Abstract
Ipsilateral actions of pyramidal tract (PT) neurons are weak but may, if strengthened, compensate for deficient crossed PT actions following brain damage. The purpose of the present study was to examine whether transcranial direct current stimulation (tDCS) may strengthen ipsilateral PT actions; in particular those relayed by reticulospinal neurons co-excited by axon collaterals of fibres descending in the ipsilateral and contralateral PTs and of reticulospinal neurons descending in the medial longitudinal fascicle (MLF). Effects of tDCS were assessed in acute experiments on deeply anaesthetized cats by comparing postsynaptic potentials evoked in hindlimb motoneurons and discharges recorded from their axons in a ventral root, before, during and after tDCS. tDCS was consistently found to facilitate joint actions of ipsilateral and contralateral PTs, especially when stimulated together with the MLF. Both EPSPs and IPSPs evoked in motoneurones and the ensuing ventral root discharges were facilitated even though the facilitatory effects of tDCS were not sufficient for activation of motoneurons by ipsilateral PT neurons alone. Facilitation outlasted single tDCS periods by at least a few minutes and effects evoked by repeated tDCS by up to 2 hours. The results of this study thus indicate that tDCS may increase the contribution of ipsilateral PT actions to the recovery of motor functions after injuries of contralateral PT neurons and thereby assist rehabilitation provided that cortico-reticular and reticulo-spinal connections are preserved.
Keywords: Transcranial polarization (tDCS), Motor Cortex, Reticular Formation, Plasticity
Introduction
Ipsilateral corticospinal actions are mediated via descending neurons projecting to the spinal cord as well as via supraspinal relay neurons. Different strategies might thus be used to increase the contribution of ipsilateral corticospinal actions to movements when contralateral actions are deficient. Stimulation of the ipsilateral motor cortex as well as a more intense use of the ipsilateral extremities were demonstrated to improve actions evoked by spinally projecting cortical neurons (Raineteau et al., 2002; Martin et al., 2007; for most recent references see Nishimura & Isa, 2012; Carmel et al., 2013; Carmel et al., 2014; Isa et al., 2013). The transmission between the corticospinal neurons and their relay neurons may also be facilitated, e.g. by 4-Amino-pyridine (4AP; Jankowska et al., 2005) or additional input to the involved neurons (Edgley et al., 2004; Jankowska & Stecina, 2007; Stecina & Jankowska, 2007). Considering the possibility of further facilitation by transcranial direct current stimulation (tDCS), the aim of the present study was to examine to what extent ipsilateral actions of corticospinal neurons relayed by reticulospinal neurons may be enhanced by tDCS.
We expected the ipsilateral corticospinal actions to be enhanced by tDCS taking into account the evidence for long-lasting effects of tDCS on not only cortical neurons (see e.g. Bindman et al., 1964; Purpura & McMurtry, 1965; Stagg & Nitsche, 2011) but also on subcortical neurons, including reticulospinal neurons (Bolzoni et al., 2013a; Bolzoni et al., 2013b). Another reason was the evidence that at least some reticulospinal neurons are excited not only by contralateral but also by ipsilateral pyramidal tract cortical neurons (He & Wu, 1985; Canedo & Lamas, 1993; Edgley et al., 2004; Stecina & Jankowska, 2007; Baker, 2011). However, even if facilitation of ipsilateral PT actions relayed by reticulospinal neurons by tDCS appeared to be likely, the degree and the timing of the facilitation, or experimental conditions under which the facilitation is optimized could not be predicted.
In order to restrict the ipsilateral actions of corticospinal neurons to actions mediated by reticulospinal neurons with axons descending in the Medial Longitudinal Fascicle (MLF), we used the technique of spatial facilitation to activate reticulospinal neurons. To this end we used stimuli that by themselves failed to induce motor responses from either the ipsilateral or the contralateral medullary pyramid (PT) or the MLF, but became effective when applied jointly, by activating reticulospinal neurons co-excited by axon-collatera of PTs and of the MLF. Effects of iPT stimuli were compared during and after application of tDCS using the polarity (anodal tDCS), timing and intensities previously found to facilitate activation of reticulospinal neurons (Bolzoni et al., 2013b). The mutual facilitation of synaptic actions of nearly synchronous ipsilateral and contralateral PT and/or MLF stimuli evoked during and after tDCS made it unlikely that the effects of the iPT stimuli were relayed by other than reticulospinal neurons (for further arguments see the first section of the discussion).
Methods
Ethical approval
All experiments were approved by a regional Ethics Committee for Animal Research (Göteborgs Djurförsöksetiska Nämnd) and comply with EU and NIH guidelines for animal care. The animals were bred and housed under veterinary supervision at the Laboratory of Experimental Biomedicine at Sahlgrenska Academy where the experiments were carried out.
Preparation
The experiments were performed on 11 deeply anaesthetised cats weighing 2.7–3.5 kg. Anaesthesia was induced with sodium pentobarbital ( i.p.; Apoteksbolaget, Sweden or Apotek Produktion & Laboratorier AB Stockholm, Sweden; 40 mg/kg, i.p.) and maintained with intermittent doses of α-chloralose (i.v. Rhône-Poulenc Santé, France; 5 mg/kg administered every 1–3 hours, up to 65 mg/kg, i.v.). Additional doses of α-chloralose were given when motor reactions were evoked during dissection and when increases in the continuously monitored blood pressure or heart rate were evoked by any experimental procedures. During recordings, neuromuscular transmission was blocked by pancuronium bromide (i.v. Pavulon, Organon, Sweden; 0.3 mg/kg i.v. supplemented with about 0.2 mg kg h−1) and the animals were artificially ventilated. Mean blood pressure was maintained at 100–130 mm Hg and end-tidal CO2 at 4–4.5% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml kg h−1). Body temperature was kept at about 37.5°C by servo-controlled heating lamps. The experiments were terminated by an overdose of pentobarbital i.v. and formalin perfusion.
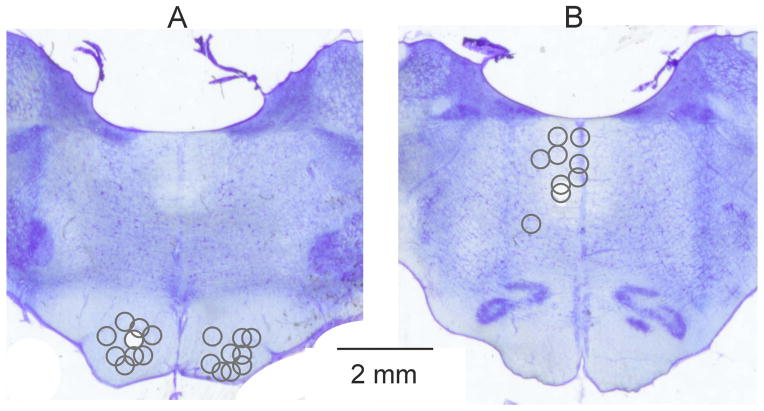
Following the initial vein, artery and tracheal cannulation the caudal lumbar and sacral segments of the spinal cord were exposed by laminectomy, and selected nerves in the ipsilateral hind limb were dissected free. These included the quadriceps (Q), the gastrocnemius soleus (GS) and the posterior biceps-semitendinosus (PBST) muscle nerves. The head was fixed in a stereotactic frame and the cerebellum was exposed by craniotomy to allow inserting electrodes into the left and right pyramidal tract (PT) and the left medial longitudinal fascicle (MLF) to stimulate axons of corticospinal and reticulospinal neurons respectively. The electrodes were inserted at an angle of 20° from vertical (tip directed rostrally), PTs and MLF being aimed at Horsley-Clarke coordinates, P6-8, L1.3, H-10 and P9, L0.8, H-5 respectively. The electrodes were left at locations from which distinct descending volleys evoked at stimulus intensities of 20 μA or less were recorded from the surface of the spinal cord at the C1/C2 segments. To this end an area of 1–2 mm2 over the lateral funiculus between the first and second vertebrae was made accessible by widening the space between these vertebrae, thereby allowing one silver ball recording electrode to be in contact with the spinal cord surface, with a reference electrode inserted in a neck muscle. At the end of the experiments the stimulation sites were marked with electrolytic lesions, the locations of which were verified on 100 μm thick sections, cut in the plane of the electrode insertions using a vibratome, mounted on slides, counterstained with cresyl violet and scanned (see Fig. 1).
Figure 1. Sites of brainstem stimulation.
Reconstructions of the locations of the stimulating electrodes in the pyramidal tracts (A) and in and lateral to the medial longitudinal fascicle (B). The stimulation sites defined by electrolytic lesions made at the end of the experiments are indicated on representative sections of the medulla at the levels of the superior (A) and inferior (B) olives respectively. The circles in (A) and (B) correspond to the centres of the lesions and their diameters to the distances of estimated spread of current, within 0.2–0.3 mm from the electrode tip for 20–30 μA (see Fig. 11 in Gustafsson & Jankowska, 1976).
