Abstract
Enhancing the quantity and quality of cancellous bone with anabolic pharmacologic agents may lead to more successful outcomes of non-cemented joint replacements. Using a novel rabbit model of cancellous bone loading, we examined two specific questions regarding bone formation at the bone-implant interface: (1) does the administration of intermittent PTH, a potent anabolic agent, and mechanical loading individually and combined enhance the peri-implant cancellous bone volume fraction; and, (2) does surgical trauma enhance the anabolic effect of PTH on peri-implant bone volume fraction. In this model, PTH enhanced peri-implant bone volume fraction by 30% in loaded bone, while mechanical loading alone increased bone volume fraction modestly (+10%). Combined mechanical loading and PTH treatment had no synergistic effect on any cancellous parameters. However, a strong combined effect was found in bone volume fraction with combined surgery and PTH treatment (+34%) compared to intact control limbs. Adaptive changes in the cancellous bone tissue included increased ultimate stress and enhanced remodeling activity. The number of proliferative osteoblasts increased as did their expression of pro-collagen 1 and PTH receptor 1, and the number of TRAP positive osteoclasts also increased. In summary, both loading and intermittent PTH treatment enhanced peri-implant bone volume, and surgery and PTH treatment had a strong combined effect. This finding is of clinical importance since enhancing early osseointegration in the post-surgical period has numerous potential benefits.
Keywords: Cancellous bone, Bone adaptation, Implant, PTH, loading, surgery
1.0 Introduction
Fixation of total joint replacements without the use of cement requires a viable and biomechanically robust cancellous bone bed for a total joint implant to osseointegrate properly. A variety of host factors, such as cancellous bone quantity and quality, determine if the cancellous bone is sufficient for the successful osseointegration of an implant. Bone quality and quantity can be modulated by a number of pharmacological agents and by mechanical loading of peri-implant bone tissue. In the United States intermittent PTH is currently the only FDA-approved anabolic pharmacological agent for enhancing bone formation and improving bone strength in osteoporotic and osteopenic individuals. The anabolic effect is site specific and occurs primarily at cortico-cancellous sites. In addition, PTH enhances early screw fixation through increased bone apposition [1, 2], suggesting that similar effects may be present at the bone-implant interface.
In a variety of animal models, applied mechanical loading can directly enhance cancellous bone formation and inhibit bone loss by increasing bone formation and bone mass [3–6]. Our group previously demonstrated increased bone volume fraction and altered bone architecture when loading was applied directly to healthy cancellous tissue in a rabbit model [7, 8]. In further studies, when an implant was included in the model, loading increased the amount of bone tissue and decreased the amount of fibrous tissue in the loaded porous implant [9].
While surgical trauma may be linked to enhancing tissue quality surrounding implants with intermittent PTH administration, the exact mechanism is unclear [10–12]. In a canine model with loaded implants, the surgical insult dominated the early healing response [13]. One possible explanation is that the particulate bone debris generated during preparation of the bony bed prior to the insertion of an implant may contain growth factors that stimulate osteogenesis. A second potential explanation is that the inflammatory reaction induced by the surgical trauma to the tissues results in stimulatory cytokine and chemokine expression [14].
We have developed a rabbit model for the study of functional adaptation to an implant in cancellous bone under a well-controlled mechanical loading. Increased bone volume fraction, altered trabecular architecture, and enhanced mineral apposition rate were seen after 4 weeks of cyclic loading at 1 MPa [7–9]. On the other hand, how different anabolic treatments can be combined with loading to enhance the cancellous bone bed for implant surgery is still unclear. In this current study, we used our rabbit loading model to examine two specific hypotheses about tissue formed at the bone-implant interface: (1) PTH and mechanical loading individually and combined will enhance the peri-implant cancellous bone volume fraction; and (2) surgical trauma will enhance the anabolic effect of PTH on peri-implant bone volume fraction.
2.0 Materials and Methods
A previously validated rabbit model for adaptation in surgically-exposed cancellous bone was used to explore (1) the individual and combined effects of loading and PTH and (2) the effects of PTH and surgery. We characterized the tissue microarchitecture, histology and biomechanical properties, and cellular activity and localization of expression. All experiments were carried out with IACUC approval at the Hospital for Special Surgery.
