for oxLDL uptake by endothelial cells (EC) by Dr. Tatsuya Sawamura [8, 9]. Subsequent studies showed that LOX-1 is also expressed by monocytes/macrophages [10], smooth muscle cells (SMC) [10], cardiomyocytes [11], fibroblasts [12], adipocytes [13], airway epithelial cells [14], interferon γ-conditioned dendritic cells [15], and platelets [16]. Most importantly, LOX-1 is expressed in atheroma-derived cells and is observed in large abundance in human and animal atherosclerotic lesions [17]. Furthermore, it is intriguing that the major three hypotheses of atherosclerosis (i.e., oxidative modification hypothesis, response-to-injury hypothesis, and retention hypothesis) converged by the versatile functions of LOX-1 [18]. Like CD36, LOX-1 acts as a cell surface SR that participates in the binding, endocytosis, and proteolytic degradation of oxLDL. LOX-1, however, does not share any homology with other SR. LOX-1 mediates a spectrum of pro-atherogenic cellular responses implicated in the pathogenesis of atherosclerosis, including endothelial dysfunction, phagocytosis of aged apoptotic cells, vascular inflammation, foam cell formation, collagen deposition, and adipocyte cholesterol metabolism [13, 19]. Basal LOX-1 expression in EC is relatively low; it is dynamically regulated by pro-inflammatory cytokines, vasoconstrictive peptides, and other pathological stimuli relevant to atherosclerosis in vitro [19, 20]. In vivo, LOX-1 is upregulated in multiple disease states, such as hypertension, diabetes, hyperlipidemia, and ischemia/reperfusion injury [20]. The in vitro and in vivo regulators of LOX-1 are summarized in Table 1. LOX-1 is known to bind with high affinity to a broad spectrum of structurally distinct ligands besides oxLDL [18]. These ligands include activated platelets, advanced glycation end-products (AGEs), apoptotic bodies, bacteria, C-reactive protein (CRP) and various forms of modified LDL [18, 21, 22] (Table 2).
Table 1.
In vitro and in vivo stimuli of LOX-1 expression/activation
| In vitro | |
| Pro-inflammatory cytokines | Stimuli in hypertension |
| Tumor necrosis factor-α (TNF-α) | Angiotensin II (Ang-II) |
| Interleukin-1 (IL-1) | Endothelin-1 (ET-1) |
| Lipopolysaccharides (LPS) | Aldosterone |
| C-reactive protein (CRP) | Fluid shear stress |
| Interferon-γ (IFN-γ) | Transforming growth factor-β (TGF-β) |
| Atherogenic stimuli | Hyperglycemic stimuli |
| Oxidized LDL (oxLDL) | High glucose (HG) |
| Homocysteine (Hcy) | Advanced glycation end-products (AGEs) |
| Lysophosphatidylcholine (LPC) | Other |
| Palmitic acid (PA) | Human cytomegalovirus (HCMV) |
| In vivo | |
| Atherosclerosis | Hypertension |
| Hyperlipidemia | Ischemia reperfusion injury |
| Diabetes mellitus | Psychological stress |
| Obesity | Transplantation |
| HIV infection | Heart failure |
Table 2.
The pathophysiologic ligands of LOX-1
| Modified LDL | Other ligands |
|---|---|
| Oxidized LDL | C-reactive protein (CRP) |
| 15-Lipoxygenase modified LDL | Advanced glycation end-products (AGEs) |
| Carbamylated LDL | Heat shock protein 60 (HSP60) |
| Hypochlorite-modified HDL | 4-Hydroxy-2-nonenal–histidine adduct |
| Remnant-like lipoprotein particle | N-(4-oxononanoyl)lysine (ONL) |
| Electronegative LDL-L5 | Activated platelets |
| 4-Hydroxy-2-nonenal (HNE)-LDL | Aged/apoptotic cells |
| Glycoxidized LDL | Cardiolipin |
Lectin-like oxLDL receptor-1 is a type II membrane glycoprotein comprised of 273 amino acids. LOX-1 consists of a short N-terminus cytoplasmic domain, transmembrane domain, neck domain, and a C-type lectin-like domain [23]. It is synthesized as a 40-kDa precursor protein with N-linked high mannose-type carbohydrate, which is further glycosylated and processed into a 50-kDa mature form [24]. By analyzing site-specific N-linked glycosylation using mass spectrometry, one potential glycosylation site of recombinant human LOX-1 on asparagine 139 (Asn-139) has recently been identified [25]. Direct evidence for the involvement of LOX-1 in atherogenesis has been obtained using knockout/transgenic animal models and adenoviral gene transfer. Lectin-like oxLDL receptor-1 transgenic mice crossed with Apolipoprotein-E knockout mice (LOX-1tg/ApoE−/−) on a high-fat diet display augmented oxLDL uptake, oxidative stress, and accelerated infiltration of macrophages in the heart and vessels compared to control littermates [26]. In addition, overexpression of LOX-1 in EC promotes atherogenesis in the common carotid artery of the ApoE−/− mouse model [27]. Further, LOX-1−/−/LDL-R−/− mice display reduced atherosclerotic lesions, compared to LDL-R−/− mice with a comparable serum LDL level [28]. However, ectopic expression of LOX-1 in the liver of ApoE−/− mice ameliorates the development of atherosclerotic lesions, with a transient reduction in plasma oxLDL by removing oxLDL from the circulation and reducing systemic oxidative stress [29].
LOX-1 causes endothelial activation and dysfunction
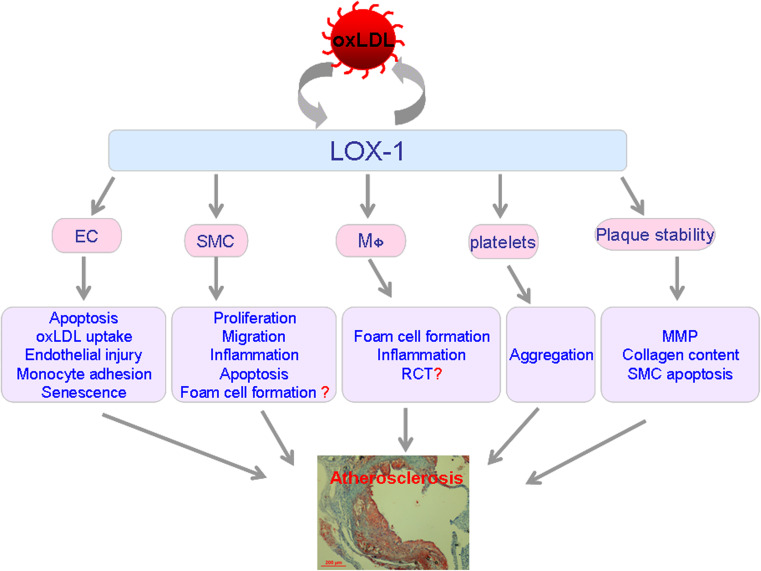
Lectin-like oxLDL receptor-1 regulates the pathogenesis of atherosclerosis by targeting multiple cellular events (Fig. 1). Activation of LOX-1 by oxLDL results in endothelial activation and dysfunction, characterized by reduced endothelium-dependent relaxation, increased monocyte adhesion to EC, as well as the apoptosis and senescence of EC.
Fig. 1.
Schematic diagram illustrating the pivotal role of scavenger receptor LOX-1 in atherosclerotic plaque formation and destabilization. Functions of LOX-1 in EC, SMC, monocytes/macrophages, platelets, and plaque instability are summarized. After binding to LOX-1, oxLDL stimulates endothelial injury, senescence, apoptosis, and oxLDL uptake, also promotes EC to produce adhesion molecules, and recruits leukocytes to the site of injury. Persisted endothelial dysfunction leads to enhanced permeability allowing adherent monocytes to penetrate the lining EC. Differentiated macrophages in the sub-intimal space accumulate oxLDL and convert it to lipid-laden foam cells, which form the necrotic core of the plaque. Intensified inflammation and oxLDL accumulation may result in the proliferation, migration, and foam-cell formation of SMC. As the atherosclerotic plaques develop to the advanced stage, LOX-1 mediates platelet aggregation as well as platelet–endothelium interaction. Finally, LOX-1 leads to plaque destabilization by modulating plaque components (MMP expression, the content of collagen and SMC, and the apoptosis of SMC). EC endothelial cells, SMC smooth muscle cells, RCT reverse cholesterol transport, MMP matrix metalloproteinase. Question mark represents important aspects remaining unknown
Nitric oxide-mediated dilation and oxLDL uptake by endothelial cells
Endothelial cells play an essential role in regulating vascular tone. Lectin-like oxLDL receptor-1 is the major receptor for oxLDL uptake by EC [8]. Oxidative inactivation of nitric oxide (NO) by reactive oxygen species (ROS) decreases the biological activity of NO by generating cytotoxic peroxynitrite (ONOO−), which has been recently found in human atherosclerotic lesions. Arginase II, an enzyme responsible for the hydrolysis of arginine into ornithine and urea, regulates endothelial nitric oxide synthase (eNOS) activity by competing for the common substrate l-arginine [30]. OxLDL, through the endothelial LOX-1 receptor, small GTPase RhoA (Ras homolog gene family member A), and ROCK (Rho-associated coiled-coil containing protein kinase), activates arginase II, down-regulates NO, and contributes to vascular dysfunction [31]. In addition, LOX-1 mediates oxLDL uptake by EC by inducing the activation of protein kinase C (PKC) β2 and c-Jun N-terminal kinases (JNK), as well as the subsequent phosphorylation of 66-kDa isoform of Shc adaptor proteins (p66Shc) [32]. OxLDL also impairs endothelium-dependent NO-mediated dilation of coronary arterioles by activation of a signaling cascade involving LOX-1 and NADPH oxidase. Anti-LOX-1 antibodies, given in vivo, restore NO-mediated coronary arteriolar dilation in atherosclerosis-prone ApoE−/− mice, but do not affect the endothelium-dependent vasodilation in wild-type mice [33]. Consistent with this observation, mesenteric arteries from mice overexpressing LOX-1 and on a high-fat diet have preserved vascular smooth muscle relaxation, but impaired endothelium-dependent relaxation via reduced vascular NO availability related to the exaggerated formation of ROS and decreased eNOS expression [34]. Recent in vitro evidence has shown that a complex of LOX-1 and membrane type 1 matrix metalloproteinase (MT1-MMP) contributes to ROS formation and eNOS downregulation [35]. More recently, it has been shown that HDL from patients with stable coronary artery diseases (CAD) or acute coronary syndrome (ACS) can activate LOX-1, triggering endothelial PKCβ2 activation, which in turn inhibits Akt (Ser473) phosphorylation, eNOS (Ser1,177) phosphorylation, and eNOS-dependent NO production [36]. Collectively, LOX-1 represents a central role in regulating NO-mediated vascular reactivity.