Stimulation and recording
PTs and MLF were stimulated with constant current stimuli. Trains of 2–6 monopolar stimuli were delivered via tungsten electrodes (impedance 30–150 kΩ) against the reference electrode in one of the neck muscles. The stimulus parameters (0.2 ms duration, intensities of most often 50–100 μA), and inter-stimulus intervals of 2.5 or 3.3 ms were chosen to ensure that these stimuli induced not only direct but also indirect activation of descending fibres. Current intensities ≤ 100 μA were previously verified to be below the critical intensities at which spread of current occurs between the left and right PTs or between the MLF and PTs (Jankowska et al., 2006). Peripheral nerves were stimulated with constant voltage stimuli of 0.2 ms duration at intensities expressed in multiples of threshold (T) stimuli, i.e. stimuli inducing barely visible afferent volleys in records from the dorsal root entry zone.
Records of the activity of hindlimb motoneurons were obtained in two ways. In 6 experiments postsynaptic potentials of PT and MLF origin were recorded from motoneurons within the GS and PBST motor nuclei in the caudal part of the L7 segment. The motoneurons were identified by antidromic activation following stimulation of the GS or PBST nerves. The records were obtained using glass micropipettes broken to about 1.5 μm external diameter (“sharp electrodes”) filled with 2M KCitrate (resistance of 3–7 MOhms) and a conventional high input resistance amplifier. In 5 experiments the degree of activation of lumbar motoneurons was estimated from records of spike discharges of motoneuron axons running in the L7 or S1 ventral roots (VR), which allowed comparison of responses of populations of motoneurons. To this end the ventral root were transected distally and mounted on pairs of silver wire electrodes in a paraffin oil pool.
Descending volleys evoked by MLF or combined PT and MLF stimulation were recorded at the level of the L7 segment. The volleys were recorded monopolarly with one electrode in contact with the intact dura mater over the dorsal columns within the L7 segments, and the reference electrode in contact with one of the neck or back muscles.
Both single records and averages of 10–50 records were stored on-line (with the time resolution of 30 μs per address) and were analysed off-line.
Parameters of tDCS
The transcranial polarization was applied as described by Bolzoni et al. (2013b) to the area over the contralateral pericruciate region about 3–10 mm from the midline, corresponding to the human sensori-motor cortex. Anodal current was routinely used, except in a few control polarization series, as anodal tDCS facilitates activation of reticulospinal neurons in the cat (Bolzoni et al., 2013b) in contrast to the rat in which the cathodal tDCS is facilitatory while anodal tDCS is depressive (Bolzoni et al., 2013a). The polarizing current was applied via 3% agar-agar in saline contained in a chamber attached to the skull. The bottom of the chamber was fitted to the shape of the skull after the frontal sinus was opened and sealed. It was shaped as an elipse with diameters of 14.8 mm and 18 mm and a contact area of about 200 mm2. The reference electrode was in contact with about twice that area, between the ipsilateral (left) lateral aspect of the skull and the temporal muscles, about 20 mm caudal and 20 mm lateral from the electrode over the sensori-motor cortex.
Current intensities were continuously monitored. Stimuli of 0.2 mA were routinely used but they were sometimes increased to 0.5 mA in order to verify that the conditions of polarization were optimal. The intensity of 0.2 mA stimuli corresponded to about 1 μA mm−2. This intensity exceeded the current intensity used in humans (0.3 μA mm−2, in total 1 mA over 35 cm2) but falls within the range of intensities used in acute experiments on anaesthetized rats (up to 57 μA mm−2; see Table 1 in Brunoni et al., 2011). The higher current intensity compared to that used in humans was warranted by the significant drop in current density within the target area at depth as the size of the electrode is decreased (Miranda et al., 2009; see also Rahman et al., 2013).
Polarizing current was routinely applied during 6–7 periods of 5 minutes separated by 5 minutes intervals, to allow a comparison of direct effects of the polarization and the development of effects seen during the post-polarisation periods. In the following we will refer to effects of tDCS evoked during tDCS without specifying the period of application of tDCS (unless otherwise indicated) and to effects seen at least 10–30 min after the last tDCS as the post-polarization effects.
Analysis
Effects of tDCS were estimated from changes in postsynaptic potentials evoked in individual motoneurons or in ventral root responses evoked by submaximal stimuli applied within the PTs and MLF. Repeated series of 10 or 20 stimuli were delivered at 2–3Hz, alternately to PTs and MLF, or by using overlapping short trains of PT and MLF stimuli. Responses to these stimuli were recorded during and after tDCs, and compared to those evoked before tDCS. The comparison included effects of a brief train of stimuli and it was noted after which stimuli the responses appeared and how their areas and latencies were affected by tDCS. Software for sampling and analysis developed by E. Eide, T. Holmström and N. Pihlgren (University of Gothenburg) was used for measurements of latencies and areas and to calculate the differences between the areas of the tested potentials from their averaged records.
Differences between data sets were assessed for statistical significance by using Student’s t-test, Mann-Whitney Rank Sum Test, z-test or ANOVA with the adequate (Holm-Sidak or Dunn’s) post-hoc tests for determining differences to control (using Statistica 5.1, StatSoft or SigmaPlot 12.5 Systat software INC). For all tests the overall significance level was set at p<0.05.
Results
Experimental strategy
Facilitation of activation of reticulospinal neurons by iPT neurons during and after tDCS was estimated from effects of spacial facilitation at the level of reticulospinal neurons resulting in postsynaptic actions on hindlimb motoneurons or in activation of these motoneurons. The principles of spacial facilitation as applied to the organization of neuronal networks in animals (Lundberg, 1975; Burke, 1999; Jankowska, 2012) and humans (Pierrot-Deseilligny & Burke, 2012) have been repeatedly outlined. We will, therefore, point out only two consequences of the use of this indirect technique for the analysis of the results of this study. Firstly, that it is highly sensitive, i.e. that the generation of action potentials in neurons in which spatial facilitation occurs may depend on minute changes in input to these neurons occurring at the critical level of transition from subthreshold to suprathreshold membrane potentials generating action potentials. Secondly, that changes in the input critical for spatial facilitation have to be optimized in each individual case because the use of too weak and/or not properly timed stimuli may fail to bring the postsynaptic neurones to the threshold for generation of action potentials, while the use of too strong stimuli will prevent disclosing converging presynaptic actions. Facilitation of converging synaptic actions of corticospinal and reticulospinal neurones on reticulospinal neurons could thus not be examined under strictly standardized conditions. For these reasons, we choose the strategy of examining as many as possible effects of tDCS in order to verify our hypothesis, rather than one or two strictly repeatable and easiest to quantify effects. We considered that consistently found different manifestations of facilitation of synaptic actions of iPT on reticulospinal neurons by tDCS would considerably increase the confidence in its occurrence, even if they were found in different combinations. These manifestations included larger postsynaptic potentials recorded in individual motoneurons, shorter latencies of these potentials, the appearance after fewer stimuli in a train and in a higher percentage of motoneurons, as well as the corresponding changes in responses of populations of motoneurons recorded in the ventral roots.
Effects of tDCS on intracellularly recorded motoneurons
Weak facilitation by tDCS of postsynaptic actions of selectively activated ipsilateral PT neurons
During and after application of tDCS EPSPs evoked by selective stimulation of the ipsilateral PT were found in only12 of 66 (18%) of motoneurons. This proportion was slightly larger than the proportion of motoneurons tested under control conditions (3 of 42; 7%), but the difference was not found to be statistically significant (Table 1). Most convincing evidence for facilitation by tDCS was obtained in three of the 12 motoneurons which were recorded from both prior and during application of tDCS. In all three of these motoneurons the earliest iPT stimuli in the train evoked EPSPs during or after but not before tDCS, as illustrated in Fig. 2A–C with EPSPs appearing after the 1st and 2nd stimuli only during tDCS. Furthermore, EPSPs evoked by later stimuli were larger after than before tDCS which is illustrated with larger EPSPs after the 4th stimuli and may be best appreciated from the superimposed records in Fig. 2C. Unfortunately, in view of the longlasting effects of tDCS, this kind of comparison could only be made in the first motoneuron tested in an experiment but the proportion of motoneurons in whichEPSPs were evoked by the 1st, 2nd or 3rd stimuli during and after than before tDCS was much greater in the whole sample of 15 motoneurons, as summarized in Fig. 2L. The figure shows also that EPSPs evoked under control conditions and either during or after tDCS were evoked within the same range of latencies (open symbols in Fig. 2L).
Table 1.
