Abstract
In polarized, migrating cells, stress fibers are a highly dynamic network of contractile actomyosin structures composed of bundles of actin filaments held together by actin cross-linking proteins such as α-actinins. As such, α-actinins influence actin cytoskeleton organization and dynamics and focal adhesion maturation. In response to environmental signals, α-actinins are tyrosine phosphorylated and this affects their binding to actin stress fibers; however, the cellular role of α-actinin tyrosine phosphorylation remains largely unknown. We found that non-muscle a-actinin1/4 are critical for the establishment of dorsal stress fibers and maintenance of transverse arc stress fibers. Analysis of cells genetically depleted of α-actinin1 and 4 reveals two distinct modes for focal adhesion maturation. An α-actinin1 or 4 dependent mode that uses dorsal stress fiber precursors as a template for establishing focal adhesions and their maturation, and an α-actinin-independent manner that uses transverse arc precursors to establish focal adhesions at both ends. Focal adhesions formed in the absence of α-actinins are delayed in their maturation, exhibit altered morphology, have decreased amounts of Zyxin and VASP, and reduced adhesiveness to extracellular matrix. Further rescue experiments demonstrate that the tyrosine phosphorylation of α-actinin1 at Y12 and α-actinin4 at Y265 is critical for dorsal stress fiber establishment, transverse arc maintenance and focal adhesion maturation.
Keywords: α-Actinin, Phosphorylation, Stress fiber, Focal adhesion
Introduction
Stress fibers are bundles of polymeric actin filaments and contractile myosin that are essential for the adhesion of cells to extracellular matrix (ECM), and regulate cell morphology and cell movement [1]. Based on their subcellular localization and interaction with focal adhesions (FAs), they have been divided into three classes: ventral stress fibers, dorsal stress fibers (or radial stress fibers), and transverse arcs (reviewed in [2,3]). Ventral stress fibers lie along the base of the cells and attach to focal adhesions at each end. They play important roles in cell adhesion and cell contraction. Transverse arcs are curved actomyosin bundles that form beneath the dorsal surface of the cells and do not directly associate with focal adhesions. In migrating cells, transverse arcs show typical retrograde flow from the leading edge towards the nucleus where they dissemble [4–6]. Dorsal stress fibers are non-contractile stress fibers attached to FAs at one end and rise from FAs towards the dorsal surface [6,7]. Dorsal stress fiber assembly has been shown to play an important role in promoting FA maturation which is required to form tensin rich fibrillar adhesions used in ECM remodeling [8].
In motile cells these three types of stress fibers assemble by distinct mechanisms [6,2,3]. The dorsal stress fibers elongate from focal adhesion dorsally to form short filaments that contain α-actinin. Clusters of myosin are only occasionally incorporated into dorsal stress fibers and when so can displace α-actinin. mDia1-mediated actin polymerization contributes to the elongation of dorsal stress fibers. Transverse arcs are generated from α-actinin cross-linked actin filaments and originate in the lamellipodia meshwork. Arp2/3 nucleated actin polymerization is required for the assembly of transverse arcs [6]. These filaments are joined endwise with myosin bundles to form contractile transverse arcs. Transverse arcs form from myosin clusters at the leading edge of the cells and travel backwards through lamella to associate with the loose network of actin filaments at the base of protrusions. Contraction of the myosin generates cell tension leads to their reorganization into actin bundles where α-actinin can be incorporated [9,10,2]. Ventral stress fibers form through the endwise joining of two dorsal stress fibers such that they are attached to focal adhesions at both ends [6]. Ventral stress fibers may also be generated from the fusion of short FA attached stress fibers [5,11].
Focal adhesions are large integrin-based, dynamic macromolecular structures that connect the extracellular matrix with the intracellular actin cytoskeleton. Both mechanical force and biological signals are transmitted by FAs [12]. As such the organization and function of FAs and cellular stress fibers are interrelated. In the lamellipodia of migrating cells, nascent adhesions initially form. These are short lived structures that either turnover or mature centripetally into elongated FAs [13,14]. mDia1 or Arp2/3 nucleated actin polymerization in the lamellipodium is required for the assembly of nascent adhesions, and in concert with the actin cross-linking proteins α-actinin and myosin II, form the precursors that serve as templates for the maturation of nascent adhesions into FAs [13,15]. This process of FA maturation in response to actomysoin generated cell tension modulates adhesion dynamics. While this describes FA formation and maturation, the role of the α-actinin in these processes is not fully appreciated.
α-actinins are ubiquitously expressed cytoskeleton proteins that cross-link actin filaments at adherens junctions in epithelia and focal adhesions at the leading edge of migrating cells [16–19]. α-actinins also interact with a number of other focal adhesion-associated proteins including vinculin [20] and integrins [21,22]. As such they are thought to contribute to the linkage of actin filaments to focal adhesions [23,12]. In mammalian cells, there are four α-actinin proteins. α-actinin1 and 4 are ubiquitously expressed, while α-actinin2 and 3 are muscle specific [24]. They are components of all three types of stress fibers [6]; however, their precise role in the formation and maintenance of stress fiber integrity has not been determined.
α-actinins are also phosphoproteins. α-actinin1 is phosphorylated by FAK at Y12 and dephosphorylated by PTP1B [25,26]. Phosphorylation at Y12 reduces α-actinin1's affinity for actin [25,27]. In response to EGF, α-actinin4 is phosphorylated predominantly at Y4 and Y31. Like α-actinin1, phosphorylation at these two sites in α-actinin4 decreases its binding to actin [28]. α-actinin4 can also be phosphorylated at Y265 [28–30] and this also increase its affinity for actin [28].
To determine the role of α-actinins 1 and 4, and their tyrosine phosphorylation status, in actin stress fiber establishment, maintenance, and FA maturation we used lentiviral mediated small hairpin RNAi (shRNAi) to deplete both in U2OS cells. We found that α-actinin1 and 4 are required for dorsal stress fiber formation and transverse arc maintenance. In their absence, FAs are established from transverse arcs precursors and partially matured at both ends of transverse arcs. Mutant rescue analysis indicates that tyrosine phosphorylation of Y12 in α-actinin1, or Y265 in α-actinin4 is the key to the establishment of dorsal stress fibers, the maintenance of transverse arc fibers, and FA maturation.
Materials and methods
Antibodies and cell lines
Monoclonal anti-α-actinin1 (A5044, clone BM-75.2), polyclonal anti-Zyxin, monoclonal anti-vinculin (V9131) antibodies and Fibronectin were purchased from Sigma. Polyclonal anti-α-actinin4 antibody was from Santa Cruz. Polyclonal antibody against GFP was from Molecular Probes, monoclonal anti-VASP was from Transduction laboratory (V40620), polyclonal anti p-FAK (Y397), p-paxillin (Y118), FAK, were from BioSource International Inc. Type I collagen was from BD Bioscience. Human osteosarcoma cell line U2OS and derived cells were maintained in DMEM-10 (DMEM containing 10% FBS and pen/strep, glutamine) and HEK-293-T cells in DMEM-10.