Role of LOX-1 in monocyte adhesion to endothelial cells
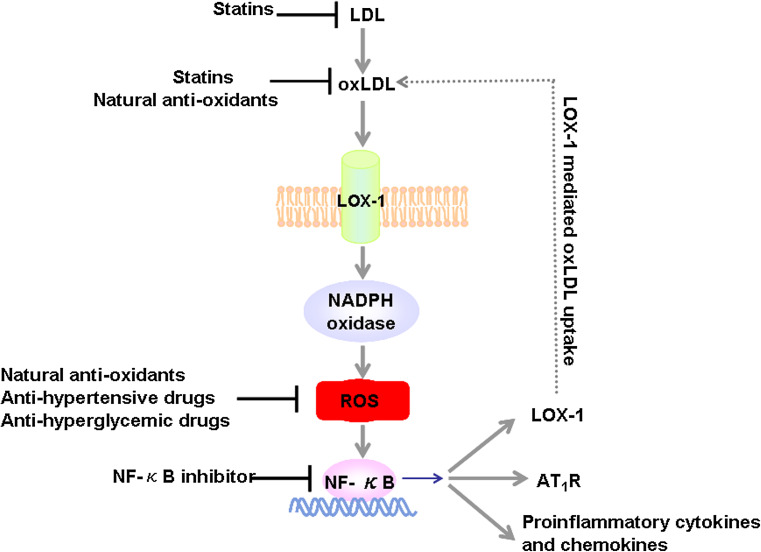
Recruitment of leukocytes from the bloodstream into the arterial intima is a crucial step in the development of atherosclerosis [37]. Incubation of human coronary artery endothelial cells (HCAEC) with oxLDL markedly increases monocyte chemoattractant protein-1 (MCP-1) production as well as monocyte adhesion to HCAEC. This response was inhibited by a human LOX-1 antisense RNA, suggesting that LOX-1 is a key factor in oxLDL-mediated monocyte adhesion to HCAEC [38]. In vivo, anti-LOX-1 antibody efficiently suppresses leukocyte infiltration and protein exudation in a model of low-dose endotoxin-induced uveitis. In situ videomicroscopic analyses of leukocyte interactions with retinal veins revealed that anti-LOX-1 antibody reduced the number of rolling leukocytes and increased the velocity of rolling, suggesting that LOX-1 functions as a vascular-tethering ligand [39]. Additional evidence indicates that binding of oxLDL to LOX-1 activates redox-sensitive nuclear factor-kappa B (NF-κB) signaling pathway, a master regulator in enhanced expression of various adhesion molecules, thus, promoting monocytes adhesion to EC (Fig. 2). However, LOX-1-mediated monocyte adhesion to EC can be attenuated by aspirin and pravastatin, either alone or in combination [40].
Fig. 2.
Positive feedback loop involving the oxLDL/LOX-1 signaling pathway and LOX-1 modulators. LDL is oxidized within the vascular wall under atherogenic conditions to form oxLDL. The binding of oxLDL to LOX-1 activates the NADPH oxidase on the cell membrane that results in the quick increase of intracellular ROS formation. Increased ROS activates the redox-sensitive NF-κB signaling pathway, generating three responses: (1) increases binding to LOX-1 promoter, therefore, increases LOX-1 expression and amplifies LOX-1-mediated oxLDL uptake; (2) enhances Ang-II type 1 receptor expression; (3) augments downstream pro-inflammatory cytokine and chemokine expression, such as P-selectin, E-selectin, VCAM-1, ICAM-1, and MCP-1, resulting in increased recruitment of monocytes to endothelial cells. LOX-1 modulators are demonstrated to function at each critical step of this feedback loop. oxLDL oxidized LDL, ROS reactive oxygen species, AT 1 R Ang-II type 1 receptor, NF-κB nuclear factor-kappa B
Endothelia cell apoptosis and senescence
Lectin-like oxLDL receptor-1 mediates oxLDL-induced apoptosis of EC, which can be inhibited by LOX-1 knockdown, LOX-1 inhibitors (i.e., polyinosinic acid and carrageenan), and NF-κB inhibition [41]. OxLDL decreases expression of anti-apoptotic proteins c-IAP-1 (inhibitory apoptotic protein-1) and Bcl-2, as well as activates caspase-9 and caspase-3, which relates to the degradation of c-IAP-1 and Bcl-2. However, LOX-1 knockdown and caspase-9 inhibition both mediate oxLDL-induced apoptosis of EC [42]. These findings suggest that oxLDL binding to LOX-1 decreases expression of anti-apoptotic proteins such as Bcl-2 and c-IAP-1 and activates apoptotic signaling pathways involving caspase-9 and caspase-3, leading to apoptosis of EC [43]. Consistent with these data, an electro-negative LDL fraction, L5, via LOX-1, attenuated Akt phosphorylation and suppressed Bcl-2 expression. L5 also selectively inhibited Bcl-xL expression and eNOS phosphorylation, but increased expression of Bax, Bad, and TNF-α. Blocking Akt phosphorylation increased LOX-1 expression, suggesting a modulatory role of Akt in LOX-1 synthesis [44]. Senescence of EC is another prominent aspect in the pathobiology of endothelial dysfunction as LOX-1 mRNA and protein content were decreased in senescent EC (by repetitive passaging) and in aortas of aged mice (50 weeks old). Compared to early cultures, late-passage human umbilical vein endothelial cells (HUVEC) also exhibit nuclear translocation of NF-κB p65 subunit and reciprocal shifts in Bax and Bcl-2 protein content, resulting in an almost twofold increase in Bax/Bcl-2 ratio and a threefold increase in apoptotic response to TNF-α [45].
LOX-1 mediates smooth muscle cell proliferation and migration
Another important characteristic of atherosclerotic plaques is the proliferation and migration of smooth muscle cells (SMC). OxLDL-induced LOX-1 expression stimulates SMC growth and proliferation via NF-κB- and JNK-signaling pathways in cultured rat SMC. Low concentrations of oxLDL (10 μg/ml) stimulated proliferation of cultured SMC, which was inhibited by LOX-1 antisense mRNA. In addition, increased LOX-1 expression was also observed in the neointima of human coronary arteries after balloon angioplasty. In human restenotic lesions, double immunofluorescence staining showed the co-localization of LOX-1 and proliferating cell nuclear antigen (PCNA) [46]. These results suggest that LOX-1 mediates oxLDL-induced SMC proliferation and plays a role in neointimal formation after vascular injury. Consistent with these data, LOX-1 expressed in SMC is involved in intimal hyperplasia in a rat model of balloon injury. However, in rats administered anti-LOX-1 antibody intravenously every 3 days after balloon injury, intimal hyperplasia, oxidative stress, and leukocyte infiltration were markedly suppressed [47]. Direct evidence of LOX-1 in SMC proliferation and migration is obtained from LOX-1−/− mice. Genetic deletion of LOX-1 in ApoE−/− mice leads to a significant reduction in SMC proliferation and migration [28], and accumulation of inflammatory cells and collagen [48]. Thus, through LOX-1, oxLDL caused the proliferation and transmigration of SMC from media to subendothelial spaces, creating an environment in the vascular wall for the SMC to become foam cells. However, the significance of oxLDL binding or uptake through LOX-1 in SMC and resultant foam-cell formation remains to be determined.