2.1 Study Design
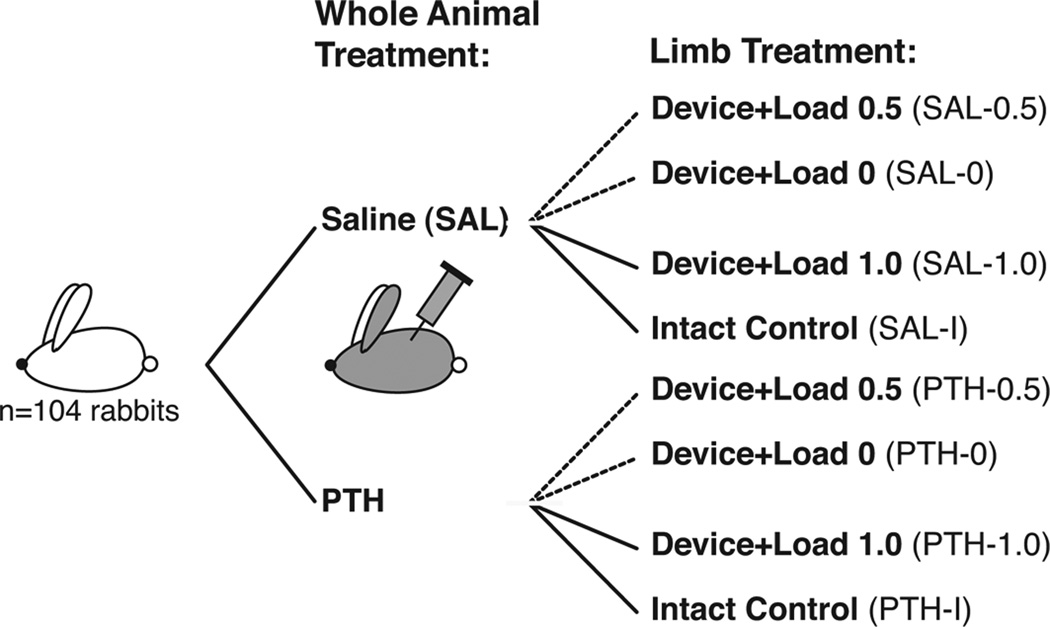
One hundred and four New Zealand White rabbits (minimum age 7 months old, 3.81 ± 0.15 kg body mass) underwent surgical implantation of a custom loading device on the lateral aspect of the distal femur. Half of the rabbits were treated with PTH (20 µg/kg, SQ, 5 days/week, Lilly, Indianapolis, Indiana), and the other half received saline injections for 4 weeks starting the day after surgery (Fig. 1). In total eight experimental groups were examined (n=26 limbs/group): saline-treated non-operated, intact control limbs (SAL-I), and saline-treated limbs implanted with the loading device receiving three different load levels: no load (SAL-0), 0.5 MPa load (SAL-0.5) and 1.0 MPa load (SAL-1.0); and, PTH-treated non-operated intact control limbs (PTH-I) and PTH-treated limbs implanted with the loading device receiving three different load levels: no load (PTH-0), 0.5 MPa load (PTH-0.5) and 1.0 MPa load (PTH-1.0)
Figure 1.
Study design with treatment groups (PTH or Saline) and pair-wise design with loaded, non-loaded and intact limbs.
2.2 Cancellous Loading Device and In Vivo Protocol
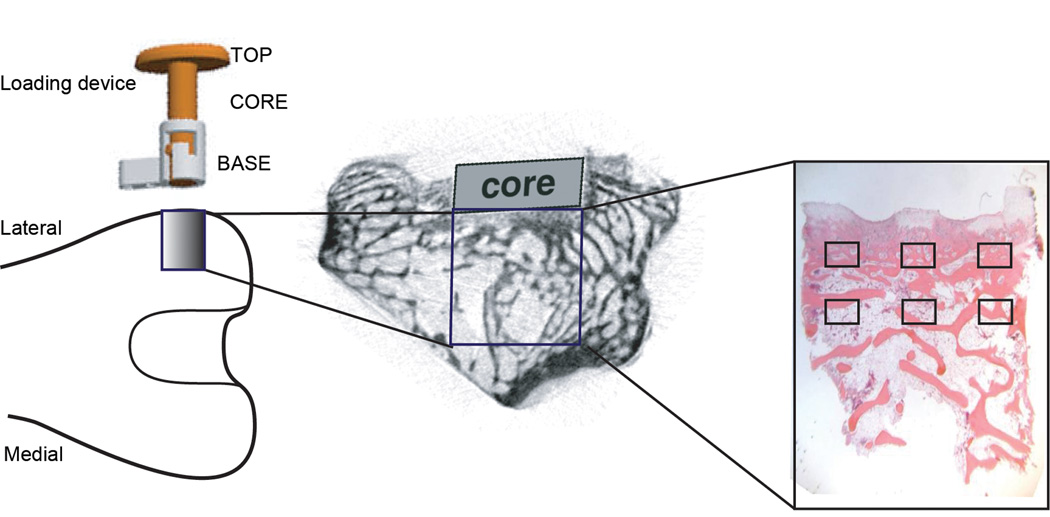
The loading device was inserted into the distal femurs of skeletally mature male New Zealand white rabbits, as detailed previously (van der Meulen et al. 2006, 2009; Willie et al. 2010). The device consisted of a stationary base mounted on the lateral femoral condyle with two bicortical screws, a movable loading core (5 mm diameter), and a top (Fig. 2). During surgery to place the device, the surface of the cortical bone was milled down to ensure direct contact of the core with the underlying cancellous bone prior to attaching the base to the cortex. Transcutaneous loading was applied under isoflurane anesthesia (2%, 1L/minute). During loading, the core slid within the base to compress the underlying bone with a load of known magnitude. The loading device included feedback control (National Instruments, Labview v8.2) using an in-line force transducer (Sensotec Precision Miniature Load Cell, Model 31) to control the actuator (Lynch et al. 2010). Mechanical loading of the right limb was initiated immediately post-operatively at either 0.5 or 1.0 MPa for 50 cycles at 1 Hz per day, 5 days/week for 4 weeks. The left limb was not loaded but had the same device implanted in half the rabbits (no load groups: SAL-0 and PTH-0).
Figure 2.
Schematic of loading device (left) on femur and lateral view of distal femur showing device position and core location (middle) and location of the underlying cancellous bone (right) with histological areas of interests.
To label active bone formation, fluorochrome labels were administered 14 (Xylenol orange, 30 mg/kg, IV) and 3 (0.1% Calcein, 15 mg/kg, IV) days pre-euthanasia. Upon completion of the 4-week experiment, animals were euthanized by barbituate overdose (Sodium Pentobarbital 26%/Isopropyl alcohol 10% euthanasia solution, 2.0 ml, IV).