Proportions of motoneurons in which EPSPs from the ipsilateral PT were found before and after tDCS.
| Proportions of motoneurons | |||||
|---|---|---|---|---|---|
| Motoneurons with EPSPs from: | before | after | p | ||
| iPT alone | 3/42 | 7% | 12/66 | 18% | ns |
| co PT alone | 14/42 | 33% | 18/66 | 27% | ns |
| iPT & coPT | 14/40 | 35% | 36/58 | 55% | ns |
| iPT & MLF | 11/34 | 32% | 42/61 | 69% | * |
| coPT & MLF | 12/36 | 33% | 39/61 | 64% | * |
First row, data for motoneurons in which iPT stimuli were evoking oligosynaptic, most likely disynaptic EPSPs by themselves, like in Fig. 2A. Second row, similar data for EPSPs evoked from coPT. Third row, data from motoneurons in which excitatory effects from iPT were deduced from mutual facilitation of oligosynaptic EPSPs evoked from iPT and coPT, i.e. when joint actions from iPT and coPT exceeded the sum of their separate actions, as in Fig. 2G or Fig. 4C. Fourth row, data from motoneurons in which excitatory effects from iPT were deduced from facilitation of EPSPs evoked from the MLF, as in Fig. 5AB and DE. Fifth row, as in the fourth row, but for excitatory effects from co PT, illustrated in Fig. 5C and F. The facilitation is attributed to spatial facilitation occurring at a premotoneuronal, most likely reticular level. Differences between proportions of motoneurons were tested for significance using z-test. They are indicated in the last column (* p<0.01, ns p≥0.05).
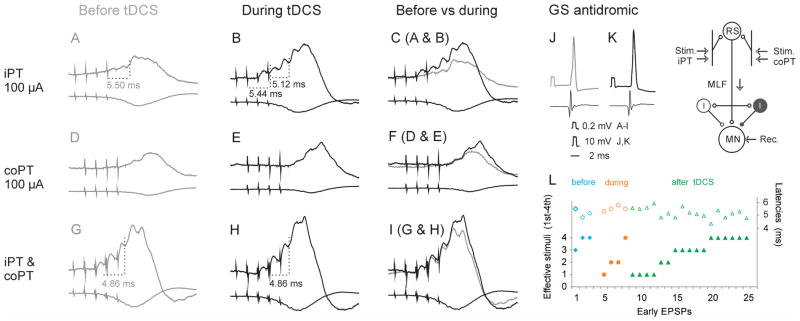
Figure 2. Facilitation of EPSPs evoked from iPT by tDCS.
(A–I), Intracellular records from a GS motoneuron before (left column) and during (middle column) anodal tDCS. In each panel the upper traces are averaged records (n=20) from the motoneuron and the lower records from the cord dorsum. Upper, middle and lower row records show effects of stimulation of ipsilateral PT, contralateral PT and of overlapping trains of stimuli applied in both iPT and coPT, as indicated. In (C), (F) and (I) are superimposed records from periods before and during application of tDCS. J and K, antidromic action potentials from the motoneuron obtained just before records in (A) and (B) respectively. Records in (A) were taken at the membrane potential 6 mV lower than in (B). The rectangular voltage calibration pulses in J and K are for intracellular records as indicated. Time calibration is for all records. In this and in all of the following figures the negativity is down in intracellular records and up in records from the cord dorsum. (L) A plot summarizing higher efficacy of ipsilateral PT stimulation during and after than before tDCS, as reflected in the appearance of EPSPs following earlier stimuli in the train (left ordinate, filled symbols. These were evoked at similar latencies from the effective iPT stimuli (right ordinate, open symbols). Data for 3 motoneurons recorded before and during tDCS and 12 motoneurons recorded during or after tDCS (with more than one early EPSPs evoked in individual motoneurons). The diagram indicates the stimulation and recording sites and the underlying convergence of iPT and coPT on reticulospinal neurons providing input to motoneurons, either directly or via spinal excitatory and inhibitory interneurons.
Latencies of EPSPs evoked by iPT stimuli were measured as illustrated in Fig 3. When an EPSP appeared after the second but not after the first stimulus in a train, its latency was measured from the second stimulus. Likewise when another EPSP appeared when the number of stimuli was increased from three to four its latency was measured from the fourth stimulus. Mesured in this way latencies of EPSPs evoked from iPT fell within the same range (4.29–6.98, mean 5.38±0.16 ms SEM; n=19) as the latencies of EPSPs evoked by co PT stimulation (4.54–6.66, mean 5.54 ±0.09 ms; n=29). No statistically significant differences were found between them using Student’s t-test for 2 samples assuming equal variances.
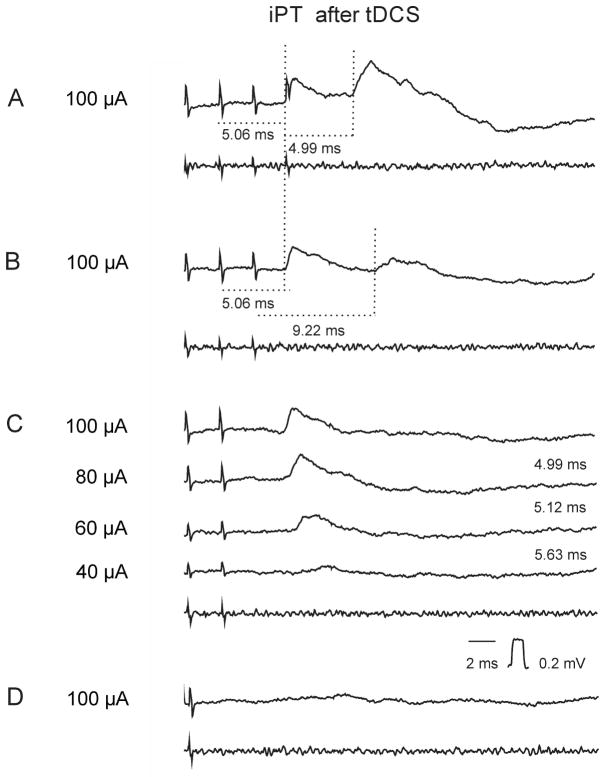
Figure 3. Examples of EPSPs linked to the early iPT stimuli.
Intracellular averaged records of EPSPs evoked from iPT in a GS motoneuron after tDCS (upper traces; n=20) and records from the cord dorsum (bottom traces). (A), (B), (C) and (D), effects of decreasing numbers of stimuli at intensities indicated to the left. Other indications are as in Fig. 2.
Latencies within these ranges are fully consistent with the disynaptic coupling indicated in Fig. 2. Latencies of the earliest disynaptic actions of PT neurons on hindlimb feline motoneurons were previously found to be 4.70±0.06 ms (Stecina & Jankowska, 2007) and synaptic actions involving one or two additional synaptic delays would be evoked with about 1 and 2 ms longer latencies respectively.
Joint actions from the ipsilateral and contralateral PTs are stronger after tDCS
Joint actions of iPT and coPTs should increase the probability of disclosure of facilitatory actions of tDCS in view of previous evidence that reticulospinal neurons relay indirect synaptic actions of the two PTs on motoneurons (Edgley et al., 2004; Stecina & Jankowska, 2007). Spatial facilitation of synaptic actions of the ipsilateral and contralateral PT neurons on reticulospinal neurons, as indicated in the diagram in Fig. 2, should thus bring reticulospinal neurons closer to the firing threshold. Any additional facilitation induced by application of tDCS (Bolzoni et al., 2013a; Bolzoni et al., 2013b) should therefore contribute to reaching this threshold. Consequently, EPSPs and/or IPSPs should be evoked in greater proportions of motoneurons by combined stimulation of ipsilateral and contralateral PTs. Theyshould also be larger than the sum of EPSPs/or IPSPs evoked from each of the PTs separately and be evoked at shorter latencies.
The proportion of motoneurons in which EPSPs were evoked by joint actions of iPT and coPT was indeed greater than of motoneurons in which EPSPs were evoked from iPT alone and the proportion of activated motoneurons further increased following tDCS. It increased from 35% to 55% although this increase was not found to be statistically significant (Table 1). The degree of facilitation varied both before and after tDCS; in extreme cases EPSPs appeared upon combined iPT and coPT stimulation even though separate iPT and coPT stimuli failed to evoke EPSPs by themselves, as illustrated in Fig. 2G for EPSPs evoked by the first 3 stimuli in the train and in Fig. 4C. Such combined actions further increased during tDCS (Fig. 2H, for EPSPs evoked by 4th stimuli) and appeared at shorter latencies, which is another manifestation of facilitation of iPT actions by tDCS.
Figure 4. Examples of PSPs evoked by combined stimulation of the ipsilateral and contralateral PTs.
(A) and (B), Records from a GS motoneuron before and during application of tDCS respectively. C, Records from a PBST motoneuron after application of tDCS. In each panel the records were obtained upon stimulation of contralateral PT, ipsilateral PT and both of them conjointly at 100 μA. The diagram indicates the stimulation and recording sites and the underlying convergence of iPT and coPT on reticulospinal neurons providing input to motoneurons, either directly or via spinal interneurons. Other indications are as in Fig. 2. Note that in B an IPSP appears upon combined stimulation of iPT and coPT during but not before tDCS (and not upon iPT or coPT stimuli alone). Note also that in C an EPSP is evoked by combined but not by separate iPT and coPT stimuli.