LOX-1 mediates cholesterol uptake and macrophage foam-cell formation
OxLDL is taken up by monocyte-derived macrophages and SMC through a variety of SR, such as SR-AI/II, CD36, SR-BI, macrosialin/CD68, and LOX-1, and results in the formation of foam cells [43]. LOX-1 plays a crucial role in oxLDL-triggered pathological transformation of vessel wall components. Importantly, LOX-1 is absent in monocytes (human peripheral blood monocytes and the THP-1 cells), but induced in differentiated macrophages [49, 50]. OxLDL was taken up via LOX-1 in macrophages stimulated with lysophosphatidylcholine [51], palmitic acids [52], high glucose [53], and oxLDL itself [51]. It is noteworthy that LOX-1 does not alter the uptake of oxLDL in unstimulated macrophages and is not essential for the pro-survival effect of oxLDL in these cells. However, LOX-1 expression is highly inducible by pro-inflammatory cytokines, and if that occurred in macrophages within atheromas, LOX-1 substantially increased oxLDL uptake by lesional macrophages [51], further confirming the specificity of LOX-1 to modulate oxLDL uptake in macrophages.
LOX-1 and plaque stability
Disruption of unstable atherosclerotic plaques and subsequent formation of occlusive thrombi are the primary causes of ACS, which includes unstable angina pectoris, nonfatal myocardial infarction (MI), and fatal MI. Mounting evidence suggests the involvement of LOX-1 in the destabilization of histologically unstable atherosclerotic plaques. LOX-1 is intensively expressed in atherosclerotic plaques with thin fibrous caps and macrophage-rich lipid core areas in Watanabe heritable hyperlipidemic (WHHL) rabbit with advanced atherosclerotic lesions. Specifically, LOX-1 modulates plaque stability by affecting matrix metalloproteinases (MMP) expression/activity, apoptosis of SMC, and collagen content within the atherosclerotic plaques, as expanded upon below.
Increases in MMP expression/activity
OxLDL induces apoptosis of SMC and elicits inflammatory responses in the vascular wall including expression of MMP. OxLDL increases the expression/activity of MMP-1 (collagenase) and MMP-3 (stromelysin-1) in a concentration- and time-dependent manner, without significantly affecting expression of tissue inhibitors of metalloproteinases (TIMP) [54]. Expression/activity of MMP-2 and MMP-9 was increased in the LDLR−/− mice, but not in mice with LOX-1 deletion [48]. There is also evidence showing that the LOX-1/MT1-MMP axis plays a crucial role in RhoA and Rac1-dependent signaling pathways upon oxLDL stimulation, suggesting that this axis may be a promising target for treating endothelial dysfunction [35]. Incubation of human aortic endothelial cells (HAEC) with oxLDL increases LOX-1 expression and enhances MMP-9 production. However, treatment with an anti-LOX-1 antibody or with antioxidants inhibits these effects. Induction of LOX-1 and LOX-1-mediated MMP-9 expression involves endothelin-1 production and NF-κB activation [55].
Enhanced apoptosis of SMC
Apoptosis of SMC in the fibrous cap has been implicated in the destabilization of atherosclerotic plaques and plaque rupture [56]. High concentrations of oxLDL caused apoptosis of SMC, leading to plaque instability and rupture [57]. This process is largely mediated by LOX-1, and can be prevented by anti-LOX-1 monoclonal antibodies [58]. oxLDL (>60 μg/ml) induced apoptosis in SMC, a response which is inhibited by anti-LOX-1 antibody [46, 59]. In human advanced atherosclerotic plaques, macrophages and SMC in the intima express LOX-1, which is co-localized with Bax in both the fibrous cap and shoulder regions of atherosclerotic plaques [59]. OxLDL upregulated LOX-1 expression through phosphorylation of extracellular signal-regulated kinases (ERK) in SMC, and a neutralizing anti-LOX-1 monoclonal antibody, blocked LOX-1—mediated cellular uptake of oxLDL, and prevented oxLDL—induced apoptosis. It was also reported that oxLDL induced apoptosis (3 h) of SMC in a dose-dependent manner, with a maximal effect at a concentration of 300 μg/ml. OxLDL (100 μg/ml), but not native LDL, stimulated ROS production rapidly (≤5 min) and the ROS level remained elevated for at least 45 min [57]. Therefore, LOX-1 may play an important role in oxLDL-induced apoptosis in SMC.
Modulation of collagen accumulation in atherosclerotic plaques
In stable atherosclerotic plaques, there is abundant collagen content covering the fibrous cap, rendering the plaques resistant to rupture. LOX-1 plays an important role in angiotensin-II (Ang-II) and transforming growth factor-β1 (TGF-β1)-stimulated fibroblast growth and collagen synthesis. In LDLR−/− mice, deletion of LOX-1 resulted in a marked reduction in collagen accumulation in atherosclerotic plaques [48]. Similarly, TGF-β1-mediated increase in collagen synthesis was markedly attenuated in the LOX-1−/− mouse cardiac fibroblasts as well as in wild-type mouse cardiac fibroblasts treated with a specific anti-LOX-1 antibody [60]. This decline in collagen deposition in association with a reduction in atherosclerosis has raised the issue of plaque instability as a result of LOX-1 deletion. Future studies should be conducted to determine the effect of LOX-1 deletion on plaque stability in advanced human atherosclerosis by examining plaque composition.
LOX-1 contributes to platelet activation
Lectin-like oxLDL receptor-1 is expressed on the surface of human platelets in an activation-dependent manner. OxLDL binding to platelets can be inhibited by anti-LOX-1 antibody [16]. In addition, LOX-1 also accumulates at the site of thrombus within the atherosclerotic plaque of patients with unstable angina pectoris [16]. The presence of LOX-1 on activated platelets suggests that LOX-1 might be critically involved in thrombus formation. LOX-1 blockade inhibited ADP-induced platelet aggregation in a concentration- and time-dependent manner. In addition, LOX-1 is important for ADP-stimulated inside-out activation of platelet αIIbβ3 and α2β1 integrins (fibrinogen receptors). Mechanistically, LOX-1 inhibition of integrin activation was mediated by inactivating PKC [61]. Treatment with aspirin (1–10 mM) and pravastatin (1–5 μM) reduced platelet LOX-1 expression, with a synergistic effect of the combination of low-dose aspirin and pravastatin on decreasing malondialdehyde (MDA) release and enhancing NO release [61]. Endothelial LOX-1 also functions as an adhesion molecule for platelets by mediating the platelet-endothelium interaction [62]. Notably, binding of activated platelets to LOX-1 resulted in enhanced release of endothelin-1 [62] via interaction of LOX-1 and CD40 [63], and inactivated NO via increased intracellular production of superoxide [64] from EC, thereby directly causing endothelial dysfunction.
LOX-1 signaling pathways
OxLDL elicits wide-ranging effects on multiple signaling pathways implicated in atherosclerosis. As the cellular effect of oxLDL is mainly dependent on specific binding of oxLDL to the LOX-1 receptor, numerous signal transduction pathways are affected by LOX-1 activation: NADPH oxidase [48], mitogen-activated protein kinases (p38, ERK1/2, JNK) [65–67], PKC [54, 66], RhoA/Rac1 [35], Akt [41, 44], protein tyrosine kinase (PTK) [66], Ang-II type 1 receptor (AT1R) [12], sirtuin-1 (SIRT1) [68], octamer-binding protein-1 (Oct-1) [69, 70], activator protein-1 (AP-1) [71] and NF-κB [53]. It is noteworthy that LOX-1-mediated NF-κB activation by oxLDL is crucial for increasing expression of adhesion molecules and chemokines including E- and P-selectins, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and MCP-1, which are crucial for the initiating event of monocyte adhesion to EC [23, 56]. This was corroborated by a recent report showing that LOX-1 abrogation resulted in a broad inhibition of NF-κB target genes, such as VCAM-1 [72]. Following binding to LOX-1 in EC, oxLDL induced the generation of ROS, thereby activating NF-κB but inactivating NO [73, 74]. As oxLDL is a potent activator of NF-κB and there are putative NF-κB-binding sites located in the LOX-1 gene promoter [75], it is conceivable that oxLDL binding to LOX-1 can result in the activation of redox-sensitive NF-κB via NADPH oxidase-ROS signaling pathways. NF-κB activation can induce upregulation of pro-inflammatory mediators, AT1R as well as LOX-1 itself, which in turn increase LOX-1-mediated oxLDL uptake, thus amplifying the effects of atherogenic oxLDL (Fig. 2).