2.3 Sample Distribution and Analyses
Immediately after euthanasia, a 5-mm × 5-mm (D × H) cylindrical core of cancellous bone was removed directly below the loading device in the lateral distal femur (Starlite Industries, Core Drill 101055). In intact limbs, cores were taken from the same location on the femur and included the cortex. All cancellous cores (n=208) were first scanned by microcomputed tomography (microCT). Thereafter, cores were prepped for subsequent tissue or cellular analyses. Samples for dynamic histomorphometry were dehydrated and embedded in polymethyl methacrylate (PMMA). Samples for cellular analyses were fixed, decalcified and embedded in paraffin for cellular analyses.
2.3.1 Static Histomorphometry
MicroCT scans were performed at 19µm isotropic voxel resolution on all cancellous core specimens (Scanco Medical, Bassersdorf, CH). Mineralized tissue was segmented from soft tissue and background using a global threshold of 3200 mg HA/cc. A 4-mm diameter volume of interest was analyzed. Cancellous outcome measures included bone volume fraction (BV/TV), trabecular thickness (Tb.Th) and separation (Tb.Sp), and tissue mineral density (TMD).
2.3.2 Mechanical Testing
104 core specimens (13 specimens/group) underwent compression testing to evaluate the ultimate strength of each specimen. Prior to testing, the lateral end of the cores was trimmed with a low-speed diamond wafering saw (Isomet 1000, Buehler, Lake Bluff, IL) under constant irrigation to create 4 mm height cores. Our previous data indicated the maximum loading effect occurred within 4 mm of the loaded surface. Cortical bone was removed with the diamond saw prior to testing for the intact controls (SAL-I/PTH-I).
The cores were tested in uniaxial compression to failure at 0.5% strain/sec (0.02 mm/sec) at room temperature using custom designed platens in a servohydraulic testing system (MiniBionix, MTS Systems Corporation, Eden Prairie, MN). To reduce frictional effects, the platens were lubricated with low viscosity oil. Load and displacement data were collected digitally at 10 Hz and converted to stress and strain by normalizing by cross sectional area and initial length, respectively. Ultimate stress and modulus were determined from these data.
2.3.3 Dynamic Histomorphometry
The dynamic trabecular bone response to mechanical loading, PTH, and surgery was assessed in two non-consecutive sections (7 µm thick) separated by 40 µm. Each section was first imaged at low magnification (10× lens) using an epifluorescent microscope (Nikon Microphot-FXA, Japan) to visualize calcein and Xylenol Orange double labeled surfaces in the trabeculae. Sections were then imaged using a 20× lens and images of at least three paired labels per section were captured (BIOQUANT Image Analysis Corp., Nashville, TN). The distance between the paired labels was measured at three evenly separated locations (ImageJ v1.38, National Institutes of Health, Bethesda, Maryland, USA). The trabecular mineral apposition rate (MAR, µm/day) was calculated as the average of the two sections measurements average distance divided by the time period [15], between the two label injections, 11 days.
2.3.4 Localization of cellular activity
To quantify the cellular response of bone to mechanical loading and PTH, sections were analyzed with immunohistochemical staining for pro-collagen 1 (PC1) (250ng/2µl; SP1.D8, Developmental Studies Hybridoma Bank, Iowa City, IA) and parathyroid hormone receptor 1 (PTHR1) (1:50; ab3271, Abcam, Cambridge, MA), two markers of osteoblast activity, and proliferating cell nuclear antigen (PCNA) (1:100; sc-56, Santa Cruz, Santa Cruz, CA), a marker of cell division, and with tartrate-resistant acid phosphatase assay (TRAP) (Sigma-Aldrich, St. Louis, MO), a marker of osteoclast activity.
For immunohistochemistry sections were deparaffinized, rehydrated, blocked for endogenous peroxidase with 3% H2O2 for 30min, followed by hot citrate buffer for 30 min for antigen retrieval and then blocked with protein block solution for 20 minutes (X0909, Dako, Carpinteria, CA). Primary antibodies diluted in PBS were allowed to react overnight in a humid chamber, followed by incubation of biotinylated secondary antibody (PK-6102, Vectastain Laboratories Inc., Burlingame, CA) for 1 hour. After 15 minutes of incubation with horseradish perioxidase-conjugated avidin–biotin complex, the reaction was completed using diaminobenzidine (D5905, Sigma, St. Louis, MO) for 20 minutes. PC1 and PTHR1 sections were counterstained with hematoxylin (nuclear stain), while PCNA sections were counterstained with eosin, and dehydrated and coverslipped. TRAP staining was performed according to the manufacturer's instructions (cat # 387-A, Sigma, St. Louis, MO). Sections were counterstained with hematoxylin, dehydrated and coverslipped.
To determine the percentage of positively labeled osteoblasts and the osteoclast density, three regions (250 µm wide × 187 µm thick) 0.5 mm apart from each other were analyzed on one section under light microscopy (Nikon Microphot-FXA, Japan). The three regions were imaged 0.5 mm below the surface of the loading device and analyzed using a 20× objective. The number of PC1, PTHR1, and PCNA positively labeled osteoblasts were counted based on the cell's location on bone surface, positive staining and cuboidal shape. To determine the osteoclast density, multinucleated cells (containing at least three nuclei) stained with TRAP were considered positive. The osteoblast and osteoclast numbers were calculated as an average of cells per area (250 µm × 187 µm), based on three sections with three regions counted per section. All observers were blinded.