The latencies of EPSPs evoked by joint actions of iPT and coPT decreased by 0.2 – 0.4 ms when measured from the effective stimulus, e.g. 4.86 ms in Fig 2H as compared to 5.12 ms in Fig 2B. In addition, such EPSPs were evoked by the 5th or 4th coPT & iPT stimuli before tDCS but by the 2nd 3rd or 4th stimulus after tDCS. On the average, preceding coPT stimuli by iPT stimuli, or vice versa, reduced the latencies of EPSPs from the first stimulus in the train by 2.42 ms before tDCS but by 4.08 ms during or after tDCS (p<0.001; t-test for two samples assuming equal variances; data for 10 and 15 motoneurones respectively).
IPSPs evoked by joint stimulation of the ipsilateral and contralateral PTs during and after tDCs were usually more distinct than when evoked by separate iPT or coPT stimuli under control conditions, or appeared only to joint iPT and coPT stimuli, as exemplified in Fig. 4AB. IPSPs with distinct onset appeared at latencies of 6.08±0.15 ms from the effective stimulus (n=25), which were on the average 0.9 ms longer and significantly different (p<0.01, t-test) from latencies of EPSPs of PT origin. When compared to latencies of disynaptic EPSPs evoked from the MLF (4.10±0.08 ms), latencies of EPSPs and IPSPs of PT origin were about 1 and 2 ms longer, indicating only one additional synaptic delay for the EPSPs and two additional synaptic delays for the IPSPs, as indicated in the diagram of the most direct pathways between the PTs and motoneurons in Fig. 2.
Joint actions from the ipsilateral PT and the MLF are stronger after tDCS
In a further attempt to increase the probability of disclosing facilitatory effects of tDCS in pathways between iPT and motoneurons we compared synaptic actions from iPT stimulated conjointly with not only coPT but also with the MLF. As collaterals of fibres descending in the MLF provide strong input to reticulospinal neurons, these neurons should be brought even closer to the threshold for activation by adding MLF stimulation than when only the two PTs are stimulated.
The net effects of iPT stimuli combined with MLF stimuli were assessed from differences between disynaptic EPSPs evoked by combined stimuli (Fig. 5BE), as compared to when MLF was stimulated alone (Fig. 5AD). More marked differences following tDCS, or a higher proportion of activated motoneurons, were taken to indicate a larger contribution from iPT after tDCS and thus a higher degree of facilitation of activation of reticulospinal neurons by iPT.
Figure 5. Comparison of PSPs evoked by stimulation of MLF alone and when preceded by stimulation of iPT or co PT after tDCS.
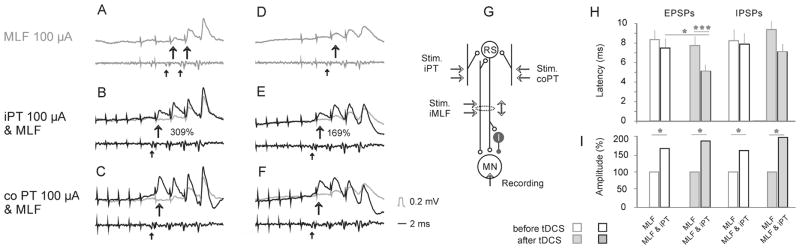
(A–C and D–F), intracellular records from two GS motoneurons penetrated in the same experiment after tDCS (upper traces; averages of 20 records) and the corresponding records from the cord dorsum. Upper row, effects of a train of four stimuli applied in the MLF alone. Middle and bottom row, effects of the same stimuli preceded by four stimuli applied to either iPT or co PT, as indicated, with EPSPs evoked by MLF alone (grey) superimposed. Large arrows indicate the earliest EPSPs. Small arrows indicate MLF volleys followed by these EPSPs. The increases of EPSPs evoked by second stimulus in % of control are indicated in B and E. Other indications are as in Fig. 2. (G), the diagram showing the stimulation and the recording sites and the involved inhibitory interneurons. (H), decreases in the latencies (mean latencies and SEM; from the first MLF stimulus) of EPSPs and IPSPs evoked by MLF or by joint iPT and MLF stimuli. After tDCS the decreases in latencies of the joint iPT-MLF actions (shaded columns) were significantly larger with respect to EPSPs evoked by both iPT and MLF stimuli under control conditions and only MLF stimuli after tDCS (*p<0.05, t-test paired data; *** p<0.001 t-test two samples). (I), increases in amplitudes of EPSPs and IPSPs evoked by MLF stimuli when combined with iPT stimuli (* p<0.05 t test for either paired data or two samples). In (H) and (I) are data for 10 and 12 motoneurons in which EPSPs were recorded before or during/after tDCS and in 9 and 14 motoneurons in which effects of tDCS were compared on IPSPS.
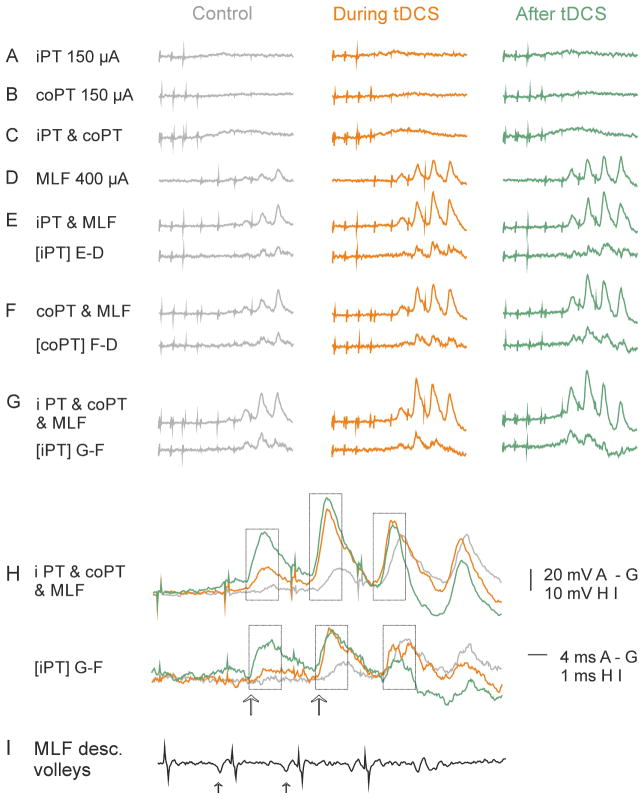
As shown in Table 1, stimulation of iPT after tDCS was found to facilitate synaptic actions evoked from the MLF in 32% of motoneurons before tDCS but in 69% of motoneurons after tDCS (difference significant at p<0.01, z-test). EPSPs evoked by joint actions of iPT and MLF, or coPT and MLF were facilitated in similar proportions of motoneurons, 32% vs 33% before and 69% vs 64% after tDCS respectively, both differences being statistically significant. Similar degree of facilitation of effects of stimulation of iPT and of coPT after tDCS is further illustrated in Fig 5B, C, E and F. The facilitatory effects are reflected in larger amplitudes of EPSPs evoked from iPT or coPT stimulated jointly with MLF (black) than of EPSPs of MLF origin (grey). In particular they show that both iPT and coPT could make EPSPs to appear following the earlier originally ineffective MLF stimuli after tDCS (arrows in Fig. 5B,C and E,F). On the average iPT stimuli shortened latency of EPSPs of MLF origin from the first stimulus by 0.85 ms before tDCS and by 2.63 ms during and after tDCS (p<0.05; t-test paired data from 10 and 12 motoneurons respectively; Fig 5H). Amplitudes of EPSPs from the MLF were increased by PT stimuli to a different extent depending on their amplitude, the medium size EPSPs within the range of 150–200% but the smallest EPSPs several-fold (Fig. 5I).
Facilitation of IPSPs evoked from the MLF by iPT stimuli was likewise more effective during and after than before tDCS. In the total sample of 9 and 14 motoneurons recorded before and during or after tDCS the difference was most marked in the latencies of IPSPs measured from the first MLF stimulus (Fig. 5H). These were shortened by iPT stimuli by 0.36 ms in the control sample but by 2.26 ms after tDCS (though not statistically significant, p>0.5). The peak amplitude of IPSPs evoked by the first effective stimuli in these two samples increased then to a similar extent (to 187% and 197% respectively; statistically significant, p<0.5). However, IPSPs evoked in the same sample of motoneurons by the second effective stimuli were almost twice more enhanced after tDCS than those evoked by the first stimuli. The estimates of the degree of facilitation of the IPSPs depended also on the state of the motoneurons after the penetration. More representative might therefore be parallel changes in the latency and the size of the IPSPs found in 3 motoneurons recorded from before and during tDCS. Thus in the motoneuron illustrated in Fig. 6, IPSPs following the third MLF stimuli from the end were only weakly facilitated by iPT and/or coPT stimulation before tDCS and during the first periods of tDCS (Fig. 6B), but they substantially increased during the 3rd and 4th periods of tDCS and after tDCS, when the first IPSPs appeared following an earlier stimulus (arrows in Fig. 6C–E). Stimulation of iPT combined with stimulation of coPT and MLF resulted in the appearance of IPSPs following two earlier stimuli (indicated by the two arrows in Fig. 6D) and these IPSPs were also much larger. As joint actions of coPT and MLF (third column) were not much stronger than of MLF alone, the facilitatory effects in the last column must have been linked to the additional actions of iPT.