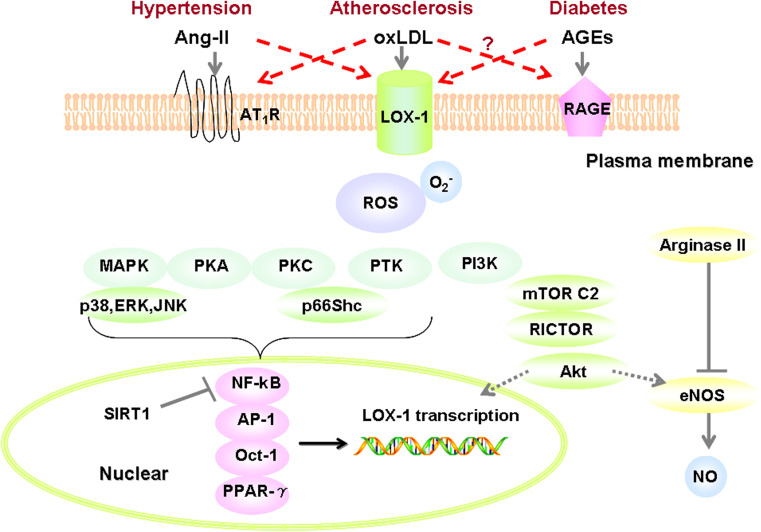
There is strong evidence for a mutually facilitatory cross-talk between Ang-II and oxLDL, in that AT1R expression is upregulated by oxLDL in EC through NF-κB activation [76]. Likewise, Ang-II has been shown to facilitate oxLDL uptake by EC via upregulating LOX-1, which is completely blocked by pre-treatment of HCAEC with losartan [77, 78]. Although oxLDL and Ang-II both induce LOX-1 expression in HCAEC, the underlying mechanisms of LOX-1 promoter activation are different: oxLDL, by activating Oct-1, induces LOX-1 promoter activation (between nucleotide −1,599 and −1,494), while another promoter region (between nucleotide −2,247 and −2,131), is required for Ang II-induced LOX-1 promoter activation via NF-κB [70, 79]. More recently, it was shown that deacetylation of NF-κB by SIRT1 in macrophages suppressed LOX-1 expression, thereby preventing macrophage-derived foam-cell formation [68]. Interestingly, Shiu et al. [80] recently demonstrated that AGEs, after binding to receptor of AGEs (RAGE), induce LOX-1 expression via phosphatidylinositol 3-kinases (PI3K)-mammalian target of rapamycin complex 2 (mTORC2)-Akt signaling pathway. Taken together, LOX-1 represents an important mechanistic converging point of Ang-II-, oxLDL-, and AGEs-mediated signaling pathways (Fig. 3).
Fig. 3.
LOX-1 signaling mechanistically links atherosclerosis, hypertension, and diabetes. oxLDL binding to LOX-1 affects NO catabolism by two mechanisms: (1) increased ROS production, which not only reacts with intracellular NO, resulting in the formation of cytotoxic peroxynitrite (ONOO−) but also down-regulates eNOS, thereby decreasing NO bioavailability; (2) oxLDL, through LOX-1 receptor activates arginase II, competing with eNOS for use of the substrate l-arginine, thus, down-regulating NO formation, and contributing to vascular dysfunction. Persistently increased ROS can activate multiple signaling pathways within vascular cells, such as PI3K/Akt, MAPK (p38, ERK and JNK), PKA, PKC, PTK, and p66Shc. These pathways further activate the redox-sensitive NF-κB pathway, increase NF-κB binding to LOX-1 promoter, and orchestrate LOX-1 expression in atherosclerosis. Alternatively, SIRT1 de-acetylates NF-κB to suppress the expression of LOX-1. LOX-1 is also the target gene of other transcription factors, such as AP-1, Oct-1, and PPAR-γ. AGEs act as a ligand of LOX-1 and can upregulate LOX-1 expression via PI3K/mTORC2/Akt pathway. In light of the cross-talk between AT1R and LOX-1, and potentially between RAGE and LOX-1, LOX-1 may provide a mechanistic link between atherosclerosis, hypertension, and diabetes. Ang-II angiotensin-II, oxLDL oxidized LDL, AGEs advanced glycation end-products, AT 1 R Ang-II type 1 receptor, RAGE receptor of advanced glycation end-products, NO nitric oxide, eNOS endothelial NO synthase, SIRT1 sirtuin-1, mTORC2 mammalian target of rapamycin complex 2, PI3K phosphatidylinositol 3-kinases, MAPK mitogen-activated protein kinases, ERK extracellular regulated protein kinases, JNK c-Jun N-terminal kinase, PKA protein kinase A, PKC protein kinase C, PTK protein tyrosine kinase, p66Shc 66-kDa isoform of Shc adaptor proteins, NF-κB nuclear factor-kappa B, AP-1 activator protein-1, Oct-1 octamer-binding protein 1, PPAR-γ peroxisome proliferator-activated receptor-gamma
Soluble LOX-1 and LOX-1 index in prognosis of cardiovascular diseases
Like many cell-surface receptors with a single trans-membrane domain, LOX-1 also undergoes post-translational proteolytic cleavage, which occurs within the “neck” region of the extracellular domain, releasing a 187-residue polypeptide known as soluble LOX-1 (sLOX-1) [81]. sLOX-1 was identified in conditioned media and can be measured in serum and plasma. The serum level of sLOX-1 may reflect LOX-1 expression. Inflammatory cytokines, including interleukin-18, TNF-α, and CRP, stimulate the release of sLOX-1 [81–83]. Circulating sLOX-1 is associated with inflammatory markers such as interleukin-6, TNF-α, and CRP [84]. LOX-1 gene polymorphisms were associated with sLOX-1 levels after being adjusted for age, sex, race, and body mass index [85]. Moreover, circulating sLOX-1 levels were elevated in patients with ACS [86]. Under conditions associated with plaque instability or rupture, elevated thrombin and protease activity have been implicated in the processing of membrane LOX-1 to generate sLOX-1. Thus, circulating sLOX-1 may not only be a prognostic biomarker of plaque destabilization but also a predictor of ACS recurrence or death [87]. Compared with troponin T and high-sensitivity CRP (hs-CRP), two established diagnostic biomarkers and predictors for ACS, sLOX-1 seems to reflect better the vulnerable atherosclerotic plaque [88].
LOX-1 ligand containing ApoB (LAB) of more than 2,000 healthy subjects was measured in a community-based cohort study [89]. During the 11-year follow-up period, the hazard ratio of ischemic stroke was increased in those with the highest LAB level. “LOX Index”, defined as a multiplication of LAB and sLOX-1, can reflect the biological activity of LOX-1 ligands, also proved to be a novel predictive marker for ischemic stroke, and to a lesser extent, coronary heart disease [89]. LAB showed significant correlations with smoking and triglycerides [90]. In hypercholesterolemic patients, baseline LAB levels did not correlate with LDL cholesterol and was significantly lowered by pitavastatin [91].
Classification of novel LOX-1 modulators
Lectin-like oxLDL receptor-1 emerges as a novel therapeutic target in cardiovascular pathologies including atherosclerosis [23, 92–95]. Over the past decade, it has become a major focus of research to aim to identify and pharmacologically characterize compounds that modulate LOX-1 function, especially phytochemicals from natural products. Vascular LOX-1 modulators are categorized as follows (Fig. 2).
Naturally occurring antioxidants
Oxidative modification of LDL is generally believed to play a pathologic role in the development of atherosclerotic plaques. Promising therapeutic strategies that would lower plasma oxLDL levels, including inhibition of oxLDL formation and removal of oxLDL from the circulation, may contribute to the prevention of atherosclerosis [29]. Antioxidants reduce the generation of oxLDL and the extent of atherosclerosis in experimental animal models. However, the antiatherogenic effects of antioxidant therapy are controversial because most clinical trials have yielded negative results [96]. In recent years, several natural compounds that inhibit LOX-1 have been identified, providing further insight into LOX-1 regulation. These compounds include tanshinone II-A [19], curcumin [97], berberine [98, 99], epigallocatechin gallate (EGCG) [100], and resveratrol [101]. We have previously demonstrated that tanshinone II-A, the most abundant diterpene quinone isolated from Salvia miltiorrhiza (Danshen), attenuates LDL oxidation and macrophage foam cell formation in diet-induced experimental atherosclerosis [102, 103]. Further observations indicate that tanshinone II-A inhibits oxLDL-induced LOX-1 expression in macrophages and decreases foam cell formation via ROS-NF-κB inhibitory effects [19]. Therefore, it is conceivable that antioxidants may inhibit LOX-1 expression at least through two mechanisms: firstly, reduction of the circulating oxLDL level, thus inactivating the LOX-1 signaling pathway, and secondly, since, in general, antioxidants have NF-κB inhibitory effects, antioxidants may reduce transcription of LOX-1 by interfering with NF-κB binding to the LOX-1 promoter.
Statins
Lowering LDL cholesterol by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) prevents the progression of atherosclerosis and decreases cardiovascular mortality. Increasing evidence suggests that statins may also enhance plaque stability. In EC, inhibition of LOX-1 by statins is associated with multiple antiatherosclerotic effects, such as reduced uptake of oxLDL, decreased apoptosis of EC, reduced monocyte-EC adhesion, upregulation of eNOS, and inhibition of Ang-II-converting enzyme (ACE) expression. LOX-1 mRNA expression in monocytes is appreciably suppressed by lovastatin [10]. It was also shown that simvastatin and atorvastatin inhibited oxLDL-induced LOX-1 expression through increasing Akt activity in HCAEC, which results in reduced binding (uptake) of 125I-oxLDL into HCAEC [41]. In addition, pravastatin is reported to downregulate LOX-1 expression in cultured human macrophages and SMC [104]. Likewise, administration of rosuvastatin reduced the upregulation of LOX-1 and AT1R and associated NADPH oxidase and NF-κB activation in LDLR−/− mice [105]. However, it remains unknown whether the inhibitory effect of statins on LOX-1 is related to the statin-mediated, cholesterol-lowering effect. Recent evidence suggests a previously unrecognized role of membrane cholesterol in modulating LOX-1 activity and suggests that statins protect vascular endothelium against the adverse effects of oxLDL by disruption of membrane rafts and impairment of LOX-1 receptor function [106]. Taken together, these data suggest that statins may exert their atheroprotective effects via downregulating LOX-1 expression in vascular lesions, extending the pleiotropic effects of statins.