2.4 Statistical Analysis
We examined the two primary hypotheses statistically (SPSS, version 19). For both hypotheses, cancellous bone volume fraction below the loading device was the primary outcome. The data was roughly normal and the variance was not significantly different between groups. The first hypothesis was tested using a two-factor ANOVA with treatment (PTH or saline) and loading (0 MPa, 0.5 MPa or 1 MPa) as the independent variables. Significant differences due to treatment, loading or their interaction were followed by a Bonferroni post-hoc test. The second hypothesis was tested using a two-factor ANOVA with surgery (implanted or intact) and treatment (PTH or Saline). Due to the paired design and use of non-paired statistics, we tested for a correlation between the left and right limbs, and no correlations were found. For all analyses p<0.05 was considered significant. All results presented are presented as mean ± SD and are significant unless stated otherwise.
3.0 Results
3.1 Effects of mechanical loading and PTH on cancellous bone
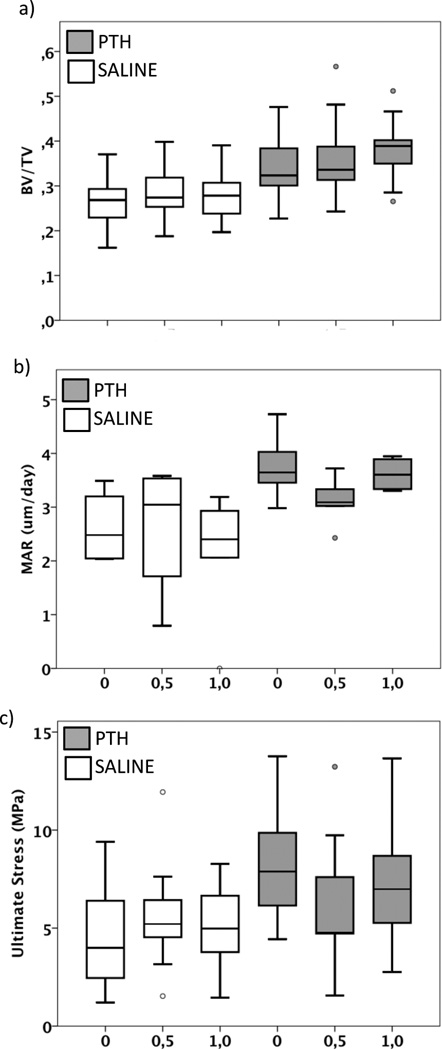
In cancellous bone, four weeks of mechanical loading increased bone volume fraction compared to non-loaded limbs. When load level was examined, bone volume fraction was increased (10%) in limbs loaded with 1 MPa (SAL-1.0/PTH-1.0) compared to limbs without applied loading (SAL-I/PTH-I) (Figure 3a). This increase was partly explained by increased trabecular thickness, with 1 MPa loading compared to 0.5 MPa loading (+12%). Trabecular number, separation and mineral apposition rate were not affected (Figure 3b). Cancellous ultimate stress was not changed with loading (Figure 3c). Loading decreased the number of proliferating cells, an effect that was mainly seen in the 0.5 MPa loaded limbs (SAL-0.5/PTH-0.5) compared to non-loaded limbs (SAL-0/PTH-0) (Figure 4a–b). The number of osteoblasts expressing PC1 and PTHR1 was not changed by loading, nor were the number of TRAP positive osteoclasts (Tables 1, 2).
Figure 3.
PTH and loading both enhanced the cancellous bone bed, but no additional effect was seen when these anabolic treatment were combined. (a) Bone volume fraction (BV/TV), (b) mineral apposition rate (MAR) and (c) ultimate stress of the cancellous bone below the loading core in saline- and PTH-treated animals. Statistical differences are presented in Table 2.
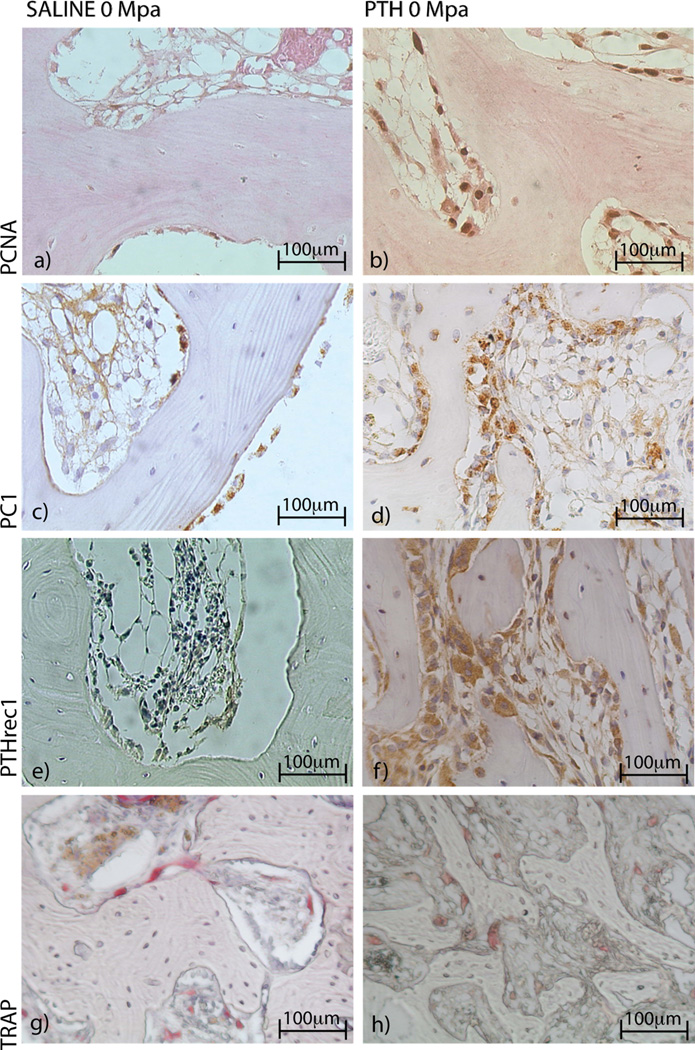
Figure 4.