Construction of lentiviral plasmids
The human α-actinin RNAi sequence used was GGGACACAGATCGAGAACATCGAAGAG. The U6-shRNA expression cassette was constructed by overlapping PCR and was cloned into lentiviral vector pFLRu-FH [31]. For control shRNA we used a fire fly luciferase target sequence 5‘GCTTACGCTGAGTACTTCGA under U6 promoter. For construction of RNAi resistant human α-actinin1 and 4 (rr-α-actinin1 or 4) cDNA, primers with three or four point mutations inside the shRNA targeted DNA sequence were designed, these mutations did not change peptide encoded. For human α-actinin1, we designed the following four primers:
5‘-AGAGAATTCCATGGACCATTATGATTCTCAGCAAAC
5‘-CCTCTTCAATATTTTCAATCTGTGTCCCCGCCTTCCGGAG
5‘-CACAGATTGAAAATATTGAAGAGGACTTCCGGGATGGCCTG
5‘-CTCGGTACCGGTAGGTCACTCTCGCCGTACAGGCGCGTG
N-terminal fragment (N-ter) and C-terminal fragment (C-ter) of rr-α-actinin 1 were obtained by PCR using primer pairs 1 and 2, or 3 and 4, respectively. Full length of rr-α-actinin1 was obtained by joint PCR using purified N-ter and C-ter mixed template and primers 1 and 4, followed by subcloning the fragment into EcoRI/ AgeI sites of pFLRu-FH, the GFP tagged rr-α-actinin1 and mutants (▲ABD, Y12F and Y12E) were obtained by PCR, subcloning into pEGFPN1 and sequence validated. For construction of rr-αactinin4, same strategy was performed, except the following primers were used:
5‘-AGATGAATTCCATGGTGGACTACCACGCGGCGAAC
5‘-CCCAAATTGAAAACATTGATGAGGACTTCCGAGAC
5‘-CAATGTTTTCAATTTGGGTGCCTGCCTTCCGCAGGTGGGAGTTG
5‘-AGTACCCCGGGCCAGGTCGCTCTCGCCATACAAG
The synthesized rr-α-actinin4 fragment was cloned into EcoRI/ XmaI sites of pFLRu and sequence validated. α-actinin4 and mutants (Y4F, Y31F, Y4F;Y31F, Y265F, Y265E) were provided by Dr. Alan Wells [28].
Lentivirus generation and cell transduction
Lentivirus production and cells transduction were as described [32]. U2OS cells were selected by puromycin (final concentration of 2.5 μg/ml, and incubated for 2 days, un-infected cells (about 30–50%) were removed, the residual cells were collected, ali-quoted in freezing medium (DMEM-10 plus 15% DMSO) and froze down, saved in – 130 °C until use. Cells from thawed vials were cultured for 3 days in the presence of puromycin before experiments. Only cells within the first 10 passages from freshly thawed cells were used for all analyses.
Preparation of U2OS cells for immunostaining
Glass cover-slips were pre-coated with fibronectin (0.5 μg/cm2) in 4 °C overnight and were washed with medium before use. The U2OS derived cells were serum starved for 4 h, trysinized, cells were resuspended in DMEM and played onto fibronectin coated glass cover-slips (1 × 104 cells/cm2). Cell adhesion were allowed up to 2 h before fixation with 4% paraformaldehyde. Immunostaining was performed using the primary antibodies as indicated, and Alexa Fluor 488, 568 or 633 conjugated secondary antibodies were applied thereafter.
Measurement of cell adhesive strength
Cell adhesive strength was measured using a radial flow detachment assay as previously described [33]. Briefly, silicon membrane was attached to circular p100 plate, cells were allowed to attach to silicone substrates coated with either collagen Type I (3.95 μg/cm2) or fibronectin (1.97 μg/cm2) to achieve density to 5000 cells/cm2. After overnight incubation (20 h), images of arbitrarily selected regions were captured using phase-contrast microscopy to determine initial cell density. The radial flow chamber was carefully assembled, gap height kept constant at 200 mm by four spacers, cells were subjected to fluid (1XPBS+1% dextran) flow (1.5 ml/second) for 10 min at ambient temperature. At the end of the experiment cells were fixed with 3.7% formaldehyde for 10 min. All adhesive strength experiments were done in triplicate.
Serial images along two mutually perpendicular diameters were taken. The average number of adherent cells at each radial position was obtained using NIH Image. The critical radial position (Rc) corresponding to 50% cell detachment was determined by fitting the data to a sigmoidal logistic curve
where f is the fraction of adherent cells, r is the radial position, and b is the inflection slope. The estimated fluid shear stress (FSS) corresponding to Rc is called the critical FSS and is the measure of attachment strength. It was estimated using the creeping flow assumption with power series expansion correction [33,34]
where tw is the wall shear stress, h is the gap height, m and r are fluid viscosity and density, Q is the volumetric flow rate, and r is the radial position.
Fluorescence recovery after photobleaching (FRAP) analysis
U2OS derived cells were transfected with Fugene6 precipitated GFP-actin, GFP-vinculin or GFP-paxillin expressing plasmid at a ratio of 3:2 (fugene6:DNA), 48 h after transfection, cells were trypsinzed and played onto fibronectin coated cover-glass (θ=40 mm) for overnight culture. The medium changed to L15-10 before FRAP. A Zeiss fluorescence confocal microscope equipped with both stage and objective heaters was equilibrated to 37 °C for 1 h before photobleach experiment began. For GFP imaging, a 488 nm line and a 60 × oil objective were used. After a pre-bleaching scan of the entire image, the regions of interest (ROIs) were bleached 15 iterations with 100% intensity of 488 nm laser line to achieve 50–80% loss of initial GFP fluorescence. After bleaching, the fluorescence recovery was monitored 40 times every 10 s for GFP-actin and GFP-vinculin, while 30 times every second for GFP-paxillin transfected cells. The recovery of GFP intensity of ROIs was measured by software, the intensity of the bleached area was normalized to a neighboring non-bleached area to diminish the error caused by normal photo-bleaching during the monitoring period. Bleached and control areas used for measurements were also outlined to contain only one focal adhesion to diminish fast intensity recovery caused by diffusion of soluble proteins. The value of intensity versus time were charted the recovery half time (t1/2) was measured from the plots.
Results
α-Actinin1 and 4 are required for dorsal stress fiber establishment, transverse arc maintenance, and focal adhesion organization
To determine whether and how non-muscle α-actinin1 or 4 affect stress fibers and FA formation and maturation we first determined the localization of each non-muscle α-actinin in mesenchymal osteosarcoma U2OS cells. We focused primarily on their localization to sites of cell-ECM adhesion and dorsal, ventral, and transverse arc actin stress fibers. To do so, cells were transiently transfected with a low, fixed amount of GFP tagged actinin1 or 4 and increasing amounts of empty vector so as to limit over-expression of exogenous tagged proteins. Two days after transfection, cells were detached and added to fibronectin coated glass coverslip, then fixed and immunostained. Both α-actinin1-GFP and 4-GFP localized to the leading edge, FAs (Vinculin), and all three types of actin stress fibers (Fig. S1).
