Abstract
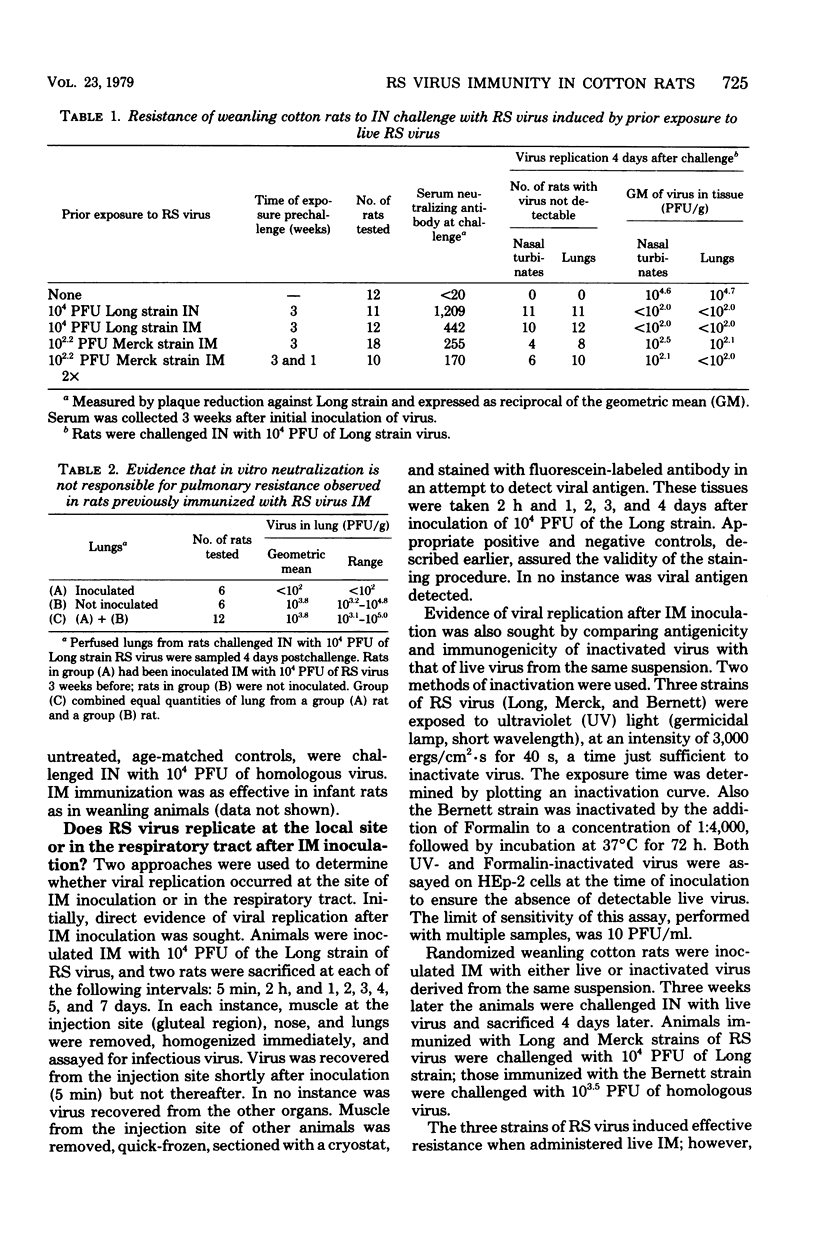
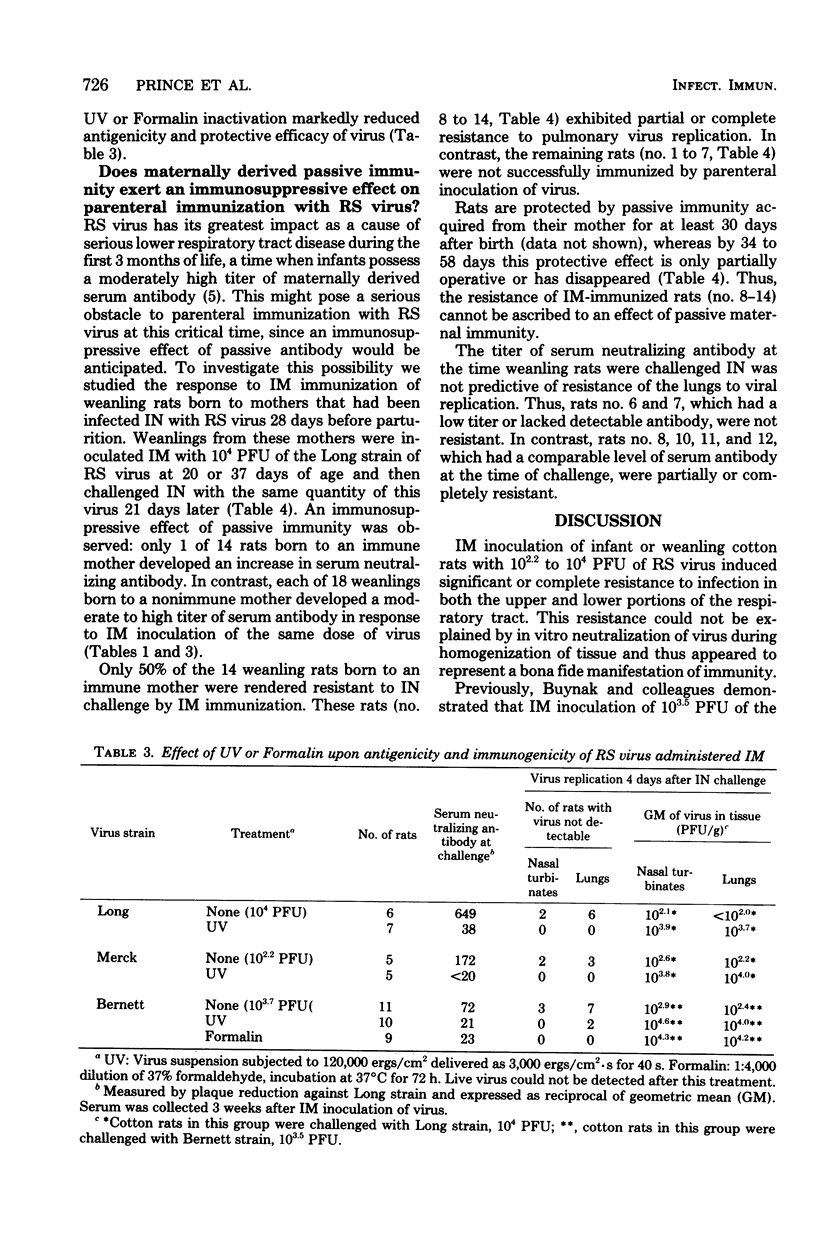
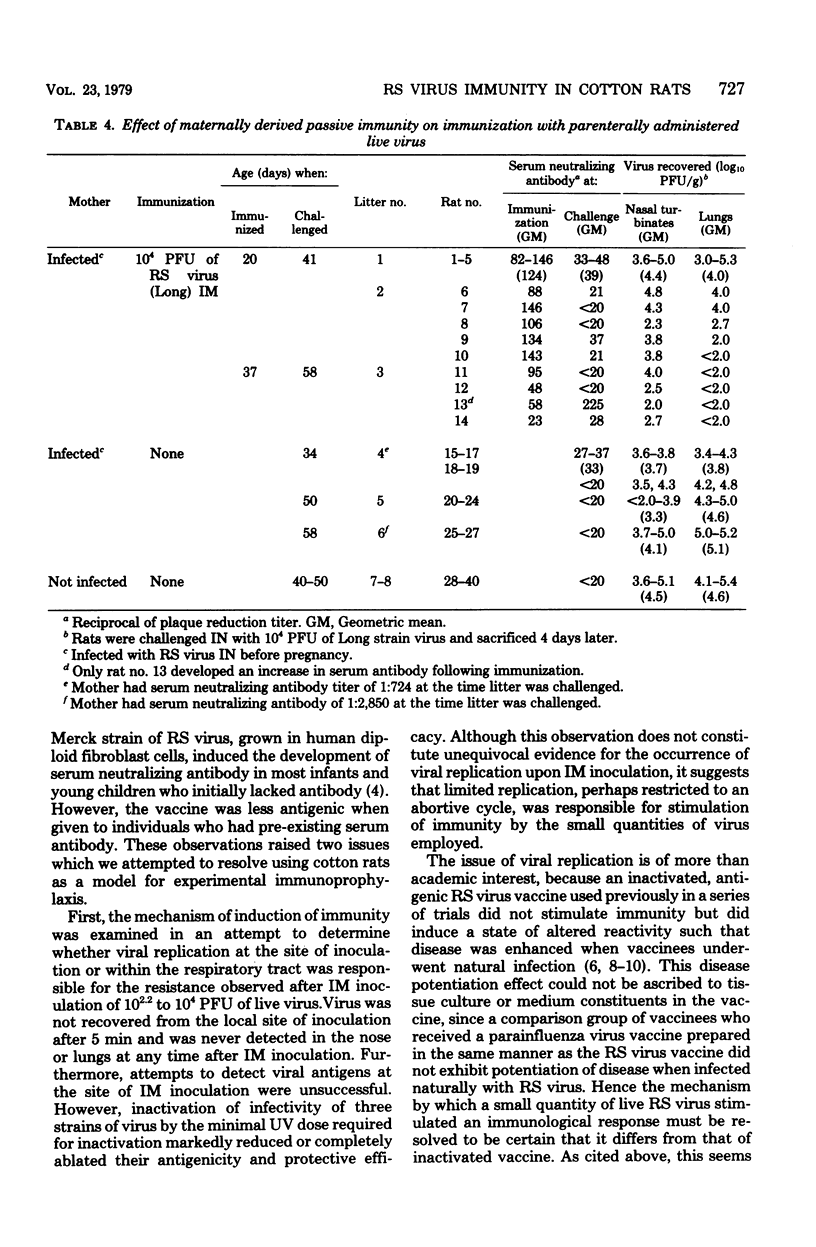
Intramuscular inoculation of infant or weanling cotton rats with 10(2.2) to 10(4) plaque-forming units of respiratory syncytial virus induced significant or complete resistance to infection in both the upper and lower portions of the respiratory tract. This resistance did not appear to be the result of in vitro neutralization of virus during homogenization of tissue. Virus was not recovered from the local site of inoculation after 5 min and was never detected in the respiratory tract of intramuscularly inoculated rats. Attempts to detect viral antigens at the site of inoculation by using indirect immunofluorescence were unsuccessful. However, inactivation of infectivity of three different strains of respiratory syncytial virus markedly reduced or completely ablated antigenicity and protective efficacy by the intramuscular route. This suggests that these viruses underwent limited replication, perhaps restricted to an abortive cycle, at the local site of