(a–b). Surgery and loading individually increased the number of proliferating cells (PCNA) compared to controls. (c–h) PC1, PTHR1 and TRAP increased in limbs following surgery compared to intact controls.
Table 1.
Cell analysis (mean ± SD) of the effect of loading combined with PTH or saline treatment.
| Saline (SAL) | PTH | |||||||
|---|---|---|---|---|---|---|---|---|
| No Load | Load | No Load | Load | |||||
| Intact | 0MPa | 0.5MPa | 1.0MPa | Intact | 0MPa | 0.5MPa | 1.0MPa | |
| PCNA | 0.06 (0.1) | 5.6 (4.8) | 1.6 (2.3) | 5.5 (4.8) | 0.83 (1.0) | 12.6 (3.0) | 6.5 (4.6) | 8.3 (4.2) |
| PC1 | 5.8 (4.9) | 8.1 (5.0) | 4.1 (2.5) | 8.6 (6.1) | 7.9 (6.5) | 14.5 (10.5) | 16.5 (4.4) | 14.1 (6.9) |
| PTHRI | 4.3 (4.0) | 3.9 (7.7) | 1.9 (1.9) | 3.5 (3.9) | 3.8 (4.2) | 10.2 (8.0) | 10.1 (7.5) | 12.2 (8.7) |
| TRAP | 0.9 (1.3) | 1.4 (1.3) | 0.5 (0.8) | 0.9 (1.2) | 1.0 (1.0) | 2.7 (2.0) | 3.9 (2.1) | 2.5 (1.3) |
Table 2.
Statistical differences (p-values) in cancellous bone parameters due to loading and PTH treatment. Results from two factor ANOVA with loading and treatment. Significant differences indicated in bold.
| Effect of Loading | Effect of PTH | Loading *PTH | |
|---|---|---|---|
| BV/TV | 0.05 | 0.000 | 0.44 |
| Tb.N | 0.82 | 0.000 | 0.86 |
| Tb.Sep | 0.72 | 0.000 | 0.28 |
| Tb.Th | 0.05 | 0.06 | 0.08 |
| MAR | 0.61 | 0.003 | 0.52 |
| Ultimate stress | 0.89 | 0.002 | 0.23 |
| PCNA | 0.02 | 0.002 | 0.50 |
| PC1 | 0.93 | 0.003 | 0.50 |
| PTHRI | 0.80 | 0.003 | 0.90 |
| TRAP OC | 0.73 | 0.000 | 0.26 |
Four weeks of treatment with PTH enhanced bone volume fraction by 30%, which was explained by a 22% increase of trabecular number and 18% decrease separation, compared to saline treated animals (Figure 3a). The mineral apposition rate in the cancellous bone was increased with PTH treatment compared to saline (Figure 3b). The adaptive changes in the cancellous bone tissue induced by PTH increased ultimate stress compared to saline treatment (Figure 3c). The number of proliferative osteoblasts was enhanced after four weeks of PTH treatment in the cancellous bone compared to saline treated controls (Figure 4a–b) (Table 1). A substantial number of osteoblasts expressed PC1 (Figure 4c–d) and PTHR1 (Figure 4e–f); the number of TRAP positive osteoclasts increased with PTH treatment compared to saline treatment (Figure 4g–h) (Table 1, 2), indicating higher remodeling activity. Combined mechanical loading and PTH treatment had no effect on any measured parameters in cancellous bone (Table 1, 2).
3.2 Effects of surgery and PTH on cancellous bone
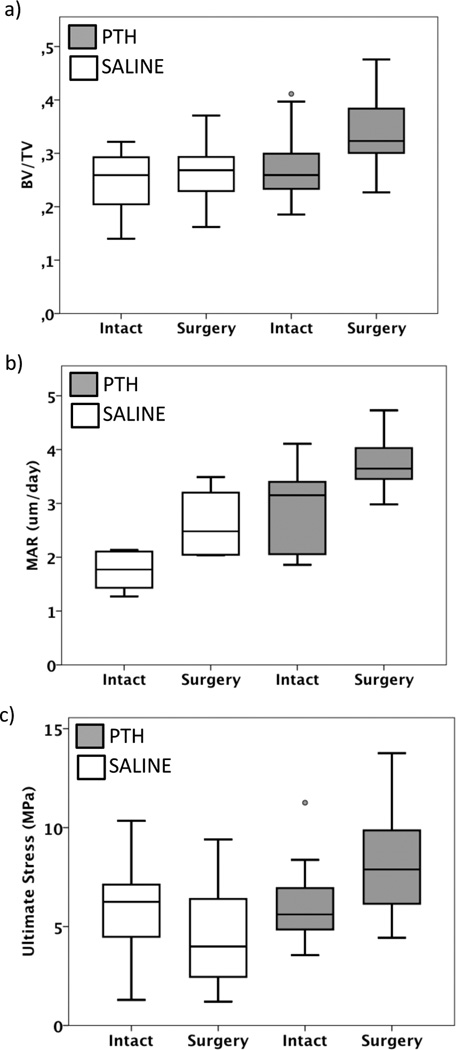
Surgery alone (SAL-0/PTH-0) enhanced the bone volume fraction (+16%) in cancellous bone (Figure 5a) compared to intact limbs (SAL-I/PTH-I), which was explained by increased numbers of trabeculae and decreased separation, but trabecular thickness was not different. Mineral apposition rate was also induced by surgery (Figure 5b). These changes did not affect the ultimate stress of the cancellous bone (Figure 5c). When the cell activity was analyzed, the numbers of proliferative osteoblasts were enhanced in the cancellous bone (Table 1). The number of osteoblasts expressing for PC1 and PTHR1 was not affected by surgery. However, a trend (p=0.08) of increased numbers of TRAP positive osteoclasts was induced by surgery (Table 1, 3).