To determine whether α-actinin1 or 4 affected FA maturation and stress fiber dynamics we depleted both α-actinin1 and 4 using lentivirus mediated shRNAi (Fig. S2B). Our lentiviral system allows for concurrent expression, in the same cell, of the shRNAi and an epitope tagged (Flag.6xHis (FH)) RNAi-resistant isoform of the targeted transcript (rr-α-actinin) (Fig. S2A). This approach control against potential off-target effects of the RNAi and limits the level of exogenous epitope tagged RNAi-resistant α-actinin relative to endogenous α-actinin level [31]. The level of rr-α-actinin1 achieved in rescue experiments was similar to the level of endogenous α-actinin1, while rr-α-actinin4 rescue was 40% of its endogenous level (Fig. S2B).
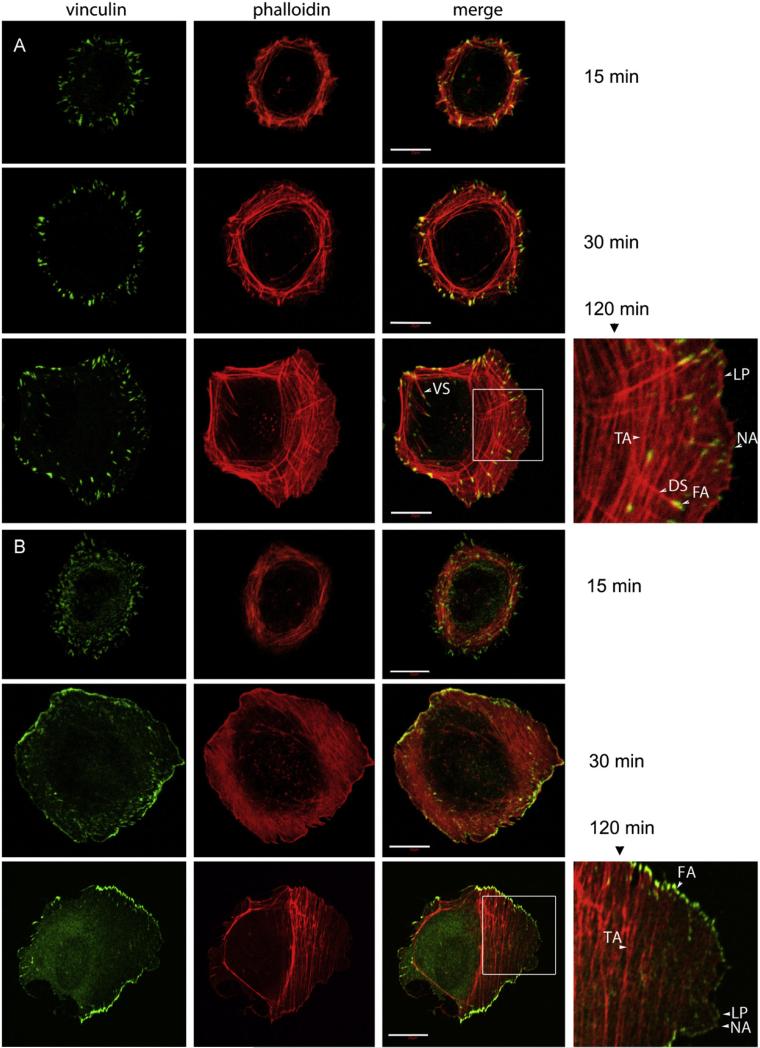
When mesenchymal cells are added to plates coated with fibronectin they undergo rapid cell spreading and ultimately develop polarized cell morphology over 2 h. Actin stress fibers and FAs form and mature during these processes [35]. Control and α-actinin1/4-depleted U2OS cells were added to fibronectin coated glass coverslips, fixed at various times after plating until 2 h and then actin stress fibers (phalloidin) and FA (Vinculin) visualized by immunofluorescence. Control cells adhere and start to spread 15 min after plating. At this time, FAs were detected at one end of nascent short dorsal actin stress fibers (Fig. 1A). Transverse arc formed ring like bundles around the cells (Fig. 1A). By 30 min, dorsal stress fibers had elongated and transverse arc stress fibers evolved from ring like bundles into individual fibers (Fig. 1A). More FAs were present and became larger. Despite these changes, cells did not polarize and migrate until 2 h. Migrating U2OS may not have apparent ventral stress fibers [3], but dorsal stress fibers and transverse arcs were easily detectable (Fig. 1A). Nascent adhesions were also present at the leading edge of polarized, migrating U2OS cells (Fig. 1A).
Fig. 1.
α-actinins are required for dorsal stress fiber establishment, transverse arc maintenance and focal adhesion maturation in spreading and polarized migrating cells. U2OS luc-shRNA (luc-sh) control cells (A) or U2OS α-actinin-shRNA (actinin-sh) cells (B) were plated onto fibronectin (Fn) coated glass coverslips and allowed adhesion for 15, 30, 120 min before fixation, immunofluorescence staining was performed for vinculin, actin stress fibers were stained with rhodomin–phallloidin, images were obtained by confocal fluorescence microscopy, scale bar represents 20 lm. Arrows in the images marked the dorsal stress fibers (DS), transverse arcs (TA), ventral stress fibers (VS), lamellipodium (LP in red), nascent adhesion (NA in green) and focal adhesion (FA).
Stress fiber dynamics and FA establishment and maturation differed in cells lacking α-actinin1/4. Fifteen minutes after addition to fibronectin-coated plates, vinculin stained FAs and smaller focal complexes were apparent at the base of small actin fragments that appeared to be equivalent to the transverse arcs observed in control cells (Fig. 1B). Although transverse arcs were aligned in multiple directions, no significant dorsal stress fibers were detected. By 30 min, α-actinin1/4-depleted cells had spread a greater distance than control cells yet still no dorsal stress fibers were apparent (Fig. 1B). FAs were present at both ends of transverse arcs. By 2 h, α-actinin1/4 depleted cells had polarized normally and started to migrate; however, in contrast to control cells dorsal stress fibers remained absent (Fig. 1B). Transverse arc precursors in the distal lamella, close to the lamellipodium, were short and nascent adhesions were associated with them. Trans-verse arcs in the proximal lamella, towards the nucleus were not smooth, rather they tended to be branched or fragmented (Fig. 1B). These data suggested α-actinin1/4 were required for the establishment of dorsal stress fibers but not transverse arcs. α-actinin1/4 were required for transverse arc maintenance, however.
