Abstract
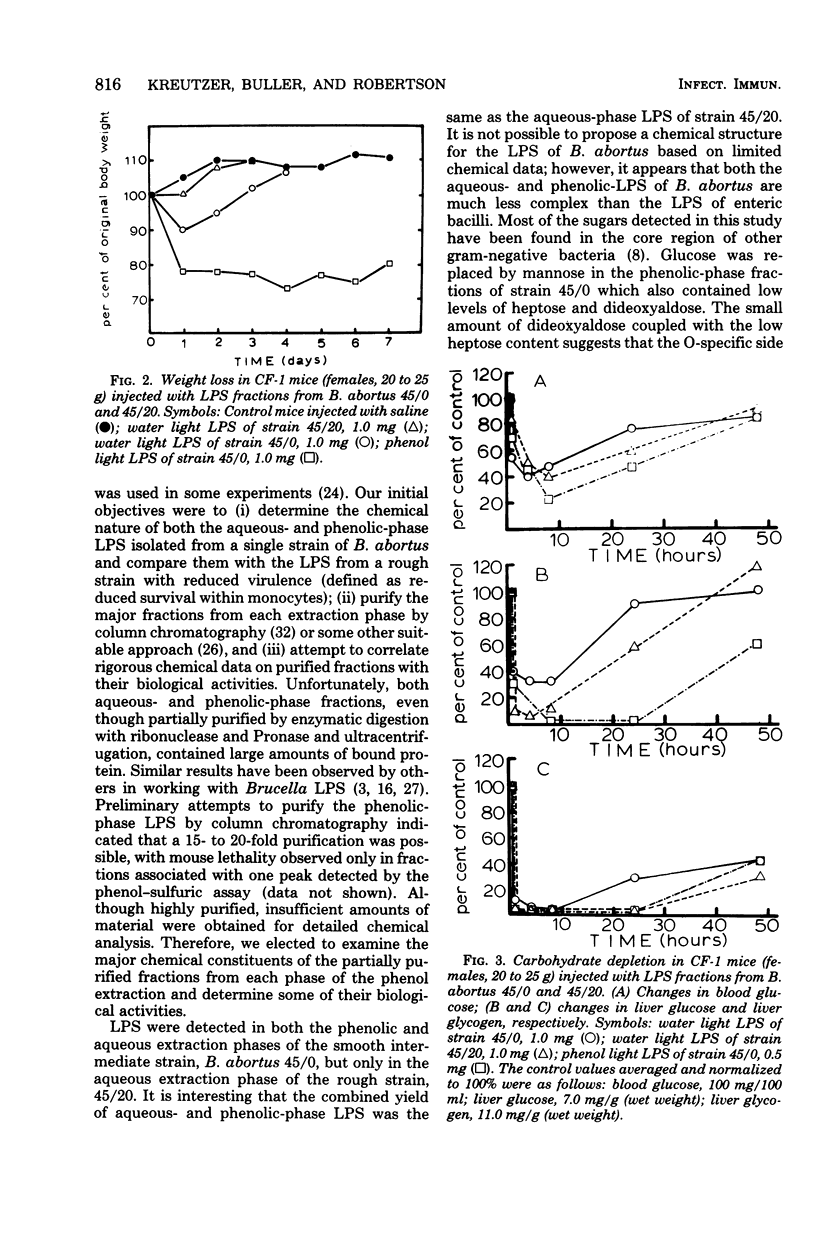
The chemical composition and some of the biological activities of lipopolysaccharides (LPS) extracted from a smooth-intermediate strain (45/0) and a rough strain (45/20) of Brucella abortus have been examined. LPS were found in both the phenolic and aqueous extraction phases of strain 45/0, but only in the aqueous phase of 45/20. The phenolic LPS contained 9- to 16-fold-lower levels of heptose and reduced amounts of dideoxyaldoses compared with aqueous fractions. The major neutral sugars were glucose, galactose, and mannose. beta-Hydroxymyristic-acid, a common marker of enteric LPS, was not detected. Fatty acids present in highest amounts were hydroxylated and nonhydroxylated species with chain lengths of 16, 18, and 20 carbons. Only the phenolic LPS of strain 45/0 exhibited mouse lethality and a curable wasting disease; however, both phenolic and aqueous fractions caused carbohydrate depletion in mice. The toxicity of aqueous LPS could not be potentiated with Pb(OAc)4. These data, coupled with the lack of mitogenic activity for B-lymphocytes, are indicative of the unique structure-function relationships of Brucella LPS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Wilson J. B. Chemical composition and biological properties of the endotoxin of Brucella abortus. J Bacteriol. 1965 Oct;90(4):895–902. doi: 10.1128/jb.90.4.895-902.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser D. V., Wheat R. W., Foster J. W., Leong D. Occurrence of quinovosamine in lipopolysaccharides of Brucella species. Infect Immun. 1974 Apr;9(4):772–774. doi: 10.1128/iai.9.4.772-774.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberg S. S. Immunity to brucella infection. Medicine (Baltimore) 1973 Jul;52(4):339–356. doi: 10.1097/00005792-197307000-00013. [DOI] [PubMed] [Google Scholar]
- Freedman H. H., Fox A. E., Willis R. S., Schwartz B. S. Induced sensitization of normal laboratory animals to Brucella abortus endotoxin. J Bacteriol. 1968 Feb;95(2):286–290. doi: 10.1128/jb.95.2.286-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Roppel J., Weckesser J., Rietschel E. T., Mayer H. Biological activities of lipopolysaccharides and lipid A from Rhodospirillaceae. Infect Immun. 1977 May;16(2):407–412. doi: 10.1128/iai.16.2.407-412.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvell B., Lindberg A. A. Serological cross-reactions between different Brucella species and Yersinia enterocolitica. Immunochemical studies on phenol-water extracted lipopolysaccharides from Brucella abortus and Yersinia enterocolitica type IX. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):113–119. [PubMed] [Google Scholar]
- Hurvell B. Serological cross-reactions between different Brucella species and Yersinia enterocolitica. Biological and chemical investigations of lipopolysaccharides from Brucella abortus and Yersinia enterocolitica type IX. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):105–112. doi: 10.1111/j.1699-0463.1973.tb02193.x. [DOI] [PubMed] [Google Scholar]