Antihypertensive agents
Calcium channel blockers (CCB) and AT1R blockers (ARB) limit the progression of atherosclerosis and decrease the incidence of cardiovascular events [105]. Calcium channel blockers that have inhibitory effects on LOX-1 include: nifedipine, which prevents the apoptosis of EC induced by oxLDL via downregulation of LOX-1 and inhibition of 32-kDa putative cysteine protease (CPP32)-like protease activity [107]; amlodipine, which improves endothelial function in Ang-II-infused rats, by inhibition of oxidative stress and LOX-1 expression [108]; diltiazem, which was reported to inhibit the apoptosis of SMC exposed to high glucose [109]. As a mutually facilitatory cross-talk exists between LOX-1 and AT1R [76], it is conceivable that ARB can inhibit LOX-1 expression. LOX-1 was upregulated by Ang-II in cultured HCAEC, contributing to increased oxLDL uptake by EC. This upregulation of LOX-1 was blocked by pre-treatment with losartan [77]. Olmesartan was also reported to attenuate the impairment of EC induced by oxLDL by downregulating LOX-1 expression [110]. Therefore, LOX-1 may provide a mechanistic link between hypertension and atherosclerosis.
Antiinflammatory drugs
Inflammation is critically involved in the pathogenesis of atherosclerosis and plaque instability. In HCAEC, aspirin (1–5 mM), but not indomethacin (0.25 mM), an non-selective inhibitor of cyclooxygenase (COX) 1 and 2, inhibited oxLDL-mediated LOX-1 expression and interfered with the effects of oxLDL in intracellular signaling (p38 activation) and subsequent MMP-1 expression/activity [111]. In acute myocardial ischemia, this could prevent endothelial dysfunction and MMP-induced plaque destabilization as well as complementing the platelet-inhibitory effect of aspirin [111]. As NF-κB regulates the expression of LOX-1 by binding to the LOX-1 gene promoter, it is possible that antiinflammatory NF-κB inhibitors can down-regulate LOX-1 expression. To date, established NF-κB inhibitors with demonstrated LOX-1 inhibitory effects include pyrrolidine dithiocarbamate (PDTC) [67], N-acetyl-l-cysteine (NAC) [53], caffeic acid phenethyl ester (CAPE) [112] and tanshinone II-A [19].
Antihyperglycemic agents
Three categories of antihyperglycemic drugs, including sulfonyl urea (metformin), biguanide (gliclazide), and peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists (thiazolidinediones, TZDs), have been widely used for the management of type 2 diabetes mellitus. These drugs demonstrate LOX-1 modulatory effects independent of their hypoglycemic or insulin-sensitizing actions. In chronic hyperglycemia seen in advanced diabetes mellitus, glucose reacts with proteins to form AGEs. AGEs have been identified as a ligand for LOX-1 and upregulate LOX-1 expression in diabetes. In EC, metformin, which has antioxidant and mTOR inhibitory effects, attenuates the expression of both RAGE and LOX-1 induced by high glucose or AGEs, possibly via inhibiting the ROS-NF-κB signaling pathway and PI3K-mTORC2-Akt pathway [80, 113]. Inhibition of LOX-1 by gliclazide [55] leads to decreased MMP-9 expression and EC apoptosis. PPAR-γ ligands (i.e., 15d-PGJ2, pioglitazone, and troglitazone), not PPAR-α ligands (i.e., Wy14643 and fenofibric acid) inhibit TNF-α-induced LOX-1 expression in EC [114]. Intriguingly, LOX-1 is a novel PPAR-γ target gene in adipocytes [13]. Treatment of obese hyperglycemic animals with PPAR-γ agonists (rosiglitazone and ciglitazone) resulted in a marked increase in adipose LOX-1 expression with a concomitant increase in uptake of oxLDL and fatty acids, as well as reduction of serum oxLDL level [13]. These data suggest a novel role for PPAR-γ, antidiabetic TZD ligands, in controlling adipocyte cholesterol metabolism through LOX-1 induction. However, TZDs also upregulate scavenger receptor CD36 [114], which complicates the pharmacological effects of PPAR-γ ligands in oxLDL uptake and resultant foam cell formation.
Conclusions and future perspectives
Lectin-like oxLDL receptor-1 is a class E multiligand scavenger receptor that is mainly upregulated by oxLDL in vascular cells. This upregulation is inhibited by anti-LOX-1 antibodies, suggesting that LOX-1 is activated by the binding of oxLDL to LOX-1, which would further enhance oxLDL uptake and the ensuing atherogenic events. This constitutes a positive feedback loop of oxLDL and LOX-1 (Fig. 2). Recently, significant progress has been made in elucidating LOX-1-mediated signaling pathways (Fig. 3). However, there are still many questions that need to be addressed, such as the role of LOX-1 in SMC-derived foam cell formation and macrophage reverse cholesterol transport (RCT), a specialized process that can remove excess cholesterol from macrophage-derived foam cells present in atherosclerotic plaques. Further research is warranted to characterize the significance of this receptor in regulating human atherosclerosis. sLOX-1 and the LOX-1 index are potentially valuable biomarkers for early diagnosis of ACS. Further studies are required to demonstrate the diagnostic value of both biomarkers in large-scale, randomized clinical trials and the effect of interventions with vasculoprotective drugs.
In light of the importance of oxLDL-LOX-1 signaling pathway in the onset and progression of atherosclerosis, antiatherogenic strategies that target oxLDL-LOX-1 interaction using small molecule inhibitors would be an exciting and promising avenue in developing therapeutic agents to alleviate the atherosclerotic process in humans. Therefore, the LOX-1 receptor may represent an attractive therapeutic target for the prevention and management of atherosclerosis-related diseases. Further elucidation of signaling pathways and novel functions of LOX-1 will definitely advance our understanding of its role in the uptake of oxLDL and the pathogenesis of atherosclerosis.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81072641); NSFC-CIHR CHINA-CANADA Joint Health Research Initiative Proposal (No. 30811120434); National Science and Technology Major Project of China “Key New Drug Creation and Manufacturing Program” (No. 2009ZX09102-152, 2011ZX09401-307); Team Item of Natural Science Foundation of Guangdong Province (No. S2011030003190); Major Project of Guangdong Province (No. 2008A030201013, No. 2012A080201007); Major Project of Department of Education of Guangdong Province (No. CXZD1006). General work in this area has also been supported by grants from the National Health and Medical Research Council of Australia, National Heart Foundation of Australia and Diabetes Australia Research Trust. Suowen Xu received the “New Investigator Award” from Ministry of Education of China. We gratefully acknowledge the contributions of investigators dedicated to the LOX-1 field, and apologize to those investigators whose work we could not cite owing to space limitations.
Conflict of interest
None declared.
Contributor Information
Suowen Xu, Phone: +1-301-5942892, Email: suo-wen.xu@nih.gov.
Peiqing Liu, Phone: +86-20-39943116, FAX: +86-20-39943026, Email: liupq@mail.sysu.edu.cn.