The effect of α-actinin1/4 depletion on FAs in polarized cells was striking. Although nascent adhesions formed in the lamelli-podium, they did not appear to mature to FAs at the lamellipodium–lamellum interface (Fig. 1B). Punctuate nascent adhesions were still observed along the transverse arc fragments at the front of lamella indicating that the maturation of focal adhesions did not occur in the front of lamella during initial arc fiber retrograde flow. FAs ultimately did form but at both ends of transverse arcs and were organized into a fused hemi-ring like adhesive structure around the cells. These results suggested that cells lacking α-actinin1/4 exhibited a distinct mode of FA maturation that used transverse arc precursors as templates not dorsal stress fiber precursors. Loss of α-actinin1/4 did not affect nascent adhesion formation but delayed nascent adhesion transition to FAs. Depletion of α-actinin1/4 did not affect the microtubule cytoskeleton (Fig. S3).
Depletion of α-actinin1/4 does not affect integrin-mediated activation of FAK and paxillin phosphorylation, but does alter FA composition of Zyxin and VASP
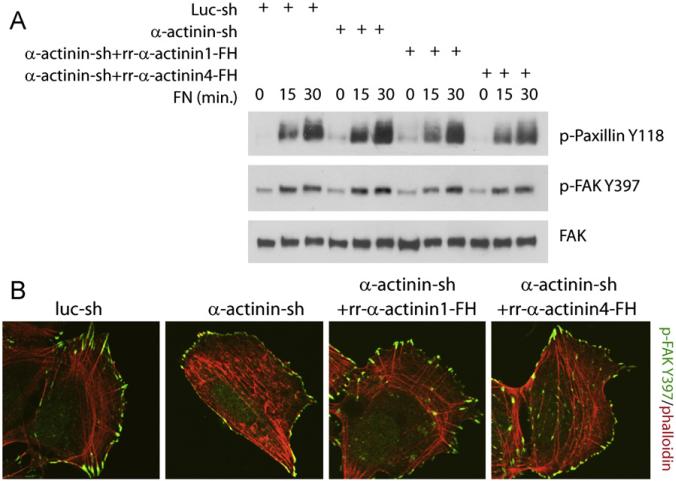
α-actinin1 affects the composition of mature FAs by impairing the activation of FAK and paxillin phosphorylation at FAs and the overall amount of integrin mediated signaling to FAK in whole cells [8]. To determine if α-actinin1/4 affected integrin mediated activation of FAK and paxillin as well as focal adhesion compositional regulation, we stimulated integrin by adding serum starved cells to fibronectin (Fn)-coated plates. In contrast to Oakes et al. [8], depletion of α-actinin1/4 in U2OS cells did not affect Fn-induced activation of FAK, as measured by pY397.FAK, or paxillin phosphorylation, as measured by pY118.paxillin (Fig. 2A). In addition, immunostaining did not show any reduction in pY397.FAK staining at focal adhesions, although there was altered subcellular localization of pY397.FAK in polarized cells (Fig. 2B). These data suggested that α-actinin1/4 did not affect activation of integrin complex components FAK and paxillin in whole cells and focal adhesions.
Fig. 2.
Depletion of α-actinins does not affect integrin mediated activation of FAK and paxillin. (A) Depletion of actinins does not affect activation of integrin signaling components FAK and paxillin in whole cells, serum starved cells were plated onto fibronectin (Fn) coated plates for time as indicated, cells were washed with ice cold 1XPBS twice and lysed afterwards. Western blots were performed using 25 lg of total cell lysate for each lane. (B) Depletion of actinins does not affect FAK activation at FA but subcellular localization of activated FAK. Cells were stained with pFAK-Y397 antibody.
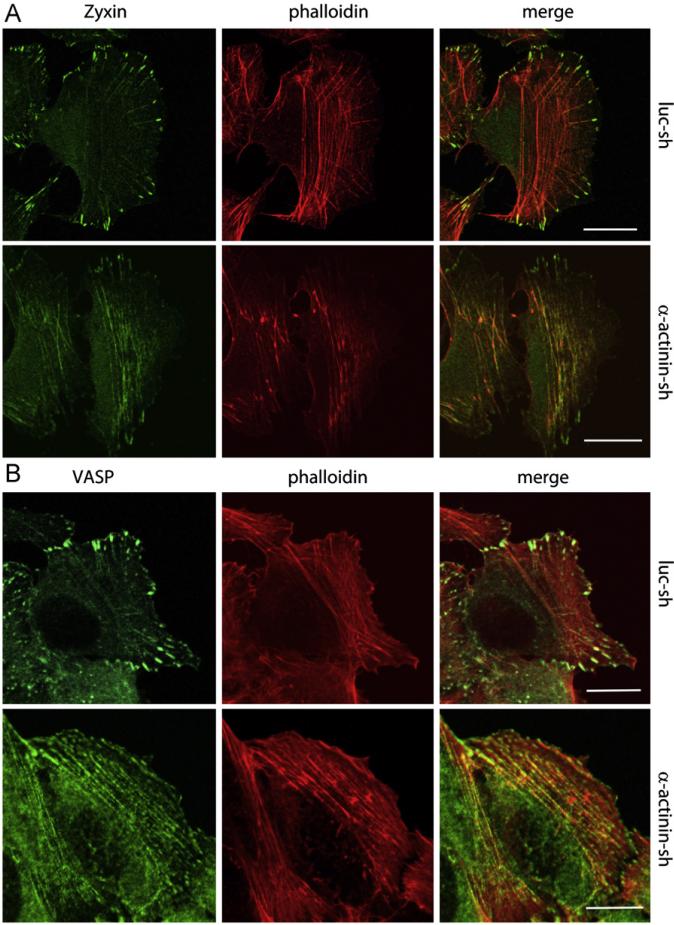
How then does α-actinin1/4 affect focal adhesion compositional maturation? Zyxin is a FA protein sensitive to tension (i.e., mechanosensor). It localizes to mature focal adhesions but is not present in nascent adhesions [36]. As such it can be considered a marker of mature FAs. Further, the presence of Zyxin at FAs is cell tension-dependent, since inhibiting cell contractility or tension leads to its translocation from FAs to stress fibers [37–39]. Other focal adhesion proteins, such as vinculin, do not show such behavior. Given that Zyxin is a binding partner of α-actinin and their interaction influences the subcellular localization of each other [40], we determined the localization of Zyxin in cells lacking α-actinin1/4. Zyxin was present at both FAs and trans-verse arcs (Fig. 3A), consistent with partial maturation of FAs in the absence of α-actinin1/4; however, compared to control cells there was a significant decrease in the amount of Zyxin detected at FAs and increased amounts present on stress fibers in the absence of any extracellular physical stimuli (Fig. 3A). VASP, a binding partner of Zyxin, also translocated to stress fibers in cells depleted of α-actinin1/4 (Fig. 3B). In sum, these results suggested that α-actinin1/4 altered focal adhesion maturation by affecting not only FA morphology and localization but also the incorporation of Zyxin and VASP into FAs.
Fig. 3.