Figure 6. Examples of facilitation of IPSPs evoked from MLF during and after tDCS.
Records from a single GS motoneuron held through tDCS application. (A), control records before tDCS by various combinations of the stimuli as indicated. Note facilitation of IPSPs evoked by the last two MLF stimuli by either co or i PT stimulated separately or jointly. (B–E), records of IPSPs evoked by the same combinations of stimuli during the 1st, 3rdth and 4th periods of tDCS and 10 min after the last tDCS as indicated. Note facilitation of IPSPs evoked by not only the last two MLF stimuli but also an earlier stimulus after the 3th tDCS and two earlier stimuli during the 4th tDCS. Large arrows indicate earliest IPSPs facilitated by iPT. Small arrows indicate MLF volleys followed by these IPSPs. Vertical dotted lines indicate onset of IPSPs evoked by MLF stimuli. Other indications are as in Fig. 2. The sites of recording and stimulation and the underlying convergence of iPT, co PT and MLF fibres on reticulospinal neurons were as indicated in the diagram in Fig. 6.
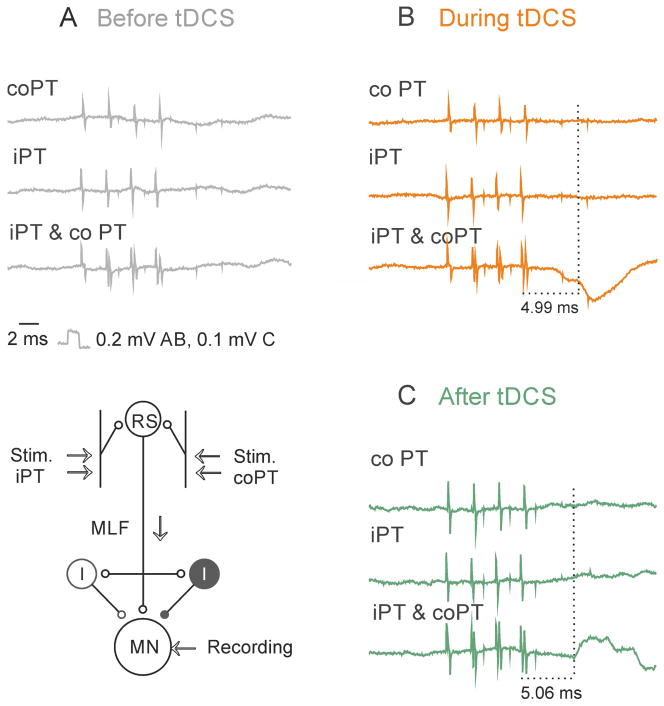
Effects of tDCS on VR discharges
Because a comparison of PSPs evoked in the same motoneurons before and after tDCS was only possible in a small sample of motoneurons, we supplemented records from individual motoneurons with records of discharges of populations of motoneurons with axons in one of the ventral roots.
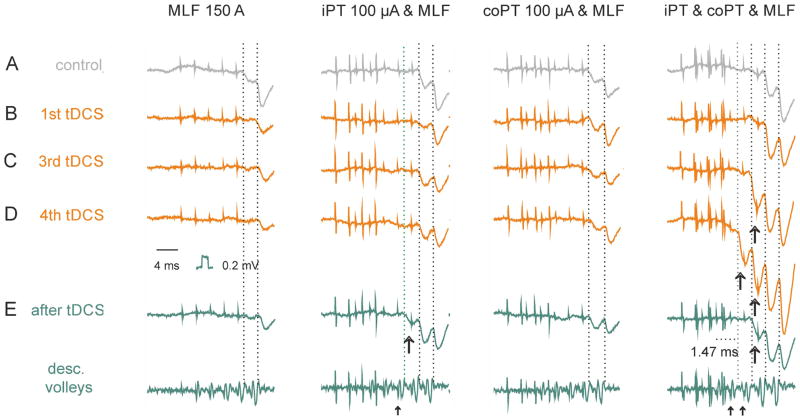
Facilitation of iPT actions by tDCS was found in all 5 experiments in which it was assessed from changes in VR discharges. Prior to tDCS VR discharges failed to appear in these experiments upon stimulation of either iPT or coPT, and even both iPT and coPT. MLF stimuli alone likewise failed to evoke any discharges in two of these experiments though weak discharges appeared when MLF stimuli were combined with PT stimuli. However, in all five experiments the situation changed dramatically during tDCS. As illustrated in Fig. 7E (middle column) MLF stimuli combined with coPT stimuli became much more effective already during the first period of tDCS and the discharges evoked by these stimuli increased and appeared at shorter latencies during and after the subsequent tDCS periods (Fig. 7E right column). Maximal facilitation was evoked when iPT stimuli were added to a combination of coPT and MLF stimuli (Fig. 7F and G, middle and right columns), indicating that jointly applied MLF, iPT and coPT stimuli activated a considerably greater number of motoneurons. In the total sample of discharges evoked in 5 experiments, PT stimuli decreased the latencies of MLF discharges from the first stimulus by 2.5 ms before tDCS, by 4.5 ms during tDCS and by 3.5 ms after tDCS respectively.
Figure 7. Examples of activation of motoneurons (VR discharges) by iPT, coPT and MLF stimulation after tDCS.
(A–F), Averaged records (n=20) of discharges of motoneurons in the S1 VR evoked by different combinations of iPT, coPT and MLF stimuli, as indicated to the left, after but not before tDCS. Left panels, control records. Middle and right panels, records during the first minute of the first and seventh periods of application of tDCS. (G), Addition of effects from iPT when stimulated together with coPT and MLF (records from row (F) minus records from row (E). Dotted vertical lines indicate the onset of discharges evoked at shortest (in F and G) and longest (in D and E) latencies. Note increase in amplitude of both the earliest and later components of VR discharges and shortening of latencies of discharges evoked by the most efficient combinations of the stimuli during tDCS. Note also that the earliest discharges after tDCS were evoked during the time window between the two dotted lines in (D–G) during which no discharges were evoked by either MLF or coPT stimuli; iPT stimulation must have been responsible for these early responses. (H), time course of changes in the areas of records of ventral root discharges following combined stimulation of MLF preceded by stimulation of both iPT and coPT, i.e. records illustrated in row (F). The measurements were made during the time window between the two dotted lines in (D–G). Ordinate, areas in % of control areas; abscissa, time in minutes from the beginning of the first period of tDCS. Dotted horizontal line indicates the original area. The diagram indicates the sites of recording and stimulation and the underlying convergence of iPT, co PT and MLF fibres on reticulospinal neurons discharging the motoneurons, either directly or via spinal interneurons.
Using changes in the area of VR discharges illustrated in Fig. 7A–G as a measure of facilitation, the time course of these changes has been plotted in Fig. 7H. The plot shows that the degree of facilitation tended to increase between the second and seventh periods of tDCS (filled circles) and during the immediately following 5 min periods (triangles). The increase continued during the first 20 minutes of post-polarization period before declining to some extent, but it remained at a level exceeding that of control records for at least 30 min. When the data from all polarization and post-polarization periods were pooled together and compared to control, they were found to be significanlly different (ANOVA, F(2, 12)=7.31, p<0.01, Holm-Sidak post-hoc test p<0.05).
Contribution of iPT effects is indicated by subtracting VR discharges evoked by coPT and MLF stimuli from those evoked by iPT, coPT and MLF stimuli (row G). Another expression of the facilitatory effects of tDCS on iPT actions was the shortening of latencies of the discharges (arrows at the level of the first dotted vertical lines) evoked when iPT stimuli were added to coPT and MLF stimuli in Fig. 7F.