- JAMES A. T. Qualitative and quantitative determination of the fatty acids by gas-liquid chromatography. Methods Biochem Anal. 1960;8:1–59. doi: 10.1002/9780470110249.ch1. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Berman D. T. Studies of Brucella lipopolysaccharide. Dev Biol Stand. 1976;31:62–67. [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Berman D. T. Endotoxic activity of rough organisms of Brucella species. Infect Immun. 1976 Jun;13(6):1638–1641. doi: 10.1128/iai.13.6.1638-1641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARLSBAD G., KESSEL R. W., DE PETRIS S., MONACO L. ELECTRON MICROSCOPE OBSERVATIONS OF BRUCELLA ABORTUS GROWN WITHIN MONOCYTES IN VITRO. J Gen Microbiol. 1964 Jun;35:383–390. doi: 10.1099/00221287-35-3-383. [DOI] [PubMed] [Google Scholar]
- Kellerman G. D., Foster J. W., Badakhsh F. F. Comparison of Chemical Components of Cell Walls of Brucella abortus Strains of Low and High Virulence. Infect Immun. 1970 Sep;2(3):237–243. doi: 10.1128/iai.2.3.237-243.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Shome B., Liao T. H., Pierce J. G. Analysis of neutral sugars by gas-liquid chromatography of alditol acetates: application to thyrotropic hormone and other glycoproteins. Anal Biochem. 1967 Aug;20(2):258–274. doi: 10.1016/0003-2697(67)90031-0. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Robertson D. C. Surface macromolecules and virulence in intracellular parasitism: comparison of cell envelope components of smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):819–828. doi: 10.1128/iai.23.3.819-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer D. L., Scheffel J. W., Draper L. R., Robertson D. C. Mitogenic activity of cell wall components from smooth and rough strains of Brucella abortus. Infect Immun. 1977 Mar;15(3):842–845. doi: 10.1128/iai.15.3.842-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Serre A., Roux J. Comparaison de la composition chimique d'une fraction lipopolysaccharidique et d'une fraction polysaccharidique isolées de Brucella melitensis. Eur J Biochem. 1969 Jun;9(2):189–198. doi: 10.1111/j.1432-1033.1969.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A., Buller C. S. The effect of phage infection on the synthesis of lipid A by Escherichia coli. Virology. 1973 Jul;54(1):80–89. doi: 10.1016/0042-6822(73)90116-5. [DOI] [PubMed] [Google Scholar]
- Lehrer S., Nowotny A. Isolation and purification of endotoxin by hydrolytic enzymes. Infect Immun. 1972 Dec;6(6):928–933. doi: 10.1128/iai.6.6.928-933.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoux G., Renoux M., Tinelli R. Influence of Brucella endotoxins on the initiation of antibody-forming spleen cells in mice immunized with sheep red blood cells. Infect Immun. 1970 Jul;2(1):1–6. doi: 10.1128/iai.2.1.1-6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoux G., Renoux M., Tinelli R. Phenol-water fractions from smooth Brucella abortus and Brucella melitensis: immunochemical analysis and biologic behavior. J Infect Dis. 1973 Feb;127(2):139–148. doi: 10.1093/infdis/127.2.139. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Wright B. G., Rebers P. A. Procedure for determining heptose and hexose in lipopolysaccharides. Modification of the cysteine-sulfuric acid method. Anal Biochem. 1972 Oct;49(2):307–319. doi: 10.1016/0003-2697(72)90433-2. [DOI] [PubMed] [Google Scholar]



