Abstract
Scanning electron microscopy, light microscopy, and quantitative culture of microorganisms in intestinal contents were used to determine the effects of oral tetracycline, the bacterial flora of conventionally reared animals (conventional), and thymus-dependent immune competency on the capacity of Candida albicans to colonize and infect the gastrointestinal tract of four groups of mice: thymus-intact conventional mice, conventional athymic mice, flora-defined athymic mice, and thymus-intact bacteria-free mice. Thymus-intact conventional mice without antibiotic treatment began to shed C. albicans less than 48 h after oral yeast challenge and were devoid of detectable yeast by day 16. Tetracycline altered the bacterial flora qualitatively and quantitatively, allowing C. albicans to colonize in less than 48 h and to persist in the gut tract for 32 days. Only 2 of 72 of these conventional mice developed candidiasis (hyphal infection). Although tetracycline altered the bacterial flora of conventional athymic (nude) mice, it was not required to allow C. albicans to colonize their gut tract to levels significantly higher than those in thymus-intact conventional mice. All conventional nude mice were consistently colonized and 14 of 24 animals showed an increased yeast colonization of the keratinized stomach, but only 3 of 24 developed gastric candidiasis. Flora-defined athymic (nude) mice had significantly lower aerobic bacterial levels and significantly higher C. albicans levels in the gut contents than conventional athymic mice. The flora-defined nude mice, however, developed gastric candidiasis by day 5. Thymus-intact bacteria-free mice were uniformly colonized and infected with C. albicans less than 48 h after oral challenge regardless of tetracycline treatment. Populations of C. albicans in the gut of bacteria-free mice were significantly higher than in the gut tract of the thymus-intact conventional or athymic mice. Gastric mycelial infection was detected in 8 of 10 bacteria-free animals 2 days after oral challenge. By 32 days, 45 of 50 mice of both tetracycline-treated and control bacteria-free groups were infected with C. albicans. These data indicate that a competive bacteria flora is more effective than an intact immune system in preventing gastric candidiasis and that an immune deficiency may allow increased yeast colonization of the keratinized and glandular stomach epithelium. Tetracycline did not appear to enhance the invasiveness or pathogenicity of C. albicans in mice even though it facilitates yeast-phase gut colonization in conventionally reared mice.
Full text
PDF

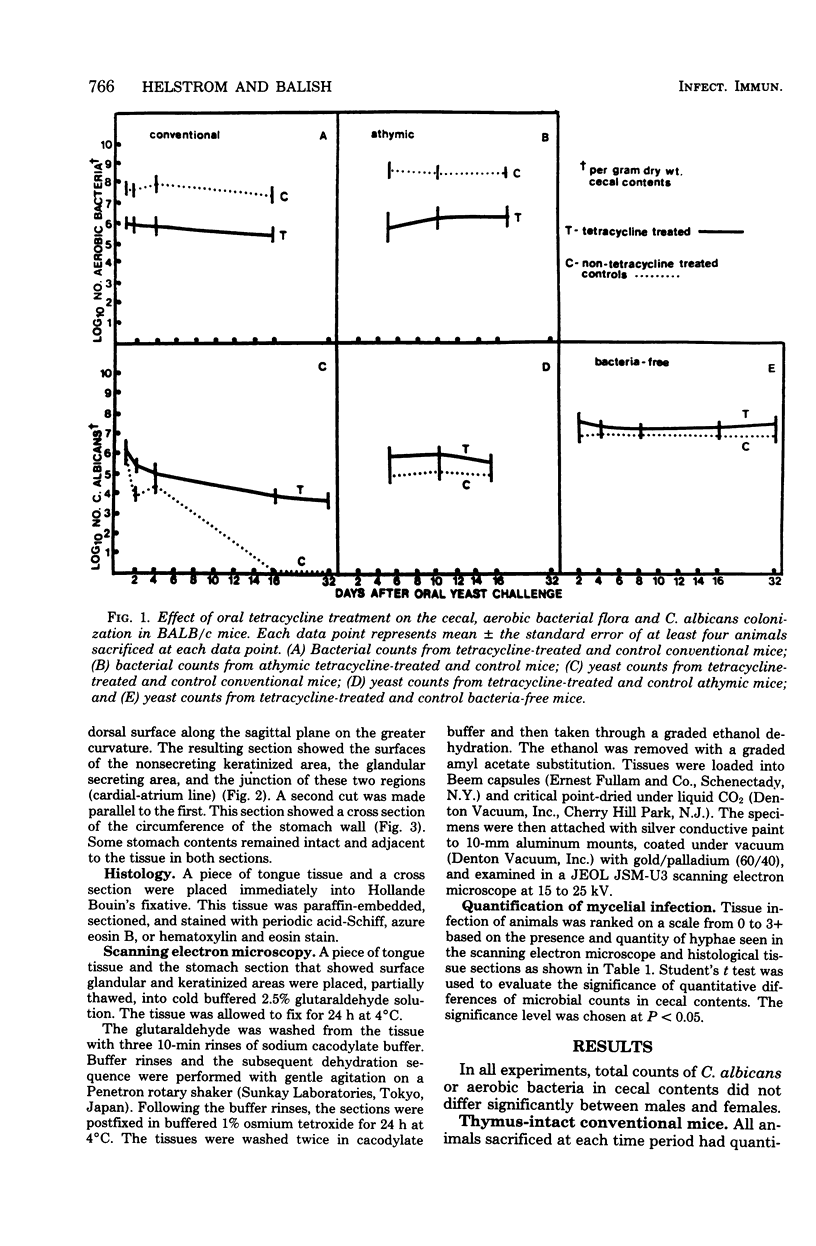








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D., Jones J. H. Life history of experimentally induced acute oral candidiasis in the rat. J Dent Res. 1971 May-Jun;50(3):643–644. doi: 10.1177/00220345710500032201. [DOI] [PubMed] [Google Scholar]
- Balish E., Phillips A. W. Growth, morphogenesis, and virulence of Candida albicans after oral inoculation in the germ-free and conventional chick. J Bacteriol. 1966 May;91(5):1736–1743. doi: 10.1128/jb.91.5.1736-1743.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. F., Balish E. Gastrointestinal microecology of BALB/c nude mice. Appl Environ Microbiol. 1978 Jul;36(1):144–159. doi: 10.1128/aem.36.1.144-159.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawson R. A. Induction of epithelial hyperplasia by Candida albicans. Br J Dermatol. 1973 Nov;89(5):497–503. doi: 10.1111/j.1365-2133.1973.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Celesk R. A., Asano T., Wagner M. The size pH, and redox potential of the cecum in mice associated with various microbial floras. Proc Soc Exp Biol Med. 1976 Feb;151(2):260–263. doi: 10.3181/00379727-151-39187. [DOI] [PubMed] [Google Scholar]