Focal adhesion compositional change of Zyixn and VASP in the absence of α-actinins. Control or actinin depleted cells were plated onto fibronectin coated glass cover slip for 2 h before fixation, immunofluorescence was performed using Zyxin (A) or VASP (B) antibodies as indicated.
Depletion of α-actinin1/4 does not affect focal adhesion and transverse arc remodeling dynamics
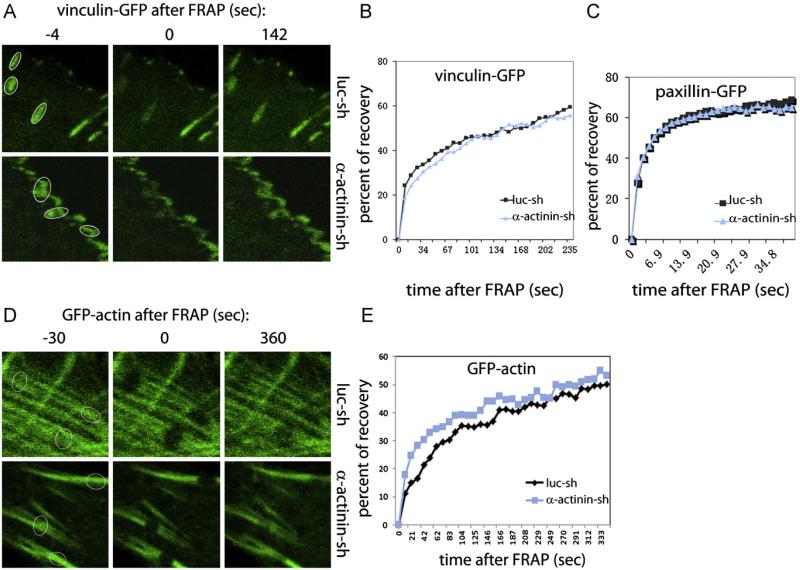
To determine if the dynamics of FAs remodeling was altered by α-actinin1/4 depletion, we performed FRAP analysis. To do so U2OS cells were transfected with GFP-tagged vinculin or paxillin to mark FAs. Cells expressing low level of GFP-tagged protein were selected so as to avoid potential perturbation to fluorescence measurements of focal adhesions. Three bleach regions of interest (ROIs) for FRAP in cells expressing GFP-vinculin were selected (Fig. 4A), but only one ROI in GFP-paxillin transfected cells was utilized (data not shown). Data from 36 ROIs were used for statistical analysis. The mean value of the recovery half time of GFP-vinculin at FAs in control U2OS cells was 142 s and this was unchanged in α-actinin1/4 depleted cells (Fig. 4B). Although FRAP recovery half time for GFP-paxillin (5.5 s) was much faster than GFP-Vinculin, there was again no significant difference in fluorescence recovery times between control and α-actinin1/4 depleted cells (p>0.1) (Fig. 4C). Therefore, α-actinin1/4 did not significantly impact mature FA remodeling dynamics.
Fig. 4.
Depletion of α-actinins does not affect vinculin, paxillin and transverse arc remodeling dynamics. (A) Focal adhesion remodeling dynamics of vinculin and paxillin unchanged in the absence of α-actinins. U2OS derived cells were transfected with GFP-vincuclin or GFP-paxillin expression plasmid and plated onto fibronectin coated cover-glass. FRAP were performed to ROIs (as indicated in the circles) and the fluorescence dynamics were monitored. Average of GFP intensity recovery percentage from 36 ROIs was plotted by setting the first GFP intensity of ROI after FRAP as 0 (C, GFP-vinculin; D, GFP-paxillin). The percent of fluorescence recovery at the recovery half time (vinculin: 142 s, paxillin: 5.5 s) was measured. (B) Transverse arc remodeling dynamics unchanged in the absence of α-actinins, procedures described in A were followed except GFP-actin was used for labeling stress fibers. Dynamic recovery of GFP intensity recovery percentage from 36 ROIs was plotted (E).
To determine if transverse arc remodeling dynamics differed in the absence of α-actinin1/4, we transiently transfected U2OS cells with a low level of GFP-actin, to label stress fibers, and then added cells onto fibronectin coated cover slips and performed FRAP analysis. We selected three bleach ROIs for FRAP on transverse arcs (Fig. 4D). Data from 36 ROIs were used for statistical analysis. The mean value of the recovery half time of GFP-actin in control cells was 330 s while in α-actinin depleted cells it was 290 s. This was not statistical different from control cells (p>0.1) (Fig. 4E). Therefore, α-actinin1/4 did not impact transverse arc remodeling dynamics.
Focal adhesions that formed in the absence of α-actinin1/ 4 exhibit reduced cell adhesion to collagen I and fibronectin
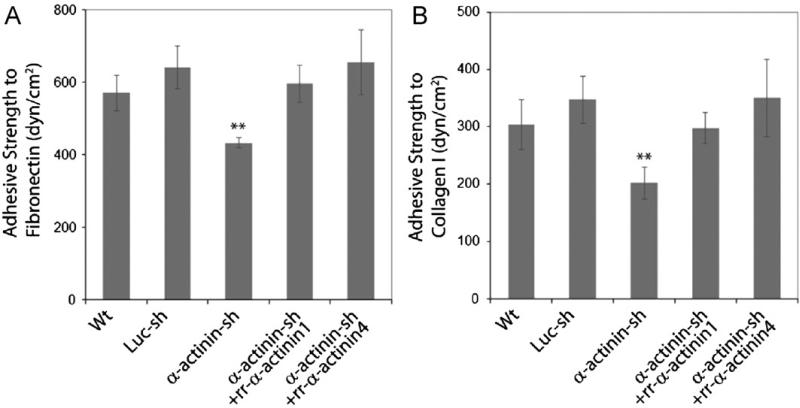
Integrin based focal adhesion assembly contributes to cell adhesion strengthening by distributing bond forces along the cell–matrix interface [41]. The functional role of each FA component in governing the cell adhesion strength can differ [33,42,43]. Given that α-actinin affects FA maturation, we asked if FAs formed in the absence of actinin1/4 exhibited altered adhesive strength to ECM components. Control and α-actinin1/4-depleted U2OS cells were placed on fibronectin or collagen I coated silicon membranes pre-attached to circular p100 plates. Stable cell adhesions were formed by overnight incubation and then images of arbitrarily selected regions were captured using phase-contrast microscopy to establish initial cell density. Cells were then subjected to radial fluid flow for 10 min, cells fixed, and serial images along two mutually perpendicular diameters taken. The average number of adherent cells at each radial position was obtained using NIH Image. The critical radial position (Rc) corresponding to 50% cell detachment was determined and cell adhesive strength to ECM calculated (see Methods). Compared to control cells, a–actinin1/4 depleted cells were statistically less adherent to both fibronectin (32.5% reduction) (Fig. 5A) and collagen I (41.77% reduction) (Fig. 5B). Importantly, the reduction in adhesive strength was rescued by rescue with either α-actinin1 or 4 (Fig. 5A and B). This result indicated that integrin based FAs formed in the absence of α-actinin1/4 exhibited less adhesive strength to the ECM proteins collagen I and fibronectin.