Figure 5.
Combining surgical insult with PTH treatment had an additive effect compared to PTH treatment in intact limbs. (a) Bone volume fraction (BV/TV), (b) mineral apposition rate (MAR) and (c) ultimate stress of the cancellous bone below the loading core or corresponding location in intact limbs found in saline- and PTH-treated animals. Statistical differences are presented in Table 3.
Table 3.
Statistical differences (p-values) in cancellous bone parameters due to surgery and PTH treatment. Results from two factor ANOVA with surgery and treatment. Significant differences indicated in bold.
| Effect of Surgery | Effect of PTH | Surgery * PTH | |
|---|---|---|---|
| BV/TV | 0.001 | 0.001 | 0.01 |
| Tb.N | 0.000 | 0.004 | 0.006 |
| Tb.Sep | 0.001 | 0.023 | 0.072 |
| Tb.Th | 0.42 | 0.12 | 0.77 |
| MAR | 0.01 | 0.001 | 0.94 |
| Ultimate stress | 0.65 | 0.008 | 0.01 |
| PCNA | 0.000 | 0.005 | 0.02 |
| PC1 | 0.18 | 0.20 | 0.50 |
| PTHRI | 0.30 | 0.33 | 0.25 |
| OC | 0.08 | 0.27 | 0.35 |
Four weeks of PTH treatment increased bone volume fraction in the cancellous bone by 21% (Figure 5a), with increased numbers of trabeculae and decreased trabecular separation, but no effect on thickness. Also the mineral apposition rate was increased (Figure 5b). The change in bone structure increased in ultimate stress (Figure 5c). PTH enhanced the number of proliferative cells, but the number of osteoblasts expressing PC1 and PTHrec1, or numbers of TRAP positive osteoclasts was not increased (Table 1,3).
Compared to the individual effect of surgery (+6%) (SAL-0) and PTH (+9%) (PTH-I) on bone volume fraction, surgery and PTH treatment showed a strong combined effect (+34%) (PTH-0) compared to intact control limbs (SAL-I) (Figure 5a). This effect was mainly due to increased number of trabeculae (+21%), with no change in trabecular thickness. The mineral apposition rate was not affected (Figure 5b). Furthermore the ultimate stress increased by 84% with surgery and PTH combined, compared to increases of 35% with surgery or 24% with PTH alone, when compared with intact control limbs (Figure 5c). The number of proliferative osteoblasts increased to 12.6±3.0 per region of interest, with combined PTH and surgery, compared to 0.06±0.1 positive cells in intact control limbs (Table 1, 3). The number of positive cells expressing PC1 and PTHR1, or numbers of TRAP positive osteoclasts were not enhanced, when surgery and PTH treatment were combined.
4.0 Discussion
The quantity and quality of the cancellous bone surrounding a total joint implant is critical for initial fixation and long-term success. Mechanical loading is a well-known anabolic stimulus for bone formation. Combining mechanical loading with intermittent administration of parathyroid hormone (PTH) treatment, the most promising anabolic agent currently available, has the potential for dramatically improving initial and long-term efficacy of osseointegration. Thus, understanding synergistic interactions between two strong anabolic stimuli may lead to better treatment strategies for total joint replacement patients.
In the present study, we found individual effects of loading and PTH treatment on cancellous bone mass, but no synergy. On the other hand, we found a synergistic effect when PTH was combined with surgery. Clinically enhancing early osseointegration in the post-surgical period has numerous potential benefits and is an important finding. Previous results have been conflicting, showing both synergistic and absence of effect when these two anabolic stimuli were combined. However, this discrepancy can be explained by several factors such as; time of administration, dosage level, or time point for evaluation.
PTH had a strong anabolic effect on loaded cancellous bone. The bone volume fraction, mineral apposition rate and mechanical strength were enhanced. At the cellular level, both the osteoblastic and osteoclastic activity increased indicating a higher remodeling activity. While, we were not able to demonstrate an enhanced mechanical strength by loading alone, we were able to confirm the anabolic effect of loading by bone volume fraction and cellular activity. Combining compressive loading with PTH has shown synergistic effects in the cancellous bone in not only rat vertebra [16, 17], and the intact rat tibia [18], but also in fracture healing [19]. However, we did not see this effect in the current study. The lack of the synergy may be due to the low individual effect of mechanical loading seen in the current study. However, the timing of PTH administration and the duration of the mechanical stimuli could also explain our data. The PTH levels may not have been physiologic when the loading was applied. In the current study PTH was administrated before the mechanical stimuli, but we did not measure the maximum plasma level before applying mechanical stimuli. However, knowledge is limited as to the ideal level required before loading or the PTH levels to get a synergistic response.