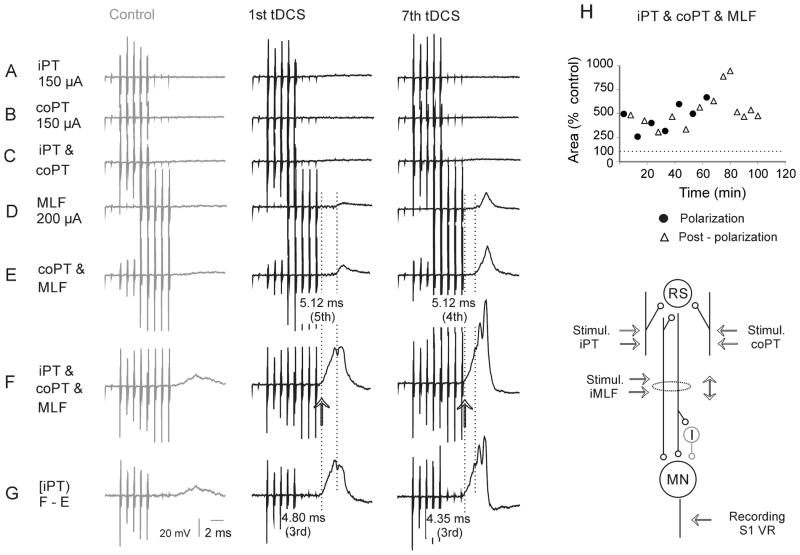
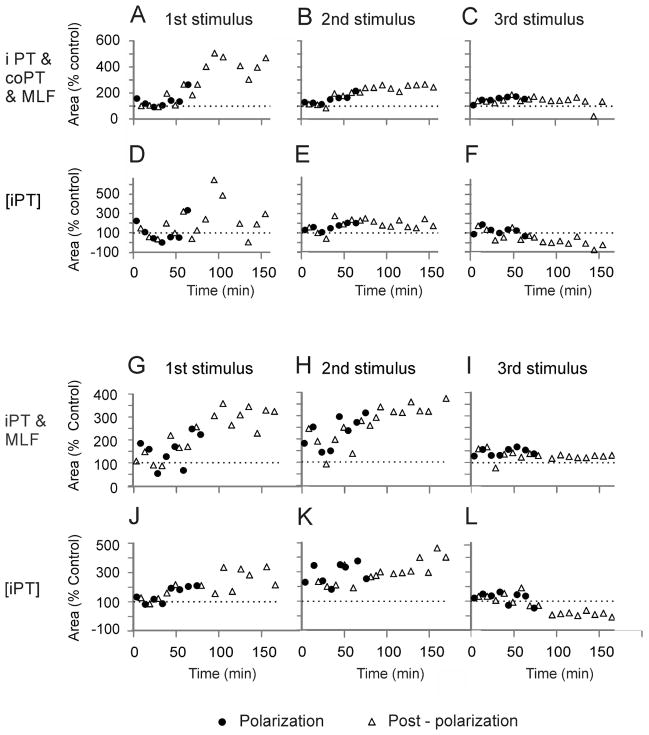
In three of the five experiments in which MLF stimuli evoked weak responses already before tDCS, though not from PTs (Fig. 8A–D), iPT stimuli enhanced them to a greater extent during and after than before tDCS (Fig. 8E) and to a similar extent as coPT stimuli (Fig. 8F). The superimposed records in Fig. 8H show that the facilitation of discharges following the first and the second MLF stimuli was particularly marked (arrows) while facilitation of EPSPs evoked by the third stimuli in a train was less potent. This is also reflected in the plots of timing of tDCS effects on VR discharges examined during 2 hours’ postpolarization periods in two experiments (Fig. 9A–F and G–L). When data from all polarization or postpolarization periods were pooled together and compared to control data, only the effects of the 2nd stimulus in a train were found to be significantly different (ANOVA F(3, 28) = 19.328, p<0.01, ANOVA F(3, 26) = 4.522 p=0.011 for Fig. 9B and E respectively, and ANOVA F(3, 34) = 18.065, p<0.01, ANOVA F(3, 32) = 5.036, p=0.006, for Fig. 9H and K respectively, all with post-hoc tests to control at p<0.05, Holm-Sidak method).
Figure 8. Facilitation of activation of a population of motoneurons (VR discharges) by iPT stimuli combined with coPT and MLF stimuli during and after tDCS.
(A–G), Averaged records (n=20) of discharges of motoneurons from the S1 VR evoked by different combinations of iPT, coPT and MLF stimuli as in Fig. 7. Net contributions from iPT are indicated by lower traces - computer generated differences between records indicated to the left. Left panels, control records. Middle and right panels, records during the 7th period of tDCS and 30 min after its last application. (H), superimposed expanded records from (G), illustrating both increases in the discharges and the shortening of their latencies. (I), records of descending volleys from the cord dorsum. Arrows in (H) indicate strongest effects of tDCS. Arrows in (I) indicate MLF volleys followed by EPSPs shown in (H). For the time course of effects of tDCS see Fig. 9A–C.
Figure 9. Time course of facilitation of activation of motoneurons (VR discharges) by successive co PT and MLF stimuli preceded by iPT stimuli during and after tDCS.
(A–C), Time course of changes in the areas of discharges exemplified in Fig. 8(G) and (H), following the 1st, 2nd and 3rd MLF stimuli preceded by iPT and coPT, respectively; the areas were measured within the time windows indicated by dotted boxes in Fig. 8(H). (D–F), Time course of the corresponding changes in the net effects of iPT stimuli. Note that they were most consistent after the 2nd stimulus. (G–L), time course of changes in the areas of discharges evoked following the MLF stimuli preceded by only iPT stimuli evoked in another experiment. Note the generally similar effects of tDCS. The sites of recording and stimulation and the underlying convergence of iPT, co PT and MLF fibres on reticulospinal neurons discharging the motoneurons, either directly or via spinal interneurons were as indicated in the diagram in Fig. 7.
In explaining a weaker facilitation of VR discharges evoked by the last MLF stimuli in the stimulus train we would favour the possibility that facilitation of excitation of motoneurons by these stimuli after tDCS was counteracted by an increase in facilitation of IPSPs evoked in motoneurons, which would reduce EPSPs that preceded them.
Time course of facilitation of actions of iPT neurons by tDCS
The time course of the enhancement of intracellularly recorded PSPs of iPT origin by tDCS could not be defined because the size of these PSPs greatly depended on changes in the state of the penetrated motoneurons. However, this problem was obviated by using facilitation of VR discharges evoked by iPT stimuli as a measure of changes in the excitatory input from iPT to motoneurons.
The plots in Figs. 7 and 9 show that the facilitation of VR discharges developed either from the very onset of tDCS application or only during the 3rd–4th periods of tDCS. They also show that the facilitation outlasted tDCS not only during the 5 min periods immediately following the termination of tDSC but also by up to 2 hours, with a facilitation of at least 20% remaining for 30 min or more in all of the 5 experiments in which it was tested on VR discharges.
Taken together, the results of this study lead to the conclusion that the time course of facilitation of iPT related excitation of motoneurons by tDCS replicates the time course of similarly prolonged facilitation of descending volleys evoked by stimulation of axons of corticospinal neurons as well as of reticulospinal neurons within the MLF (Bolzoni et al., 2013a; Bolzoni et al., 2013b).
Control experiments and additional observations for defining mechanisms underlying enhanced activation of motoneurons
As tDCS applied above the peri-cruciate cortex is unlikely to have had any direct effects at the spinal level, the reported results leave us with supraspinal facilitatory effects of tDCS. Furthermore, as input from the coPT or the MLF stimulated separately did not suffice to activate reticulospinal neurons as efficiently as when combined with iPT stimuli, the facilitatory effects must have to a great extent involved transmission from iPT.
However, the degree to which a combination of iPT, coPT and MLF stimuli was effective might have also depended on the degree of excitability of motoneurons and any spinal relay neurons. The appearance of the facilitation from the onset of tDCS links it to tDCS but does not exclude tonic excitatory actions on spinal neurons set up by tDCS in addition to facilitation of phasic actions from the MLF and/or to changes in the excitability of spinal neurons due to other factors. Control experiments were therefore performed to allow estimates of the contribution of some of these factors.
Enhancement of VR discharges after tDCS vs increase in the excitability of motoneurons
In order to estimate tonic changes in the excitability of motoneurons we compared monosynaptic reflexes recorded from the same VR before, during and after tDCS. Monosynaptic reflexes were evoked by stimulation of the GS and PBST nerves supramaximal for group I afferents. While the amplitudes of monosynaptic reflexes varied to some extent during successive periods of recording, in all of these experiments the variations were not related to changes in VR discharges evoked by MLF stimulation. In two of five experiments experiments the facilitation of VR discharges coincided with an increase in monosynaptic reflexes. In two experiments the VR discharges were facilitated while monosynaptic reflexes decreased and in one experiment the facilitation of VR discharges coincided with both increases and decreases of monosynaptic reflexes. Hence there are no reasons to attribute facilitation of effects of stimulation of iPT on hindlimb motoneurons by tDCS to a tonic increase in the excitability of motoneurons.