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Little PJ, Osman N, O’Brien KD. Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr Opin Lipidol. 2008;19(5):448–454. doi: 10.1097/MOL.0b013e32830dd7c4. [DOI] [PubMed] [Google Scholar]
- 3.Burch ML, Zheng W, Little PJ. Smad linker region phosphorylation in the regulation of extracellular matrix synthesis. Cell Mol Life Sci. 2011;68(1):97–107. doi: 10.1007/s00018-010-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballinger ML, Nigro J, Frontanilla KV, Dart AM, Little PJ. Regulation of glycosaminoglycan structure and atherogenesis. Cell Mol Life Sci. 2004;61(11):1296–1306. doi: 10.1007/s00018-004-3389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little PJ, Chait A, Bobik A. Cellular and cytokine-based inflammatory processes as novel therapeutic targets for the prevention and treatment of atherosclerosis. Pharmacol Ther. 2011;131(3):255–268. doi: 10.1016/j.pharmthera.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 7.Yamada Y, Doi T, Hamakubo T, Kodama T. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell Mol Life Sci. 1998;54(7):628–640. doi: 10.1007/s000180050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 9.Sawamura T, Kakino A, Fujita Y. LOX-1: a multiligand receptor at the crossroads of response to danger signals. Curr Opin Lipidol. 2012;23(5):439–445. doi: 10.1097/MOL.0b013e32835688e4. [DOI] [PubMed] [Google Scholar]
- 10.Draude G, Hrboticky N, Lorenz RL. The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochem Pharmacol. 1999;57(4):383–386. doi: 10.1016/S0006-2952(98)00313-X. [DOI] [PubMed] [Google Scholar]
- 11.Iwai-Kanai E, Hasegawa K, Sawamura T, Fujita M, Yanazume T, Toyokuni S, Adachi S, Kihara Y, Sasayama S. Activation of lectin-like oxidized low-density lipoprotein receptor-1 induces apoptosis in cultured neonatal rat cardiac myocytes. Circulation. 2001;104(24):2948–2954. doi: 10.1161/hc4901.100381. [DOI] [PubMed] [Google Scholar]
- 12.Hu C, Dandapat A, Sun L, Marwali MR, Inoue N, Sugawara F, Inoue K, Kawase Y, Jishage K, Suzuki H, Hermonat PL, Sawamura T, Mehta JL. Modulation of angiotensin II-mediated hypertension and cardiac remodeling by lectin-like oxidized low-density lipoprotein receptor-1 deletion. Hypertension. 2008;52(3):556–562. doi: 10.1161/HYPERTENSIONAHA.108.115287. [DOI] [PubMed] [Google Scholar]
- 13.Chui PC, Guan HP, Lehrke M, Lazar MA. PPARgamma regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J Clin Invest. 2005;115(8):2244–2256. doi: 10.1172/JCI24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieudonne A, Torres D, Blanchard S, Taront S, Jeannin P, Delneste Y, Pichavant M, Trottein F, Gosset P. Scavenger receptors in human airway epithelial cells: role in response to double-stranded RNA. PLoS ONE. 2012;7(8):e41952. doi: 10.1371/journal.pone.0041952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parlato S, Romagnoli G, Spadaro F, Canini I, Sirabella P, Borghi P, Ramoni C, Filesi I, Biocca S, Gabriele L, Belardelli F. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115(8):1554–1563. doi: 10.1182/blood-2009-07-234468. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Kakutani M, Naruko T, Ueda M, Narumiya S, Masaki T, Sawamura T. Activation-dependent surface expression of LOX-1 in human platelets. Biochem Biophys Res Commun. 2001;282(1):153–158. doi: 10.1006/bbrc.2001.4516. [DOI] [PubMed] [Google Scholar]
- 17.Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99(24):3110–3117. doi: 10.1161/01.CIR.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T, Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc Drugs Ther. 2011;25(5):379–391. doi: 10.1007/s10557-011-6324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu S, Liu Z, Huang Y, Le K, Tang F, Huang H, Ogura S, Little P, Shen X, Liu P. Tanshinone II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages by reducing intracellular superoxide radical generation and NF-κB activation. Transl Res. 2012;160(2):114–124. doi: 10.1016/j.trsl.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95(1):89–100. doi: 10.1016/S0163-7258(02)00236-X. [DOI] [PubMed] [Google Scholar]
- 21.Pirillo A, Reduzzi A, Ferri N, Kuhn H, Corsini A, Catapano AL. Upregulation of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) by 15-lipoxygenase-modified LDL in endothelial cells. Atherosclerosis. 2011;214(2):331–337. doi: 10.1016/j.atherosclerosis.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Shiu SW, Tan KC, Wong Y, Leng L, Bucala R. Glycoxidized LDL increases lectin-like oxidized low-density lipoprotein receptor-1 in diabetes mellitus. Atherosclerosis. 2009;203(2):522–527. doi: 10.1016/j.atherosclerosis.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Ogura S, Kakino A, Sato Y, Fujita Y, Iwamoto S, Otsui K, Yoshimoto R, Sawamura T. Lox-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ J. 2009;73(11):1993–1999. doi: 10.1253/circj.CJ-09-0587. [DOI] [PubMed] [Google Scholar]
- 24.Kume N, Kita T. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in atherogenesis. Trends Cardiovasc Med. 2001;11(1):22–25. doi: 10.1016/S1050-1738(01)00079-2. [DOI] [PubMed] [Google Scholar]
- 25.Qian Y, Zhang X, Zhou L, Yun X, Xie J, Xu J, Ruan Y, Ren S. Site-specific N-glycosylation identification of recombinant human lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) Glycoconj J. 2012;29(5–6):399–409. doi: 10.1007/s10719-012-9408-z. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Arai Y, Kurihara H, Kita T, Sawamura T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ Res. 2005;97(2):176–184. doi: 10.1161/01.RES.0000174286.73200.d4. [DOI] [PubMed] [Google Scholar]
- 27.White SJ, Sala-Newby GB, Newby AC. Overexpression of scavenger receptor LOX-1 in endothelial cells promotes atherogenesis in the ApoE(−/−) mouse model. Cardiovasc Pathol. 2011;20(6):369–373. doi: 10.1016/j.carpath.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100(11):1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 29.Ishigaki Y, Katagiri H, Gao J, Yamada T, Imai J, Uno K, Hasegawa Y, Kaneko K, Ogihara T, Ishihara H, Sato Y, Takikawa K, Nishimichi N, Matsuda H, Sawamura T, Oka Y. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation. 2008;118(1):75–83. doi: 10.1161/CIRCULATIONAHA.107.745174. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab. 2001;280(1):E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 31.Ryoo S, Bhunia A, Chang F, Shoukas A, Berkowitz DE, Romer LH. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2011;214(2):279–287. doi: 10.1016/j.atherosclerosis.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, Cosentino F, Camici GG, Akhmedov A, Vanhoutte PM, Tanner FC, Luscher TF. Oxidized low-density lipoprotein activates p66Shc via lectin-like oxidized low-density lipoprotein receptor-1, protein kinase C-beta, and c-Jun N-terminal kinase kinase in human endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31(9):2090–2097. doi: 10.1161/ATVBAHA.111.229260. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27(4):871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 34.Eichhorn B, Muller G, Leuner A, Sawamura T, Ravens U, Morawietz H. Impaired vascular function in small resistance arteries of LOX-1 overexpressing mice on high-fat diet. Cardiovasc Res. 2009;82(3):493–502. doi: 10.1093/cvr/cvp089. [DOI] [PubMed] [Google Scholar]
- 35.Sugimoto K, Ishibashi T, Sawamura T, Inoue N, Kamioka M, Uekita H, Ohkawara H, Sakamoto T, Sakamoto N, Okamoto Y, Takuwa Y, Kakino A, Fujita Y, Tanaka T, Teramoto T, Maruyama Y, Takeishi Y. LOX-1-MT1-MMP axis is crucial for RhoA and Rac1 activation induced by oxidized low-density lipoprotein in endothelial cells. Cardiovasc Res. 2009;84(1):127–136. doi: 10.1093/cvr/cvp177. [DOI] [PubMed] [Google Scholar]
- 36.Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Luscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121(7):2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braunersreuther V, Mach F. Leukocyte recruitment in atherosclerosis: potential targets for therapeutic approaches? Cell Mol Life Sci. 2006;63(18):2079–2088. doi: 10.1007/s00018-006-6127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101(25):2889–2895. doi: 10.1161/01.CIR.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 39.Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, Honda Y, Butcher EC, Masaki T, Sawamura T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci USA. 2003;100(3):1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JW, Zhou SB, Tan ZM. Aspirin and pravastatin reduce lectin-like oxidized low-density lipoprotein receptor-1 expression, adhesion molecules and oxidative stress in human coronary artery endothelial cells. Chin Med J (Engl) 2010;123(12):1553–1556. [PubMed] [Google Scholar]
- 41.Li DY, Chen HJ, Mehta JL. Statins inhibit oxidized-LDL-mediated LOX-1 expression, uptake of oxidized-LDL and reduction in PKB phosphorylation. Cardiovasc Res. 2001;52(1):130–135. doi: 10.1016/S0008-6363(01)00371-6. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Mehta JL, Haider N, Zhang X, Narula J, Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94(3):370–376. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- 43.Li D, Mehta JL. Intracellular signaling of LOX-1 in endothelial cell apoptosis. Circ Res. 2009;104(5):566–568. doi: 10.1161/CIRCRESAHA.109.194209. [DOI] [PubMed] [Google Scholar]