We did find a strong effect of PTH in the cancellous bone in limbs that underwent surgery compared to intact controls. This effect was shown by increased bone volume fraction, increased mineral apposition rate, and increased strength of the cancellous bone. Also in PTH treated animals cell activity was enhanced by increased numbers of osteoblasts expressing for procollagen-1 and expression of the PTH-1 receptor. The effect of PTH on osteoblasts is mediated via binding and activation of the common cell surface receptor, PTH-1 receptor, a member of the B subfamily of G protein-coupled receptors. Also the expression of the PTH-1 receptor in osteocytes seems to be crucial for the anabolic effect [20]. Histomorphometric studies from our laboratory [21] and others [22–24] have indicated intermittent PTH increases bone formation by increasing the number of osteoblasts. The increased osteoblast numbers may be a consequence of increased osteoblast proliferation seen in present study, but also inhibition of osteoblast apoptosis, and/or reactivation of quiescent lining cells [25]. However, this effect was substantially enhanced when PTH treatment was combined with surgical trauma. This finding might be explained by the ability of PTH to differentiate already committed cells, provided by the increased cell proliferation after surgical trauma.
Surgical trauma alone increased bone cancellous bone formation, but not enough to increase the mechanical strength of the bone. Our animal model consists of a relatively minor surgical insult, but reaming of the exposed bone surface does create some trauma. Bone debris generated from the milled surface contains bone morphogenic proteins and growth factors that may influence the surrounding cancellous bone response [13]. The surgical trauma could also initiate a local inflammatory response attracting macrophages contributing to cell adhesion, cell signaling and matrix mineralization [14]. It is known that creating a healing zone with a gap [10] instead of a press-fit design [11] improves implant fixation in the cancellous bone. This peri-implant healing zone creates an important regenerative process that we also saw in our current model.
In this study we did not see any systemic effects of PTH in the intact cancellous bone. This might be due to several factors such as dosage level or time of dosing but metabolic activity could be another explanation. Several studies have shown that PTH is able to increase the cancellous bone volume in same time frame. However, often this has been done in animals [26, 27] or in patients [21, 28] with compromised bone. Implant fixation in healthy animals has a weak anabolic response of PTH in the intact bone or at a distance from the healing zone [10–12].
Previously, the contralateral limb did not show a systemic effect of loading in the contralateral limb [7, 8]. However, the systemic effect due to loading has previously been debated. In a rat model it has been shown that the contralateral non-loaded ulna had a higher cortical bone formation that was later inhibited by neuronal blockade [29]. Conversely, several studies have shown no interaction between the two limbs [18]. In present study we did not find a correlation between right and left limb when both bone and biomechanical parameters were analyzed indicating that our results are in line with previous experience in this animal model.
The role of osteoclasts in bone anabolic response induced by PTH is still not clear [23, 30]. Both surgery and PTH increased the number of osteoclasts. In intact bone, PTH increases the number of osteoblasts through both osteoclast-dependent and –independent mechanisms [23, 30]. However, in a healing zone with a larger remodeling space, bone remodeling activity of osteoblasts and osteoclasts may be uncoupled. This was shown in a rat model for metaphyseal bone healing when a combined treatment of PTH and an anti-resorptive agent resulted in an additive effect [31]. This would also explain differences in PTH action for intact bone versus bone in a trauma setting.
Understanding the cellular and biomechanical effects of anabolic agents on cancellous bone is critical in enhancing osseointegration of implants. This study has demonstrated that both loading and intermittent PTH treatment enhanced peri-implant bone volume, and that surgery and PTH treatment had a strong combined anabolic effect. Questions still remain regarding the involvement of bone remodeling in this process and future studies will address this important question.
Acknowledgments
The authors would like to thank John DiBianco, Brad Nakamura and Michael Cross for technical assistance during surgery and collecting and analyzing samples. The PTH was generously donated by Eli Lilly. This project was funded by an Orthopaedic Research and Education Foundation Career Development Grant, National Institutes of Health Grants R01-AR056802, R01-AG028664, and P30-AR046121.
References
- 1.Skripitz R, Aspenberg P. Early effect of parathyroid hormone (1–34) on implant fixation. Clin Orthop Relat Res. 2001:427–432. doi: 10.1097/00003086-200111000-00056. [DOI] [PubMed] [Google Scholar]
- 2.Skripitz R, Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg Br. 2001;83:437–440. doi: 10.1302/0301-620x.83b3.10256. [DOI] [PubMed] [Google Scholar]
- 3.Chambers TJ, Evans M, Gardner TN, Turner-Smith A, Chow JW. Induction of bone formation in rat tail vertebrae by mechanical loading. Bone Miner. 1993;20:167–178. doi: 10.1016/s0169-6009(08)80025-6. [DOI] [PubMed] [Google Scholar]
- 4.DeSouza R. Transforming possibilities of care: Goan migrant motherhood in New Zealand. Contemp Nurse. 2005;20:87–101. doi: 10.5172/conu.20.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Fritton JC, Myers ER, Wright TM, van der Meulen MC. Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone. 2005;36:1030–1038. doi: 10.1016/j.bone.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein SA, Matthews LS, Kuhn JL, Hollister SJ. Trabecular bone remodeling: an experimental model. J Biomech. 1991;24(Suppl 1):135–150. doi: 10.1016/0021-9290(91)90384-y. [DOI] [PubMed] [Google Scholar]