Fig. 5.
Cell adhesive strength to fibronectin and collagen type I is reduced in the absence of α-actinins. U2OS derived cells were plated onto fibronectin or collagen type I coated silicon substrate membranes overnight, initial images were captured, radial flow shear stress were applied, cells were then fixed and imaged. The critical radial position corresponding to 50% cell detachment was determined, adhesive strength was calculated. Data represent statistical result from three independent experiments.
Phosphorylation of α-actinin1 at Y12 and α-actinin4 at Y256 is required for dorsal stress fiber establishment, transverse arc maintenance and focal adhesion maturation
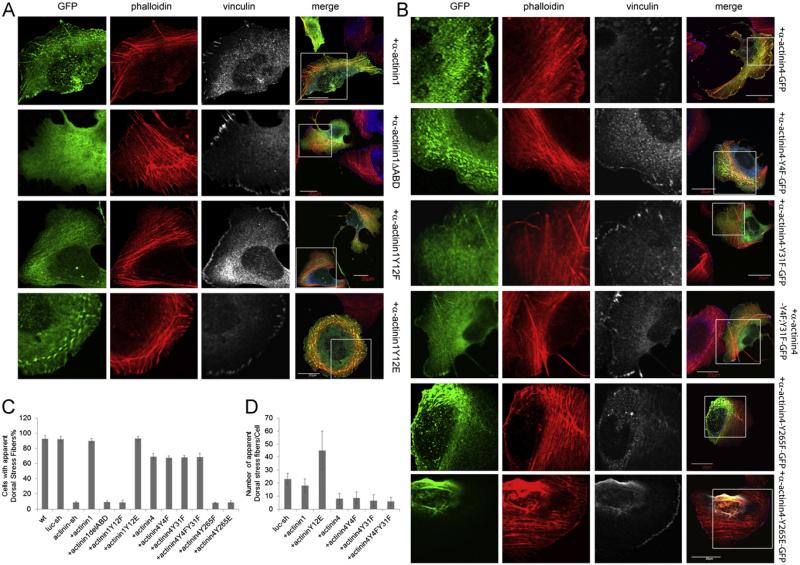
α-actinin1 contains an N-terminal actin binding domain [44,45]. To determine the structural features of α-actinin1 required for its regulation of stress fibers and FAs, we first determined if reintroduction of GFP-tagged rr-α-actinin1 rescued the α-actinin1/4 loss of function phenotypes. Reintroduction of α-actinin1 reversed major phenotypes—dorsal stress fibers were established, transverse arc morphology was maintained and FA maturation occurred at the end of dorsal stress fibers (Fig. 6A). When the actin-binding domain of aactinin1 was removed it no longer associated with actin stress fibers and did not rescue the α-actinin1/4 depleted phenotypes (Fig. 6A).
Fig. 6.
Tyrosine phosphorylation of Y12 in α-actinin1 or Y265 in α-actinin4 is required for dorsal stress fiber establishment, transverse arc maintenance and focal adhesion maturation. Rescue of stress fiber and FA defects in α-actinin depleted cells with the GFP tagged α-actinin1 wt and mutants (A), or α-actinin4 and mutants (B) as indicated, procedures described in Fig. 1 were followed, immunofluorescence staining was performed as indicated. (C) Percent of cells with apparent dorsal stress fibers and discrete FAs. About 100–150 cells with moderate GFP signal were selected for statistical analysis and plots, data represent statistical result from three independent experiments. (D) Semi-quantitative plot for number of apparent dorsal stress fibers per cell, 15 cells, was counted for each set.
To determine whether tyrosine phosphorylation at Y12 in the actin binding domain of α-actinin1 was responsible for the ability of α-actinin1 to regulate stress fibers and FAs, a GFP-tagged rr-α-actinin1.Y12F mutant was introduced into aactinin1/4 depleted cells. This mutant co-localized with actin stress fibers but did not localize to FAs (Fig. 6A) and did not rescue either the stress fiber or FA abnormalities associated with depletion of α-actinin1/4 (Fig. 6A and C). Introduction of a GFP-tagged phosphomimetic mutant (Y12E) of rr-α-actinin1 into aactinin1/4 depleted cells rescued both stress fiber and FA defects (Fig. 6A, C, and D). In fact, these cells exhibited increased numbers of dorsal stress fibers (Fig. 6A, C, and D).
α-actinin4 can be phosphorylated at Y4, Y31 and Y265 [28]. To determine if phosphorylation at these sites was important for its regulation of FA and stress fiber maintenance, we introduced GFP tagged wt rr-α-actinin4 or mutants containing Y4F, Y31F, Y4F;Y31F, Y265F or Y265E into α-actinin1/4 depleted cells. Reintroduction of wt α-actinin4 rescued the α-actinin1/4 loss of function phenotypes although much less effectively than α-actinin1, as determined by the percentage of cells rescued and the number of apparent dorsal stress fibers formed per cell (Fig. 6B, C, and D). α-actinin4 Y4F showed similar rescue capability as wt α-actinin4 (Fig. 6B). α-actinin4 Y31F and Y4F;Y31F mutants were partially cytosolic, and had less association with transverse arcs but still significant binding to dorsal stress fibers, lamellipodia and FAs (Fig. 6B). Both exhibited similar rescue potency to wt α-actinin4 (Fig. 6B, C, and D). In contrast, the α-actinin4.Y265F mutant was not able to rescue either cytoskeletal defects. Interestingly, this mutant protein tended to associate with specific actin populations: perinuclear actin and the trailing edge of the polarized cells. Unlike the Y12 phosphomimetic mutant of α-actinin1, the α-actinin4.Y265E mutant did not show rescue capability. This mutant showed similar perinuclear actin and trailing edge localization to Y265F mutant in the polarized cells, but even more exclusive from lamella actin (Fig. 6B, C, and D). Based on the observation that neither α-actinin4.Y265F nor Y265E could reconstitute subcellular localization of wt α-actinin4, it was obvious that α-actinin4.Y265E was not real phosphomimetic, glutamic residue could not substitute the function of phosphor tyrosine at this position.
These structure–function studies indicated that phosphorylation of α-actinin1 at Y12 and α-actinin4 at Y265 was critical for α-actinin regulation of stress fiber formation/maintenance and FA maturation. Moreover, differential tyrosine phosphorylation of α-actinin1 and 4 influenced their subcellular localization.