- 44.Lu J, Yang JH, Burns AR, Chen HH, Tang D, Walterscheid JP, Suzuki S, Yang CY, Sawamura T, Chen CH. Mediation of electronegative low-density lipoprotein signaling by LOX-1: a possible mechanism of endothelial apoptosis. Circ Res. 2009;104(5):619–627. doi: 10.1161/CIRCRESAHA.108.190116. [DOI] [PubMed] [Google Scholar]
- 45.Khaidakov M, Wang X, Mehta JL. Potential involvement of LOX-1 in functional consequences of endothelial senescence. PLoS ONE. 2011;6(6):e20964. doi: 10.1371/journal.pone.0020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eto H, Miyata M, Kume N, Minami M, Itabe H, Orihara K, Hamasaki S, Biro S, Otsuji Y, Kita T, Tei C. Expression of lectin-like oxidized LDL receptor-1 in smooth muscle cells after vascular injury. Biochem Biophys Res Commun. 2006;341(2):591–598. doi: 10.1016/j.bbrc.2005.12.211. [DOI] [PubMed] [Google Scholar]
- 47.Hinagata J, Kakutani M, Fujii T, Naruko T, Inoue N, Fujita Y, Mehta JL, Ueda M, Sawamura T. Oxidized LDL receptor LOX-1 is involved in neointimal hyperplasia after balloon arterial injury in a rat model. Cardiovasc Res. 2006;69(1):263–271. doi: 10.1016/j.cardiores.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Hu C, Dandapat A, Sun L, Chen J, Marwali MR, Romeo F, Sawamura T, Mehta JL. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc Res. 2008;79(2):287–293. doi: 10.1093/cvr/cvn110. [DOI] [PubMed] [Google Scholar]
- 49.Moriwaki H, Kume N, Kataoka H, Murase T, Nishi E, Sawamura T, Masaki T, Kita T. Expression of lectin-like oxidized low-density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998;440(1–2):29–32. doi: 10.1016/S0014-5793(98)01414-8. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida H, Kondratenko N, Green S, Steinberg D, Quehenberger O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J. 1998;334:9–13. doi: 10.1042/bj3340009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaeffer DF, Riazy M, Parhar KS, Chen JH, Duronio V, Sawamura T, Steinbrecher UP. LOX-1 augments oxLDL uptake by lysoPC-stimulated murine macrophages but is not required for oxLDL clearance from plasma. J Lipid Res. 2009;50(8):1676–1684. doi: 10.1194/jlr.M900167-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishiyama J, Taguchi R, Yamamoto A, Murakami K. Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis. 2010;209(1):118–124. doi: 10.1016/j.atherosclerosis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Sawamura T, Renier G. Glucose enhances human macrophage LOX-1 expression: role for LOX-1 in glucose-induced macrophage foam cell formation. Circ Res. 2004;94(7):892–901. doi: 10.1161/01.RES.0000124920.09738.26. [DOI] [PubMed] [Google Scholar]
- 54.Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107(4):612–617. doi: 10.1161/01.CIR.0000047276.52039.FB. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Renier G. The oral anti-diabetic agent, gliclazide, inhibits oxidized LDL-mediated LOX-1 expression, metalloproteinase-9 secretion and apoptosis in human aortic endothelial cells. Atherosclerosis. 2009;204(1):40–46. doi: 10.1016/j.atherosclerosis.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12(9):1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh CC, Yen MH, Yen CH, Lau YT. Oxidized low-density lipoprotein induces apoptosis via generation of reactive oxygen species in vascular smooth muscle cells. Cardiovasc Res. 2001;49(1):135–145. doi: 10.1016/S0008-6363(00)00218-2. [DOI] [PubMed] [Google Scholar]
- 58.Kume N, Kita T. Apoptosis of vascular cells by oxidized LDL: involvement of caspases and LOX-1 and its implication in atherosclerotic plaque rupture. Circ Res. 2004;94(3):269–270. doi: 10.1161/01.RES.0000119804.92239.97. [DOI] [PubMed] [Google Scholar]
- 59.Kataoka H, Kume N, Miyamoto S, Minami M, Morimoto M, Hayashida K, Hashimoto N, Kita T. Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21(6):955–960. doi: 10.1161/01.ATV.21.6.955. [DOI] [PubMed] [Google Scholar]
- 60.Hu C, Dandapat A, Sun L, Khan JA, Liu Y, Hermonat PL, Mehta JL. Regulation of TGFbeta1-mediated collagen formation by LOX-1: studies based on forced overexpression of TGFbeta1 in wild-type and lox-1 knock-out mouse cardiac fibroblasts. J Biol Chem. 2008;283(16):10226–10231. doi: 10.1074/jbc.M708820200. [DOI] [PubMed] [Google Scholar]
- 61.Marwali MR, Hu CP, Mohandas B, Dandapat A, Deonikar P, Chen J, Cawich I, Sawamura T, Kavdia M, Mehta JL. Modulation of ADP-induced platelet activation by aspirin and pravastatin: role of lectin-like oxidized low-density lipoprotein receptor-1, nitric oxide, oxidative stress, and inside-out integrin signaling. J Pharmacol Exp Ther. 2007;322(3):1324–1332. doi: 10.1124/jpet.107.122853. [DOI] [PubMed] [Google Scholar]
- 62.Kakutani M, Masaki T, Sawamura T. A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc Natl Acad Sci USA. 2000;97(1):360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakurai K, Cominacini L, Garbin U, Fratta Pasini A, Sasaki N, Takuwa Y, Masaki T, Sawamura T. Induction of endothelin-1 production in endothelial cells via co-operative action between CD40 and lectin-like oxidized LDL receptor (LOX-1) J Cardiovasc Pharmacol. 2004;44(Suppl 1):S173–S180. doi: 10.1097/01.fjc.0000166243.43616.8b. [DOI] [PubMed] [Google Scholar]
- 64.Cominacini L, Fratta Pasini A, Garbin U, Pastorino A, Rigoni A, Nava C, Davoli A, Lo Cascio V, Sawamura T. The platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells. J Am Coll Cardiol. 2003;41(3):499–507. doi: 10.1016/S0735-1097(02)02811-5. [DOI] [PubMed] [Google Scholar]
- 65.Tanigawa H, Miura S, Matsuo Y, Fujino M, Sawamura T, Saku K. Dominant-negative lox-1 blocks homodimerization of wild-type lox-1-induced cell proliferation through extracellular signal regulated kinase 1/2 activation. Hypertension. 2006;48(2):294–300. doi: 10.1161/01.HYP.0000229825.98545.5e. [DOI] [PubMed] [Google Scholar]
- 66.Li D, Yang B, Mehta JL. Ox-LDL induces apoptosis in human coronary artery endothelial cells: role of PKC, PTK, bcl-2, and Fas. Am J Physiol. 1998;275(2 Pt 2):H568–H576. doi: 10.1152/ajpheart.1998.275.2.H568. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y, Chen X. Ox-LDL-induced LOX-1 expression in vascular smooth muscle cells: role of reactive oxygen species. Fundam Clin Pharmacol. 2011;25(5):572–579. doi: 10.1111/j.1472-8206.2010.00885.x. [DOI] [PubMed] [Google Scholar]
- 68.Stein S, Lohmann C, Schafer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Boren J, McBurney MW, Landmesser U, Luscher TF, Matter CM. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J. 2010;31(18):2301–2309. doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thum T, Borlak J. LOX-1 receptor blockade abrogates oxLDL-induced oxidative DNA damage and prevents activation of the transcriptional repressor Oct-1 in human coronary arterial endothelium. J Biol Chem. 2008;283(28):19456–19464. doi: 10.1074/jbc.M708309200. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Liu Y, Liu H, Hermonat PL, Mehta JL. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) transcriptional regulation by Oct-1 in human endothelial cells: implications for atherosclerosis. Biochem J. 2006;393:255–265. doi: 10.1042/BJ20050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li L, Sawamura T, Renier G. Glucose enhances endothelial LOX-1 expression: role for LOX-1 in glucose-induced human monocyte adhesion to endothelium. Diabetes. 2003;52(7):1843–1850. doi: 10.2337/diabetes.52.7.1843. [DOI] [PubMed] [Google Scholar]
- 72.Khaidakov M, Mitra S, Kang BY, Wang X, Kadlubar S, Novelli G, Raj V, Winters M, Carter WC, Mehta JL. Oxidized LDL receptor 1 (OLR1) as a possible link between obesity, dyslipidemia and cancer. PLoS ONE. 2011;6(5):e20277. doi: 10.1371/journal.pone.0020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V, Sawamura T. The binding of oxidized low-density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem. 2001;276(17):13750–13755. doi: 10.1074/jbc.M010612200. [DOI] [PubMed] [Google Scholar]
- 74.Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. Oxidized low-density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275(17):12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 75.Nagase M, Abe J, Takahashi K, Ando J, Hirose S, Fujita T. Genomic organization and regulation of expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) gene. J Biol Chem. 1998;273(50):33702–33707. doi: 10.1074/jbc.273.50.33702. [DOI] [PubMed] [Google Scholar]
- 76.Li D, Saldeen T, Romeo F, Mehta JL. Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: the potential role of transcription factor NF-kappaB. Circulation. 2000;102(16):1970–1976. doi: 10.1161/01.CIR.102.16.1970. [DOI] [PubMed] [Google Scholar]
- 77.Li DY, Zhang YC, Philips MI, Sawamura T, Mehta JL. Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ Res. 1999;84(9):1043–1049. doi: 10.1161/01.RES.84.9.1043. [DOI] [PubMed] [Google Scholar]
- 78.Li D, Singh RM, Liu L, Chen H, Singh BM, Kazzaz N, Mehta JL. Oxidized-LDL through LOX-1 increases the expression of angiotensin converting enzyme in human coronary artery endothelial cells. Cardiovasc Res. 2003;57(1):238–243. doi: 10.1016/S0008-6363(02)00674-0. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, Liu Y, Liu H, Hermonat PL, Mehta JL. Molecular dissection of angiotensin II-activated human LOX-1 promoter. Arterioscler Thromb Vasc Biol. 2006;26(5):1163–1168. doi: 10.1161/01.ATV.0000209998.73303.b5. [DOI] [PubMed] [Google Scholar]