inoculation. An immunosuppressive effect of passive maternally derived immunity was observed. Only 50% of weanling rats possessing passive maternal serum antibody were successfully immunized by intramuscular vaccination with live virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Richardson L. S., London W. T., Sly D. L., Camargo E., Prevar D. A., Chanock R. M. Evaluation of five temperature-sensitive mutants of respiratory syncytial virus in primates: II. Genetic analysis of virus recovered during infection. J Med Virol. 1978;3(2):101–110. doi: 10.1002/jmv.1890030203. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Richardson L. S., Schnitzer T. J., Prevar D. A., Camargo E., Chanock R. M. Further characterization of the complementation group B temperature-sensitive mutant of respiratory syncytial virus. J Virol. 1977 Oct;24(1):8–12. doi: 10.1128/jvi.24.1.8-12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynak E. B., Weibel R. E., McLean A. A., Hilleman M. R. Live respiratory syncytial virus vaccine administered parenterally. Proc Soc Exp Biol Med. 1978 Apr;157(4):636–642. doi: 10.3181/00379727-157-40112. [DOI] [PubMed] [Google Scholar]
- Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- Coates H. V., Alling D. W., Chanock R. M. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966 Mar;83(2):299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Horswood R. L., Camargo E., Chanock R. M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978 Dec;93(3):771–791. [PMC free article] [PubMed] [Google Scholar]
- Richardson L. S., Belshe R. B., London W. T., Sly D. L., Prevar D. A., Camargo E., Chanock R. M. Evaluation of five temperature-sensitive mutants of respiratory syncytial virus in primates: I. Viral shedding, immunologic response, and associated illness. J Med Virol. 1978;3(2):91–100. doi: 10.1002/jmv.1890030202. [DOI] [PubMed] [Google Scholar]
- Richardson L. S., Schnitzer T. J., Belshe R. B., Camargo E., Prevar D. A., Chanock R. M. Isolation and characterization of further defective clones of a temperature sensitive mutant (ts-1) of respiratory syncytial virus. Arch Virol. 1977;54(1-2):53–60. doi: 10.1007/BF01314378. [DOI] [PubMed] [Google Scholar]