Discussion
By analyzing stress fiber establishment, maintenance and FA maturation in spreading and polarized migrating U2OS cells on fibronectin, we find that α-actinin1 and 4 are required for the establishment of dorsal stress fibers and the maintenance of transverse arcs. Maturation of FAs requires availability and integrity of dorsal or arc stress fibers. Based on these results we propose that cells exhibit two distinct modes of focal adhesion maturation and that the phosphorylation state of aactinin1/4 is critical for these functions. An α-actinin-dependent mode of FA maturation uses dorsal stress fiber precursors as a template and establish focal adhesions at one end, whereas in cells lacking α-actinin1/4 transverse arc precursors are used as a template for FA maturation and this occurs at both ends of the transverse arcs. Focal adhesions formed in the absence of α-actinin1/4 contain less Zyxin and VASP and exhibit reduced adhesion to ECM proteins. Phosphorylation at Y12 in α-actinin1 and Y265 in α-actinin4 is critical for dorsal fiber establishment, transverse arc maintenance and FA maturation.
Recently, α-actinin1 and Rac1 are found to be important for dorsal stress fiber assembly. In this work α-actinin4 seems not required for the assembly of dorsal stress fibers. But depletion of both α-actinin1 and 4 with separate siRNAs resulted in nearly undetectable actin stress fibers in U2OS cells [46]. In contrast to these findings, our results indicate that both isoforms are involved in the establishment of dorsal stress fibers yet not required for the assembly of transverse arc precursors or their transition into longer transverse arcs, although the transverse arcs formed in the absence of α-actinin1/4 do have maintenance defects. These discrepancies could be due to less potency of aactinin4 in rescue experiments, α-actinin4 rescued cells had less dorsal stress fiber formation compared to α-actinin1 rescue. Another consideration is the potential off target effects of the RNAi since they only use one RNAi for each α-actinin protein and do not rescue either knockdown with RNAi-resistant isoforms.
Dorsal stress fibers are typically non-contractile since Myosin II is not present. Myosin II only occasionally is incorporated into dorsal stress fibers, specifically as they approach or connect to transverse arcs or when converted to ventral stress [3,6]. Although dorsal stress fibers may not transmit force to FA when cells adhere to ECM proteins, their assembly still play an important role in promoting FA maturation which is required to form tensin rich fibrillar adhesions used in ECM remodeling [8].
Our results suggest that the availability of distinct stress fiber populations can determine the mode of focal adhesion maturation. Different stress fibers are assembled by distinct actin assembly mechanisms [6]. Dorsal stress fibers are assembled through formin (mDia1/DRF1) driven actin polymerization at focal adhesions [6,8] while transverse arcs, which are typically not directly anchored to FAs, are generated by endwise annealing of myosin bundles and Arp2/3 nucleated actin bundles [6]. Failure of dorsal stress fiber establishment, due to the absence of α-actinin1/4, results in FAs established at the ends of transverse arcs.
In the nanoscale architecture of integrin based FAs, FAK and paxillin are at the sub-tier closer to integrin, whereas Zyxin, VASP, and α-actinin are closer to actin [12]. Our data suggest that loss of α-actinin1/4 neither impairs incorporation of FAK, paxillin and vinculin into FAs nor activation of FAK and paxillin, but manifests altered incorporation of Zyxin/VASP, this also evidences which sub-tier of FAs that α-actinins work on. Zyxin is incorporated into FAs late in their maturation; it is not present in nascent adhesions [36]. In response to mechanical force, Zyxin translocates from FAs to actin filaments, accumulating at sites of strain-induced stress fiber damage with its binding partner VASP [47,33,48]. The accumulation of Zyxin at stress fibers or sites of strain-induced stress fiber damage is thought to be essential for the generation of traction forces and stress fiber repair, respectively. α-actinin also plays a critical role in the restoration of actin integrity at sites of local stress fiber damage [48]. In the absence of α-actinin1/4, Zyxin accumulates at stress fibers and little is present in FA that do form, a situation similar to cells sensing mechanical stress. Thus, α-actinin1/4 likely contributes to mechano-sensing by influencing Zyxin localization.
Our results suggest the presence of an α-actinin1/4-independent mode of FA maturation in U2OS cells. However, in CHO cells, actin crosslinking activity of both α-actinin and myosin II, but not myosin contractility or motor activity, were found to be critical for the initial FA maturation [13]. One possibility is the different cell types used in the studies. Nascent adhesions that formed in the lamellipodia of CHO cells had a long life span, but turned over rapidly in U2OS cells [13]. Although myosin II ATPase activity is not required for FA maturation in CHO cells, it is required in U2OS cells [46]; in addition, the role of other actin cross-linking proteins such as fascin, fimbrin, and filamin may provide varied compensatory effects in different cell types.
Phosphorylation at Y12 by FAK reduces α-actinin1's affinity for actin [25,27]. α-actinin4 is phosphorylated at Y4, Y31, and Y265. Phosphorylation at Y4 or Y31 decreases its binding to actin [28] while phosphorylation of Y265 increases its affinity for actin [28]. Based on our rescue results, it appears that there is no correlation between the affinity of α-actinin1/4 tyrosine mutants for actin and dorsal fiber rescue.
The subcellular localization of α-actinin4 Y265F and Y265E mutants highlights a special perinuclear actin network. Recently, perinuclear actin population called actin cap is proposed [49], actin cap connects cell nucleus by LINC complex and regulates cell shape, nuclear shape and cell adhesion. It appears that unphosphorylated form of α-actinin4 at Y265 preferentially associated with this special actin population, however, whether α-actinin4 is a component of actin cap and involved in the regulation of nucleus shape and function need further investigation.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants CA85839 and CA143868 to G.D.L. We thank Dr. Alan Wells and Dr. Hanshuang Shao at University of Pittsburgh School of Medicine for their generous offer of α-actinin4 mutants, and Dr. Jiancheng Hu for helpful discussion.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.yexcr.2013.02.009.