- 7.van der Meulen MC, Morgan TG, Yang X, Baldini TH, Myers ER, Wright TM, Bostrom MP. Cancellous bone adaptation to in vivo loading in a rabbit model. Bone. 2006;38:871–877. doi: 10.1016/j.bone.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Meulen MC, Yang X, Morgan TG, Bostrom MP. The effects of loading on cancellous bone in the rabbit. Clin Orthop Relat Res. 2009;467:2000–2006. doi: 10.1007/s11999-009-0897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willie BM, Yang X, Kelly NH, Han J, Nair T, Wright TM, van der Meulen MC, Bostrom MP. Cancellous bone osseointegration is enhanced by in vivo loading. Tissue Eng Part C Methods. 2010;16:1399–1406. doi: 10.1089/ten.tec.2009.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugaard H, Elmengaard B, Andreassen T, Bechtold J, Lamberg A, Soballe K. Parathyroid hormone treatment increases fixation of orthopedic implants with gap healing: a biomechanical and histomorphometric canine study of porous coated titanium alloy implants in cancellous bone. Calcif Tissue Int. 2011;88:294–303. doi: 10.1007/s00223-010-9458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugaard H, Elmengaard B, Andreassen TT, Baas J, Bechtold JE, Soballe K. The combined effect of parathyroid hormone and bone graft on implant fixation. J Bone Joint Surg Br. 2011;93:131–139. doi: 10.1302/0301-620X.93B1.24261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamo K, Miyakoshi N, Kasukawa Y, Nozaka K, Sasaki H, Shimada Y. Intermittent weekly administration of human parathyroid hormone (1–34) improves bone-hydroxyapatite block bonding in ovariectomized rats. J Bone Miner Metab. 2010;28:634–640. doi: 10.1007/s00774-010-0178-z. [DOI] [PubMed] [Google Scholar]
- 13.Hollister SJ, Guldberg RE, Kuelske CL, Caldwell NJ, Richards M, Goldstein SA. Relative effects of wound healing and mechanical stimulus on early bone response to porous-coated implants. J Orthop Res. 1996;14:654–662. doi: 10.1002/jor.1100140422. [DOI] [PubMed] [Google Scholar]
- 14.McKee MD, Pedraza CE, Kaartinen MT. Osteopontin and Wound Healing in Bone. Cells Tissues Organs. 2011 doi: 10.1159/000324244. [DOI] [PubMed] [Google Scholar]
- 15.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 16.Chow JW, Fox S, Jagger CJ, Chambers TJ. Role for parathyroid hormone in mechanical responsiveness of rat bone. Am J Physiol. 1998;274:E146–E154. doi: 10.1152/ajpendo.1998.274.1.E146. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Takai E, Zhou H, von Stechow D, Muller R, Dempster DW, Guo XE. Trabecular bone response to mechanical and parathyroid hormone stimulation: the role of mechanical microenvironment. J Bone Miner Res. 2003;18:2116–2125. doi: 10.1359/jbmr.2003.18.12.2116. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone. 2008;43:238–248. doi: 10.1016/j.bone.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Gardner MJ, van der Meulen MC, Carson J, Zelken J, Ricciardi BF, Wright TM, Lane JM, Bostrom MP. Role of parathyroid hormone in the mechanosensitivity of fracture healing. J Orthop Res. 2007;25:1474–1480. doi: 10.1002/jor.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1–34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22:495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 22.Iida-Klein A, Zhou H, Lu SS, Levine LR, Ducayen-Knowles M, Dempster DW, Nieves J, Lindsay R. Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J Bone Miner Res. 2002;17:808–816. doi: 10.1359/jbmr.2002.17.5.808. [DOI] [PubMed] [Google Scholar]
- 23.Jilka RL, O'Brien CA, Bartell SM, Weinstein RS, Manolagas SC. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J Bone Miner Res. 2010;25:2427–2437. doi: 10.1002/jbmr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma YL, Zeng Q, Donley DW, Ste-Marie LG, Gallagher JC, Dalsky GP, Marcus R, Eriksen EF. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21:855–864. doi: 10.1359/jbmr.060314. [DOI] [PubMed] [Google Scholar]
- 25.Jilka RL, O'Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44:275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Liang H, Shen Y, Wronski TJ. Parathyroid hormone stimulates cancellous bone formation at skeletal sites regardless of marrow composition in ovariectomized rats. Bone. 1999;24:95–100. doi: 10.1016/s8756-3282(98)00167-7. [DOI] [PubMed] [Google Scholar]
- 27.Watson P, Lazowski D, Han V, Fraher L, Steer B, Hodsman A. Parathyroid hormone restores bone mass and enhances osteoblast insulin-like growth factor I gene expression in ovariectomized rats. Bone. 1995;16:357–365. doi: 10.1016/8756-3282(94)00051-4. [DOI] [PubMed] [Google Scholar]
- 28.Rubin MR, Cosman F, Lindsay R, Bilezikian JP. The anabolic effects of parathyroid hormone. Osteoporos Int. 2002;13:267–277. doi: 10.1007/s001980200026. [DOI] [PubMed] [Google Scholar]
- 29.Sample SJ, Behan M, Smith L, Oldenhoff WE, Markel MD, Kalscheur VL, Hao Z, Miletic V, Muir P. Functional adaptation to loading of a single bone is neuronally regulated and involves multiple bones. J Bone Miner Res. 2008;23:1372–1381. doi: 10.1359/jbmr.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierroz DD, Bonnet N, Baldock PA, Ominsky MS, Stolina M, Kostenuik PJ, Ferrari SL. Are osteoclasts needed for the bone anabolic response to parathyroid hormone? A study of intermittent parathyroid hormone with denosumab or alendronate in knock-in mice expressing humanized RANKL. J Biol Chem. 2010;285:28164–28173. doi: 10.1074/jbc.M110.101964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aspenberg P, Wermelin K, Tengwall P, Fahlgren A. Additive effects of PTH and bisphosphonates on the bone healing response to metaphyseal implants in rats. Acta Orthop. 2008;79:111–115. doi: 10.1080/17453670710014851. [DOI] [PubMed] [Google Scholar]