Enhancement of VR discharges after tDCS vs an increase in the general state of excitability of spinal neurons
The diagrams in Figs. 4 and 5 indicate that the most directly mediated actions of iPT stimuli would require di- or trisynaptic coupling between PT fibres and motoneurons, disynaptic actions being relayed by reticulospinal neurons and trisynaptic actions by both reticulospinal neurons and spinal interneurons and tDCS could influence excitability of these neurons. Effects secondary to variations in the depth of anaesthesia could not be estimated. However, the plots of the facilitatory effects of tDCS in Fig. 7 and 9, show that the facilitation reached a certain plateau, or started to decline during the testing periods, which does not seem to comply with the changes related to the decreasing depth of anaesthesia. No correlation was either found between the periods of testing of effects of tDCS and the time elapsed since the induction of the anaesthesia, as marked facilitation by tDCS was also found when its application was delayed by one, two or three hours. The time course of effects of tDCS does not either comply with changes in the circulatory system in view of the stability of the blood pressure and of the heart rate during periods preceeding or following the plateau or decline of tDCS effects. For all these reasons we would tend to relate any tonic changes in the excitability of spinal relay neurons to effects of tDCS, rather than to the state of the animal.
Discussion
The reported results reveal that facilitatory effects of tDCS are not sufficient to increase the probability of activation of spinal motoneurons by iPT stimuli alone, at least not hindlimb motoneurons in the cat and not under conditions of acute experiments under deep anaesthesia. However we demonstrate that tDCS potently increases the probability of activation of motoneurons by joint actions of iPT and coPT stimuli and even more so by joint actions of iPT together with coPT and MLF. The facilitated actions included both di- or trisynaptically evoked EPSPs and IPSPs and are fully consistent with facilitation of activation of reticulospinal neurons by iPT neurons.
Reasons for concluding that facilitation of ipsilateral actions of PT neurons on motoneurons by tDCS involves activation of reticulospinal neurons by iPT stimuli
Previous studies revealed effects of tDCS on subcortical neurons. Thus studies in humans demonstrated that tDCS can modify actions evoked by some subcortical neurons, in particular via cortico-striatal and thalamo-cortical connections (Polania et al., 2011) and that it induces changes in the regional cerebral blood flow in the brain, including such subcortical structures as the thalamus, globus pallidus and nucleus accumbens (Lang et al., 2005) but also the red nucleus and the reticular formation (see Fig. 10 in Bolzoni et al., 2013b). In recent studies we found that subcortical effects of tDCS extend to neurons located in the mesencephalon and medulla in the cat and rat (Bolzoni et al., 2013a; Bolzoni et al., 2013b) but tDCS by itself is unlikely to affect spinal neurons located farther away, even those in the upper cervical segments.
By testing effects of tDCS on responses evoked by stimuli subthreshold when applied to either the ipsilateral or the contralateral medullary pyramid (PT) or to the MLF, but effective when applied to both the PTs and the MLF, we could link the reported effects of tDCS to neurons co-excited by axon-collaterals of PT and MLF. Of such neurons we could eliminate antidromically activated cortical neurons in view of lack of evidence for convergence of axon collaterals of iPT, coPT and reticulospinal neurons on corticospinal neurons. Lack of such convergence rendered it similarly unlikely that other subcortical neurons, possibly affected by MLF stimuli, are involved. This would be particularly unlikely for vestibulospinal neurons with direct input from reticulospinal but not from PT neurons, and especially not from the motor cortex (see Wilson et al., 1999). It would be also unlikely for propriospinal neurons in upper spinal segments which are coexcited by coPT and reticulospinal neurons (see e.g. Alstermark & Isa, 2002) but not by iPT neurons (B. Alstermark, personal communication).
In contrast, all the available evidence is compatible with the facilitation by tDCS of activation of reticulospinal neurons co-excited by iPT, coPT and MLF stimuli which in turn would be more effective in mediating di-or trisynaptic excitation of hindlimb motoneurons. Thus increases in indirect MLF evoked descending volleys recorded at the level of C1–C2 spinal segments demonstrated facilitation of transsynaptically evoked activation of reticulospinal neurons (Bolzoni et al., 2013a). In lumbar motoneurons, the earliest components of EPSPs enhanced by tDCS were delayed with respect to the indirect MLF volleys by only about 1 ms, being compatible with monosynaptic actions of these volleys, while latencies of IPSPs were about 1 ms longer and indicated actions evoked via an additional spinal interneuron. The earliest components of discharges in motoneurons’s axons were delayed with respect to EPSPs by < 1 ms and therefore compatible with a marginal supplementary delay for initiating action potentials in motoneurons and the conduction time to the ventral roots. Taken together, these delays were thus compatible with responses directly evoked by fibres stimulated in the MLF but would be too short to involve actions of additional neurons activated by axon collaterals of either the MLF or PT fibres. However, they do not exclude that later components of responses facilitated by tDCS were mediated by disynaptic rather than monosynaptic reticulospinal pathways and that the facilitation of activation of interneurons in these disynaptic pathways may be likewise enhanced by input from reticulospinal neurons.
Plasticity of coupling between iPT neurons and reticulospinal neurons
The increasingly stronger facilitatory and depressive effects of iPT stimulation on motoneurons during successive periods of tDCS replicate the time course of effects of tDCS on descending volleys in axons of subcortical neurons (Bolzoni et al., 2013b). Both might thus be compatible with accumulating background effects which outlast the previous periods of tDCS application. In addition, the slow accumulation of changes seen after the successive tDCS periods seemed to continue, at least in some experiments, after the last tDCS period, as in the larger material of (Bolzoni et al., 2013a) and is in line with observations that the duration of after-effects of tDCS in humans depends on the number and length of tDCS applications (Monte-Silva et al., 2010; Fricke et al., 2011).
Effects of tDCS may involve postsynaptic neurons as well as presynaptic fibres that provide input to them and different aspects of synaptic transmission modified by tDCS may be subject to plastic changes (Kabakov et al., 2012; Ranieri et al., 2012; Edwardson et al., 2013; Rahman et al., 2013). Modulation of transmission between PT fibres and reticulospinal neurons by tDCS would represent only one of several cases of plasticity at subcortical levels. Functional changes secondary to the use, disuse and specific training have been found at various sites, including the spinal cord (Schwab et al., 2001; Wolpaw & Carp, 2006; Martin et al., 2007; Schwab, 2010; Martinez & Rossignol, 2013). Nevertheless, very little is known about the degree of plasticity related to the operation of reticulospinal neurons, even though they are of major importance for many centrally initiated movements (see e.g. Lundberg, 1982; Jordan et al., 2008) and for motor recovery after central injuries. E.g. it has been demonstrated that reticulospinal neurons contribute to restitution of skilled digit grasping movements in the cat after transection of the rubro- and corticospinal tracts within the cervical spinal cord (Alstermark et al.,1981, cf also Pettersson et al., 2007) while in primates the role of reticulospinal neurons in recovery of digit movements is still under discussion (Lawrence & Kuypers, 1968a; b; Zaaimi et al., 2012; Isa et al., 2013).
Relevance of the results of this study for enhancing ipsilateral actions of pyramidal tract neurons by tDCS in humans
Weak ipsilateral PT actions may be strengthened when evoked concurrently with activation of reticulospinal neurons both in the cat and in primates including humans (Edgley et al., 2004; Stecina & Jankowska, 2007; Baker, 2011; Fisher et al., 2012; Zaaimi et al., 2012). As iPT actions may be further strengthened by tDCS, the reported results lead to the conclusion that tDCS may assist in substituting actions of injured contralateral PT neurons facilitate the actions of surviving neurons. Even though our results have been obtained in experiments on anaestetised cats there are should be no reasons to doubt that they would also apply to primates, including humans.
As in the cat, primate reticulospinal neurons control not only motoneurons (Riddle et al., 2009) but also interneurons in spinal premotor networks that receive input from both reticulospinal and corticospinal tract fibres (Riddle & Baker, 2010). It might thus be predicted that ipsilateral PT neurons contributing to activation of reticulospinal neurons (Fisher et al., 2012) would make as big difference in primates as in the cat and assist limb movements, together with reticulospinal neurons and other neuronal systems important for recovery of motor functions. It might also be predicted that any factors enhancing ipsilateral PT actions including tDCS would play an essential role in tiping the balance when conditions for initiating motor responses via corticospinal and/or reticulospinal neurons are critical.
Knowledge of conditions under which tDCS most effectively facilitates iPT actions should help to selecte the circumstances under which it could be used to the greatest advantage. The results of the present study indicate that tDCS would be most effective in facilitating movements mediated by reticulospinal neurons co-excited by nerve impulses from both the ipsilateral and contralateral PT neurons. In humans such co-excitation would be most likely to occur during mutually supportive bimanual movements, and when PT and reticulospinal actions are combined (see Zaaimi et al., 2012; Dragert & Zehr, 2013; Edwardson et al., 2013). It may thus be predicted that operation of neuronal networks activated during bimanual movements would be improved during tDCS and hopefully also during long-lasting post tDCS periods.
tDCS should also facilitate iPT contribution to activation of reticulospinal neurons by any other sources of input to them. Both feline and primate spinal interneurons and reticulospinal neurons are coexcited by muscle afferents (Jankowska & Stecina, 2007; Riddle & Baker, 2010). The addition of peripheral input to other sources of input to these neurons would thus increase the probability of their activation and several observations on humans already substantiate such possibilities. E.g. ipsilateral motor evoked potentials, likely to be mediated via reticulospinal tract neurons, are easier to evoke against a background muscle contractions (Alagona et al., 2001) or lengthening contractions (Howatson et al., 2011) which would provide input to both spinal interneurons and reticulospinal neurons from muscle and skin receptors. It has also been demonstrated that static arm posture can modulate voluntary distal muscle activity in the paretic upper limb in post stroke subjects as well as in neurologically intact subjects (Dominici et al., 2005; Ginanneschi et al., 2005; Hoffmann et al., 2011). The results of the present study indicate that a further beneficial effect would be expected when tDCS is applied under such conditions.