REFERENCES
- 1.Cramer LP, Siebert M, Mitchison TJ. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J. Cell Biol. 1997;136(6):1287–1305. doi: 10.1083/jcb.136.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pellegrin S, Mellor H, Actin stress fibres J. Cell Sci. 2007;120(Pt 20):3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 3.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J. Microsc. 2008;231(3):446–454. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 4.Heath JP. Behaviour and structure of the leading lamella in moving fibroblasts. I. Occurrence and centripetal movement of arc-shaped microfilament bundles beneath the dorsal cell surface. J. Cell Sci. 1983;60:331–354. doi: 10.1242/jcs.60.1.331. [DOI] [PubMed] [Google Scholar]
- 5.Small JV, Rottner K, Kaverina I, Anderson KI. Assembling an actin cytoskeleton for cell attachment and movement. Biochim. Biophys. Acta. 1998;1404(3):271–281. doi: 10.1016/s0167-4889(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 6.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006;173(3):383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath JP, Dunn GA. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J. Cell Sci. 1978;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- 8.Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 2012;196(3):363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkhovsky AB, Svitkina TM, Borisy GG. Myosin II filament assemblies in the active lamella of fibroblasts: their morpho-genesis and role in the formation of actin filament bundles. J. Cell Biol. 1995;131(4):989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verkhovsky AB, Svitkina TM, Borisy GG. Network contraction model for cell translocation and retrograde flow. Biochem. Soc. Symp. 1999;65:207–222. [PubMed] [Google Scholar]
- 11.Zimerman B, Volberg T, Geiger B. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell Motil. Cytoskeleton. 2004;58(3):143–159. doi: 10.1002/cm.20005. [DOI] [PubMed] [Google Scholar]
- 12.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468(7323):580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008;10(9):1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister JJ, Bershadsky AD, Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS One. 2008;3(9):e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J.Cell Biol. 2010;188(6):877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, Yamada Y, Chiba H, Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J. Cell Biol. 1998;140(6):1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallenius T, Luukko K, Makela TP. CLP-36 PDZ-LIM protein associates with nonmuscle alpha-actinin-1 and alpha-actinin-4. J. Biol. Chem. 2000;275(15):11100–11105. doi: 10.1074/jbc.275.15.11100. [DOI] [PubMed] [Google Scholar]
- 18.Edlund M, Lotano MA, Otey CA. Dynamics of alpha-actinin in focal adhesions and stress fibers visualized with alpha-actinin-green fluorescent protein. Cell Motil. Cytoskeleton. 2001;48(3):190–200. doi: 10.1002/1097-0169(200103)48:3<190::AID-CM1008>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Mukhina S, Wang YL, Murata-Hori M. Alpha-actinin is required for tightly regulated remodeling of the actin cortical network during cytokinesis. Dev. Cell. 2007;13(4):554–565. doi: 10.1016/j.devcel.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wachsstock DH, Wilkins JA, Lin S. Specific interaction of vinculin with alpha-actinin. Biochem. Biophys. Res. Commun. 1987;146(2):554–560. doi: 10.1016/0006-291x(87)90564-x. [DOI] [PubMed] [Google Scholar]
- 21.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J. Cell Biol. 1990;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavalko FM, LaRoche SM. Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin. J. Immunol. 1993;151(7):3795–3807. [PubMed] [Google Scholar]
- 23.Rajfur Z, Roy P, Otey C, Romer L, Jacobson K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat. Cell Biol. 2002;4(4):286–293. doi: 10.1038/ncb772. [DOI] [PubMed] [Google Scholar]
- 24.Lek M, North KN. Are biological sensors modulated by their structural scaffolds? The role of the structural muscle proteins alpha-actinin-2 and alpha-actinin-3 as modulators of biological sensors. FEBS Lett. 2010;584(14):2974–2980. doi: 10.1016/j.febslet.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 25.Izaguirre G, Aguirre L, Hu YP, Lee HY, Schlaepfer DD, Aneskievich BJ, Haimovich B. The cytoskeletal/non-muscle isoform of alpha-actinin is phosphorylated on its actin-binding domain by the focal adhesion kinase. J. Biol. Chem. 2001;276(31):28676–28685. doi: 10.1074/jbc.M101678200. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Lin SY, Neel BG, Haimovich B. Phosphorylated alpha-actinin and protein-tyrosine phosphatase 1B coregulate the disassembly of the focal adhesion kinase x Src complex and promote cell migration. J. Biol. Chem. 2006;281(3):1746–1754. doi: 10.1074/jbc.M509590200. [DOI] [PubMed] [Google Scholar]
- 27.von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 2003;22(19):5023–5035. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao H, Wu C, Wells A. Phosphorylation of alpha-actinin 4 upon epidermal growth factor exposure regulates its interaction with actin. J. Biol. Chem. 2010;285(4):2591–2600. doi: 10.1074/jbc.M109.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rush J, Moritz A, Lee KA, Guo A, Goss VL, Spek EJ, Zhang H, Zha XM, Polakiewicz RD, Comb MJ. Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 2005;23(1):94–101. doi: 10.1038/nbt1046. [DOI] [PubMed] [Google Scholar]
- 30.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Feng Y, Nie L, Thakur MD, Su Q, Chi Z, Zhao Y, Longmore GD. A multifunctional lentiviral-based gene knockdown with concurrent rescue that controls for off-target effects of RNAi. Genom. Proteom. Bioinf. 2010;8(4):238–245. doi: 10.1016/S1672-0229(10)60025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. 2010;12(6):598–604. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngu H, Feng Y, Lu L, Oswald SJ, Longmore GD, Yin FC. Effect of focal adhesion proteins on endothelial cell adhesion, motility and orientation response to cyclic strain. Ann. Biomed. Eng. 2010;38(1):208–222. doi: 10.1007/s10439-009-9826-7. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein AS, Dimilla PA. Application of fluid mechanic and kinetic models to characterize mammalian cell detachment in a radial-flow chamber. Biotechnol. Bioeng. 1997;55(4):616–629. doi: 10.1002/(SICI)1097-0290(19970820)55:4<616::AID-BIT4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 1999;112(Pt 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 36.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003;116(Pt 22):4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 37.Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell Physiol. 2006;207(1):187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 38.Guo WH, Wang YL. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol. Biol. Cell. 2007;18(11):4519–4527. doi: 10.1091/mbc.E07-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, Haselmann U, Small JV, Schwarz US, Stelzer EH. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell Sci. 2009;122(Pt 10):1665–1679. doi: 10.1242/jcs.042986. Pt 10 (2009) 1665–1679. [DOI] [PubMed] [Google Scholar]
- 40.Li B, Trueb B. Analysis of the alpha-actinin/zyxin interaction. J. Biol. Chem. 2001;276(36):33328–33335. doi: 10.1074/jbc.M100789200. [DOI] [PubMed] [Google Scholar]
- 41.Gallant ND, Michael KE, Garcia AJ. Cell adhesion strengthening: Contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell. 2005;16(9):4329–4340. doi: 10.1091/mbc.E05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumbauld DW, Shin H, Gallant ND, Michael KE, Radhakrishna H, Garcia AJ. Contractility modulates cell adhesion strengthening through focal adhesion kinase and assembly of vinculin-containing focal adhesions. J. Cell. Physiol. 2010;223(3):746–756. doi: 10.1002/jcp.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elineni KK, Gallant ND. Regulation of cell adhesion strength by peripheral focal adhesion distribution. Biophys. J. 2011;101(12):2903–2911. doi: 10.1016/j.bpj.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil. Cytoskeleton. 2004;58(2):104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- 45.Sjoblom B, Salmazo A, Djinovic-Carugo K. Alpha-actinin structure and regulation. Cell Mol. Life Sci. 2008;65(17):2688–2701. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovac B, Teo JL, Makela TP, Vallenius T. Assembly of noncontractile dorsal stress fibers requires alpha-actinin-1 and Rac1 in migrating and spreading cells. J. Cell Sci. 2012 doi: 10.1242/jcs.115063. [DOI] [PubMed] [Google Scholar]
- 47.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005;171(2):209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith MA, Blankman E, Gardel ML, Luettjohann L, Waterman CM, Beckerle MC. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev. Cell. 2010;19(3):365–376. doi: 10.1016/j.devcel.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA. 2009;106(45):19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.