- 80.Shiu SW, Wong Y, Tan KC (2012) Effect of advanced glycation end products on lectin-like oxidized low-density lipoprotein receptor-1 expression in endothelial cells. J Atheroscler Thromb. 10.5551/jat.11742 [DOI] [PubMed]
- 81.Murase T, Kume N, Kataoka H, Minami M, Sawamura T, Masaki T, Kita T. Identification of soluble forms of lectin-like oxidized LDL receptor-1. Arterioscler Thromb Vasc Biol. 2000;20(3):715–720. doi: 10.1161/01.ATV.20.3.715. [DOI] [PubMed] [Google Scholar]
- 82.Mitsuoka H, Kume N, Hayashida K, Inui-Hayashiada A, Aramaki Y, Toyohara M, Jinnai T, Nishi E, Kita T. Interleukin 18 stimulates release of soluble lectin-like oxidized LDL receptor-1 (sLOX-1) Atherosclerosis. 2009;202(1):176–182. doi: 10.1016/j.atherosclerosis.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 83.Zhao XQ, Zhang MW, Wang F, Zhao YX, Li JJ, Wang XP, Bu PL, Yang JM, Liu XL, Zhang MX, Gao F, Zhang C, Zhang Y. CRP enhances soluble LOX-1 release from macrophages by activating TNF-alpha converting enzyme. J Lipid Res. 2011;52(5):923–933. doi: 10.1194/jlr.M015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids. 2008;43(10):945–950. doi: 10.1007/s11745-008-3227-9. [DOI] [PubMed] [Google Scholar]
- 85.Brinkley TE, Kume N, Mitsuoka H, Brown MD, Phares DA, Ferrell RE, Kita T, Hagberg JM. Variation in the human lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) gene is associated with plasma soluble LOX-1 levels. Exp Physiol. 2008;93(9):1085–1090. doi: 10.1113/expphysiol.2008.042267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayashida K, Kume N, Murase T, Minami M, Nakagawa D, Inada T, Tanaka M, Ueda A, Kominami G, Kambara H, Kimura T, Kita T. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112(6):812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- 87.Kume N, Mitsuoka H, Hayashida K, Tanaka M, Kita T. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts prognosis after acute coronary syndrome—a pilot study. Circ J. 2010;74(7):1399–1404. doi: 10.1253/circj.CJ-09-0924. [DOI] [PubMed] [Google Scholar]
- 88.Kume N, Mitsuoka H, Hayashida K, Tanaka M, Kominami G, Kita T. Soluble lectin-like oxidized LDL receptor-1 (sLOX-1) as a sensitive and specific biomarker for acute coronary syndrome–comparison with other biomarkers. J Cardiol. 2010;56(2):159–165. doi: 10.1016/j.jjcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 89.Inoue N, Okamura T, Kokubo Y, Fujita Y, Sato Y, Nakanishi M, Yanagida K, Kakino A, Iwamoto S, Watanabe M, Ogura S, Otsui K, Matsuda H, Uchida K, Yoshimoto R, Sawamura T. LOX index, a novel predictive biochemical marker for coronary heart disease and stroke. Clin Chem. 2010;56(4):550–558. doi: 10.1373/clinchem.2009.140707. [DOI] [PubMed] [Google Scholar]
- 90.Uchida K, Suehiro A, Nakanishi M, Sawamura T, Wakabayashi I. Associations of atherosclerotic risk factors with oxidized low-density lipoprotein evaluated by LOX-1 ligand activity in healthy men. Clin Chim Acta. 2011;412(17–18):1643–1647. doi: 10.1016/j.cca.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 91.Matsumoto T, Fujita M, Sawamura T, Kakino A, Sato Y, Fujita Y, Matsuda H, Nakanishi M, Uchida K, Nakae I, Kanda H, Yoshida A, Miwa K, Hayashi H, Mitsunami K, Horie M. Pitavastatin reduces lectin-like oxidized low-density lipoprotein receptor-1 ligands in hypercholesterolemic humans. Lipids. 2010;45(4):329–335. doi: 10.1007/s11745-010-3402-7. [DOI] [PubMed] [Google Scholar]
- 92.Chen XP, Zhang TT, Du GH. Lectin-like oxidized low-density lipoprotein receptor-1, a new promising target for the therapy of atherosclerosis? Cardiovasc Drug Rev. 2007;25(2):146–161. doi: 10.1111/j.1527-3466.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 93.Mehta JL, Khaidakov M, Hermonat PL, Mitra S, Wang X, Novelli G, Sawamura T. LOX-1: a new target for therapy for cardiovascular diseases. Cardiovasc Drugs Ther. 2011;25(5):495–500. doi: 10.1007/s10557-011-6325-5. [DOI] [PubMed] [Google Scholar]
- 94.Lu J, Mitra S, Wang X, Khaidakov M, Mehta JL. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal. 2011;15(8):2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 95.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69(1):36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105(17):2107–2111. doi: 10.1161/01.CIR.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 97.Kang BY, Khan JA, Ryu S, Shekhar R, Seung KB, Mehta JL. Curcumin reduces angiotensin II-mediated cardiomyocyte growth via LOX-1 inhibition. J Cardiovasc Pharmacol. 2010;55(2):176–183. doi: 10.1097/FJC.0b013e3181ca4ba1. [DOI] [PubMed] [Google Scholar]
- 98.Guan S, Wang B, Li W, Guan J, Fang X. Effects of berberine on expression of LOX-1 and SR-BI in human macrophage-derived foam cells induced by ox-LDL. Am J Chin Med. 2010;38(6):1161–1169. doi: 10.1142/S0192415X10008548. [DOI] [PubMed] [Google Scholar]
- 99.Huang Z, Dong F, Li S, Chu M, Zhou H, Lu Z, Huang W. Berberine-induced inhibition of adipocyte enhancer-binding protein 1 attenuates oxidized low-density lipoprotein accumulation and foam cell formation in phorbol 12-myristate 13-acetate-induced macrophages. Eur J Pharmacol. 2012;690(1–3):164–169. doi: 10.1016/j.ejphar.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Ou HC, Song TY, Yeh YC, Huang CY, Yang SF, Chiu TH, Tsai KL, Chen KL, Wu YJ, Tsai CS, Chang LY, Kuo WW, Lee SD. EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J Appl Physiol. 2010;108(6):1745–1756. doi: 10.1152/japplphysiol.00879.2009. [DOI] [PubMed] [Google Scholar]
- 101.Chang HC, Chen TG, Tai YT, Chen TL, Chiu WT, Chen RM. Resveratrol attenuates oxidized LDL-evoked Lox-1 signaling and consequently protects against apoptotic insults to cerebrovascular endothelial cells. J Cereb Blood Flow Metab. 2011;31(3):842–854. doi: 10.1038/jcbfm.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220(1):3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 103.Xu S, Little PJ, Lan T, Huang Y, Le K, Wu X, Shen X, Huang H, Cai Y, Tang F, Wang H, Liu P. Tanshinone II-A attenuates and stabilizes atherosclerotic plaques in apolipoprotein-E knockout mice fed a high cholesterol diet. Arch Biochem Biophys. 2011;515(1–2):72–79. doi: 10.1016/j.abb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 104.Hofnagel O, Luechtenborg B, Eschert H, Weissen-Plenz G, Severs NJ, Robenek H. Pravastatin inhibits expression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in Watanabe heritable hyperlipidemic rabbits: a new pleiotropic effect of statins. Arterioscler Thromb Vasc Biol. 2006;26(3):604–610. doi: 10.1161/01.ATV.0000201073.45862.8b. [DOI] [PubMed] [Google Scholar]
- 105.Kang BY, Wang W, Palade P, Sharma SG, Mehta JL. Cardiac hypertrophy during hypercholesterolemia and its amelioration with rosuvastatin and amlodipine. J Cardiovasc Pharmacol. 2009;54(4):327–334. doi: 10.1097/FJC.0b013e3181b76713. [DOI] [PubMed] [Google Scholar]
- 106.Matarazzo S, Quitadamo MC, Mango R, Ciccone S, Novelli G, Biocca S. Cholesterol-lowering drugs inhibit LOX-1 receptor function by membrane raft disruption. Mol Pharmacol. 2012;82(2):246–254. doi: 10.1124/mol.112.078915. [DOI] [PubMed] [Google Scholar]
- 107.Sugano M, Tsuchida K, Makino N. Nifedipine prevents apoptosis of endothelial cells induced by oxidized low-density lipoproteins. J Cardiovasc Pharmacol. 2002;40(1):146–152. doi: 10.1097/00005344-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 108.Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am J Hypertens. 2004;17(2):167–171. doi: 10.1016/j.amjhyper.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 109.Rudijanto A. Calcium channel blocker (diltiazem) inhibits apoptosis of vascular smooth muscle cell exposed to high glucose concentration through lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) pathway. Acta Med Indones. 2010;42(2):59–65. [PubMed] [Google Scholar]
- 110.Zhang H, Ma G, Yao Y, Qian H, Li W, Chen X, Jiang W, Zheng R. Olmesartan attenuates the impairment of endothelial cells induced by oxidized low-density lipoprotein through downregulating expression of LOX-1. Int J Mol Sci. 2012;13(2):1512–1523. doi: 10.3390/ijms13021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehta JL, Chen J, Yu F, Li DY. Aspirin inhibits ox-LDL-mediated LOX-1 expression and metalloproteinase-1 in human coronary endothelial cells. Cardiovasc Res. 2004;64(2):243–249. doi: 10.1016/j.cardiores.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20(4):1116–1122. doi: 10.1161/01.ATV.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 113.Ouslimani N, Mahrouf M, Peynet J, Bonnefont-Rousselot D, Cosson C, Legrand A, Beaudeux JL. Metformin reduces endothelial cell expression of both the receptor for advanced glycation end products and lectin-like oxidized receptor 1. Metabolism. 2007;56(3):308–313. doi: 10.1016/j.metabol.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Chiba Y, Ogita T, Ando K, Fujita T. PPARgamma ligands inhibit TNF-alpha-induced LOX-1 expression in cultured endothelial cells. Biochem Biophys Res Commun. 2001;286(3):541–546. doi: 10.1006/bbrc.2001.5361. [DOI] [PubMed] [Google Scholar]