Acknowledgments
Funding
The work was supported by the National Institutes of Health (grant number R01 NS040863 to E.J.) and by the University of Gothenburg.
We wish to thank Dr S. Edgley and Dr I. Hammar for comments on earlier versions of this paper and Jytte Grännsjö for excellent technical assistance during the experiments and with the histological control.
Abbreviations
- C
cervical
- Co
contralateral
- EMG
electromyographic
- EPSPs
excitatory postsynaptic potentials
- IPSPs
inhibitory postsynaptic potentials
- I
ipsilateral
- L
lumbar
- MLF
medial longitudinal fascicle
- PT
pyramidal tract
- RN
Red Nucleus
- RS
reticulospinal
- T
threshold
- tDCS
transcranial direct current stimulation
- TMS
transcranial magnetic stimulation
- VR
ventral root
Footnotes
The authors have no conflict of interest to declare.
References
- Alagona G, Delvaux V, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ, Nicoletti F, Maertens de Noordhout A. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke. 2001;32:1304–1309. doi: 10.1161/01.str.32.6.1304. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T. Premotoneuronal and direct corticomotoneuronal control in the cat and macaque monkey. Adv Exp Med Biol. 2002;508:281–297. doi: 10.1007/978-1-4615-0713-0_34. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol (Lond) 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) during Current Flow and (2) in the Production of Long-Lasting after-Effects. J Physiol (Lond) 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Baczyk M, Jankowska E. Subcortical effects of transcranial direct current stimulation in the rat. J Physiol (Lond) 2013a;591:4027–4042. doi: 10.1113/jphysiol.2013.257063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Pettersson LG, Jankowska E. Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat. J Physiol (Lond) 2013b;591:3381–3399. doi: 10.1113/jphysiol.2012.244764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:96–101. doi: 10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Exp Brain Res. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Canedo A, Lamas JA. Pyramidal and corticospinal synaptic effects over reticulospinal neurones in the cat. J Physiol (Lond) 1993;463:475–489. doi: 10.1113/jphysiol.1993.sp019606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Kimura H, Berrol LJ, Martin JH. Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury. Eur J Neurosci. 2013;37:1090–1102. doi: 10.1111/ejn.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Kimura H, Martin JH. Electrical Stimulation of Motor Cortex in the Uninjured Hemisphere after Chronic Unilateral Injury Promotes Recovery of Skilled Locomotion through Ipsilateral Control. J Neurosci. 2014;34:462–466. doi: 10.1523/JNEUROSCI.3315-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Popa T, Ginanneschi F, Mazzocchio R, Rossi A. Cortico-motoneuronal output to intrinsic hand muscles is differentially influenced by static changes in shoulder positions. Exp Brain Res. 2005;164:500–504. doi: 10.1007/s00221-005-2270-5. [DOI] [PubMed] [Google Scholar]
- Dragert K, Zehr EP. High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp Brain Res. 2013;225:93–104. doi: 10.1007/s00221-012-3351-x. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson MA, Lucas TH, Carey JR, Fetz EE. New modalities of brain stimulation for stroke rehabilitation. Exp Brain Res. 2013;224:335–358. doi: 10.1007/s00221-012-3315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol (Lond) 2012;590:4045–4060. doi: 10.1113/jphysiol.2011.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–1149. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- Ginanneschi F, Del Santo F, Dominici F, Gelli F, Mazzocchio R, Rossi A. Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp Brain Res. 2005;161:374–382. doi: 10.1007/s00221-004-2084-x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol (Lond) 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XW, Wu CP. Connections between pericruciate cortex and the medullary reticulospinal neurons in cat: an electrophysiological study. Exp Brain Res. 1985;61:109–116. doi: 10.1007/BF00235626. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Schmit BD, Kahn JH, Kamper DG. Effect of sensory feedback from the proximal upper limb on voluntary isometric finger flexion and extension in hemiparetic stroke subjects. J Neurophysiol. 2011;106:2546–2556. doi: 10.1152/jn.00522.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatson G, Taylor MB, Rider P, Motawar BR, McNally MP, Solnik S, DeVita P, Hortobagyi T. Ipsilateral motor cortical responses to TMS during lengthening and shortening of the contralateral wrist flexors. Eur J Neurosci. 2011;33:978–990. doi: 10.1111/j.1460-9568.2010.07567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Kinoshita M, Nishimura Y. Role of Direct vs. Indirect Pathways from the Motor Cortex to Spinal Motoneurons in the Control of Hand Dexterity. Front Neurol. 2013;4:191. doi: 10.3389/fneur.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneurons. In: Pfaff DW, Martin E, editors. Neuroscience in the 21st Century. Springer Verlag; 2012. p. 3200. [Google Scholar]
- Jankowska E, Cabaj A, Pettersson LG. How to enhance ipsilateral actions of pyramidal tract neurons. J Neurosci. 2005;25:7401–7405. doi: 10.1523/JNEUROSCI.1838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K. Uncrossed actions of feline corticospinal tract neurones on lumbar interneurones evoked via ipsilaterally descending pathways. J Physiol (Lond) 2007;580:133–147. doi: 10.1113/jphysiol.2006.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson L-G, Edgley SA. Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones. J Physiol (Lond) 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan L, Liu J, Hedlund P, Akay T, Pearson K. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol. 2012;107:1881–1889. doi: 10.1152/jn.00715.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Control of spinal mechanisms from the brain. In: Tower DB, editor. The Basic Neurosciences. Raven Press; New York: 1975. pp. 253–265. [Google Scholar]
- Lundberg A. Inhibitory control from the brain stem of transmission from primary afferents to motoneurones, primary afferent terminals and ascending pathways. In: Sjölund B, Björklund A, editors. Brain Stem Control of Spinal Mechnisms. Elsevier Biomedical Press; Amsterdam: 1982. pp. 179–225. [Google Scholar]
- Martin JH, Friel KM, Salimi I, Chakrabarty S. Activity- and use-dependent plasticity of the developing corticospinal system. Neurosci Biobehav Rev. 2007;31:1125–1135. doi: 10.1016/j.neubiorev.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Rossignol S. A dual spinal cord lesion paradigm to study spinal locomotor plasticity in the cat. Ann N Y Acad Sci. 2013;1279:127–134. doi: 10.1111/j.1749-6632.2012.06823.x. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clin Neurophysiol. 2009;120:1183–1187. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche MA. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) J Neurophysiol. 2010;103:1735–1740. doi: 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Isa T. Cortical and subcortical compensatory mechanisms after spinal cord injury in monkeys. Exp Neurol. 2012;235:152–161. doi: 10.1016/j.expneurol.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke B. The Circuitry of the Human Spinal Cord; Spinal and Corticospinal Mechanisms of Movement. Cambridge University Press; 2012. pp. 1–632. [Google Scholar]
- Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular Activities and Evoked Potential Changes during Polarization of Motor Cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. Cellular Effects of Acute Direct Current Stimulation: Somatic and Synaptic Terminal Effects. J Physiol. 2013;591:2563–2578. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Bareyre FM, Schwab ME. Reorganization of descending motor tracts in the rat spinal cord. Eur J Neurosci. 2002;16:1761–1771. doi: 10.1046/j.1460-9568.2002.02243.x. [DOI] [PubMed] [Google Scholar]
- Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, Pilato F, Di Lazzaro V, Grassi C. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 2012;107:1868–1880. doi: 10.1152/jn.00319.2011. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol. 2010;103:2821–2832. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Leppert CA, Kaps KH, Monnier PP. Functional recovery after spinal cord injury: basic science meets clinic. Trends Neurosci. 2001;24:437–439. doi: 10.1016/s0166-2236(00)01893-2. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 2010;11:799–811. doi: 10.1038/nrn2936. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stecina K, Jankowska E. Uncrossed actions of feline corticospinal tract neurones on hindlimb motoneurones evoked via ipsilaterally descending pathways. J Physiol (Lond) 2007;580:119–132. doi: 10.1113/jphysiol.2006.122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, Zarzecki P, Schor RH, Isu N, Rose PK, Sato H, Thomson DB, Umezaki T. Cortical influences on the vestibular nuclei of the cat. Exp Brain Res. 1999;125:1–13. doi: 10.1007/s002210050651. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Carp JS. Plasticity from muscle to brain. Prog Neurobiol. 2006;78:233–263. doi: 10.1016/j.pneurobio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]