- Clark J. D. Influence of antibiotics or certain intestinal bacteria on orally administered Candida albicans in germ-free and conventional mice. Infect Immun. 1971 Dec;4(6):731–737. doi: 10.1128/iai.4.6.731-737.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Cutler J. E. Chemotactic factor produced by Candida albicans. Infect Immun. 1977 Dec;18(3):568–573. doi: 10.1128/iai.18.3.568-573.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P. Preservation of gastrointestinal bacteria and their microenvironmental associations in rats by freezing. Appl Environ Microbiol. 1976 Feb;31(2):304–312. doi: 10.1128/aem.31.2.304-312.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria A., Buckley H., von Lichtenberg F. Gastrointestinal candidiasis in rats treated with antibiotics, cortisone, and azathioprine. Infect Immun. 1976 Jun;13(6):1761–1770. doi: 10.1128/iai.13.6.1761-1770.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel R. P., Oestreicher E. J., Maley M. P., Macmillan B. G. Inhibition of Candida albicans by Escherichia coli in vitro and in the germfree mouse. J Surg Res. 1973 Jul;15(1):53–58. doi: 10.1016/0022-4804(73)90163-7. [DOI] [PubMed] [Google Scholar]
- Jones J. H., Adams D. Experimentally induced acute oral candidosis in the rat. Br J Dermatol. 1970 Dec;83(6):670–673. doi: 10.1111/j.1365-2133.1970.tb15762.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- MAIBACH H. I., KLIGMAN A. M. The biology of experimental human cutaneous moniliasis (Candida albicans). Arch Dermatol. 1962 Feb;85:233–257. doi: 10.1001/archderm.1962.01590020073009. [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Hatano H., Ohnishi N., Sasaki S., Nomura T. Establishment of Candida albicans in the alimentary tract of the germ-free mice and antagonism with Escherichia coli after oral inoculation. Jpn J Microbiol. 1969 Sep;13(3):263–276. doi: 10.1111/j.1348-0421.1969.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Phillips A. W., Balish E. Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol. 1966 Sep;14(5):737–741. doi: 10.1128/am.14.5.737-741.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Russell C., Jones J. H. Effects of oral inoculation of Candida albicans in tetracycline-treated rats. J Med Microbiol. 1973 Aug;6(3):275–279. doi: 10.1099/00222615-6-3-275. [DOI] [PubMed] [Google Scholar]
- Seelig M. S. Mechanisms by which antibiotics increase the incidence and severity of candidiasis and alter the immunological defenses. Bacteriol Rev. 1966 Jun;30(2):442–459. doi: 10.1128/br.30.2.442-459.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohnle P. G., Frank M. M., Kirkpatrick C. H. Mechanisms involved in elimination of organisms from experimental cutaneous Candida albicans infections in guinea pigs. J Immunol. 1976 Aug;117(2):523–530. [PubMed] [Google Scholar]
- Stone H. H., Geheber C. E., Kolb L. D., Kitchens W. R. Alimentary tract colonization by Candida albicans. J Surg Res. 1973 Apr;14(4):273–276. doi: 10.1016/0022-4804(73)90028-0. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Butler T. F., Johnson M. E., Gordee R. S. Colonization of the intestinal tract of conventional mice with Candida albicans and treatment with antifungal agents. Antimicrob Agents Chemother. 1976 May;9(5):787–792. doi: 10.1128/aac.9.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Srivastava K. K. Decontamination of gnotobiotic mice experimentally monoassociated with Candida albicans. Infect Immun. 1975 Dec;12(6):1401–1404. doi: 10.1128/iai.12.6.1401-1404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]















