Abstract
Background and Purpose
Improved identification of those at risk of stroke might improve prevention. We evaluated the association of the cardiac function biomarker N-terminal pro-B-type natriuretic peptide (NT-proBNP) with stroke risk in the 30,239 black and white participants of the REasons for Geographic And Racial Differences in Stroke cohort.
Methods
With 5.4 years follow-up after enrollment in 2003–7, NT-proBNP was measured in baseline blood samples of 546 subjects with incident ischemic stroke and 956 without stroke.
Results
NT-proBNP was higher with older age and in those with heart disease, kidney disease, atrial fibrillation and lower low-density lipoprotein cholesterol. Adjusting for age, race, sex, income, education and traditional stroke risk factors there was an increased risk of stroke across quartiles of NT-proBNP; participants with NT-proBNP in the top versus the bottom quartile had a hazard ratio of 2.9 (95% CI 1.9–4.5). There was no impact of added adjustment for kidney function and heart failure. Among etiologic stroke subtypes, the association was largest for cardioembolic stroke, with a hazard ratio of 9.1 (95% CI 2.9–29.2). Associations did not differ by age, sex or race, or after excluding those with baseline heart failure or atrial fibrillation. Predicted stroke risk was more accurate in 27% of participants if NT-proBNP was considered after traditional stroke risk factors (p<0.001).
Conclusion
NT-proBNP was a major independent risk marker for stroke. Considering this and other data for stroke, coronary disease, and atrial fibrillation, clinical use of NT-proBNP measurement in primary prevention settings should be considered.
Keywords: stroke, risk factor, natriuretic peptides
N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a marker of cardiac function used in detection and monitoring of heart failure. NT-proBNP is higher with atrial fibrillation1, and is a strong risk factor for future atrial fibrillation in the general population2, 3. Given the connections between cardiac dysfunction and stroke, NT-proBNP is a candidate marker of stroke risk.
A few general population studies reported associations of higher NT-proBNP with risk of first-time stroke4–6. The largest study, including 444 incident ischemic strokes, is the Atherosclerosis Risk in Communities (ARIC). This is the only study that reported on stroke subtypes, and there was a very large association with cardioembolic stroke; hazard ratio (HR) 12.6 for NT-proBNP in the top quintile7.
We evaluated the association of baseline NT-proBNP with risk of future ischemic stroke in a large population-based cohort study of black and white Americans followed for 5.4 years. Since there is little information on racial differences in NT-proBNP we also assessed whether levels were higher in blacks and thus might contribute to racial disparities in stroke risk8.
Materials and Methods
Subjects
The REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort is a national population-based cohort study evaluating racial and geographic disparities in stroke9. Between January 2003 and October 2007 30,239 individuals age 45 and older were enrolled by telephone. The telephone response rate was 33% and cooperation rate 49%, similar to other cohort studies10. Blacks and residents of the stroke belt were oversampled9; 45% men, 55% women, 58% whites, 42% blacks, 56% stroke belt residents, 44% non-stroke belt residents. Demographic, socioeconomic factors, medical history and verbal informed consent were obtained by computer-assisted telephone interview. At an in-home examination written informed consent, blood pressure, anthropomorphic measures, blood samples, electrocardiogram, and medication inventory were obtained9. Study methods were reviewed and approved by Institutional Review Boards at each study institution.
Measurements and Definitions
Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic pressure ≥90 mmHg, or self-reported hypertension with use of anti-hypertensive medications. Diabetes was defined by self-report with use of anti-diabetic medications, fasting glucose >126 mg/dL or non-fasting glucose >200 mg/dL. Left ventricular hypertrophy (LVH) was classified by electrocardiogram11. Atrial fibrillation was defined as self-report or presence on electrocardiogram. Prebaseline heart disease was defined as self-reported myocardial infarction, bypass, angioplasty or stenting, or myocardial infarction on electrocardiogram. Prebaseline stroke was defined by self-report. Heart failure was defined as presence of orthopnea and/or paroxysmal nocturnal dyspnea12.
Stroke Ascertainment
The outcome was first ischemic stroke through September 1, 2011. Participants or their proxies were contacted every 6 months by telephone to update health status8. Medical records were obtained in the case of death, suspected cerebrovascular event, or occurrence of stroke symptoms elucidated using the Questionnaire to Verify Stroke-Free Status13. After pre-review by a stroke nurse records were reviewed and validated by at least two physicians. Stroke was defined as focal neurologic symptoms lasting greater than twenty-four hours or non-focal symptoms with positive imaging for stroke. Strokes were classified as ischemic or hemorrhagic, and ischemic into etiologic subtypes of small vessel, large vessel, cardioembolic or unclassified as in other studies14. For analysis by etiologic subtype, when more than one etiology was considered present, that case was counted in each subtype group.
Case Cohort Sample
We used a case-cohort study design with mean follow up 5.4 years. Cases were 576 participants with incident ischemic stroke and no prebaseline stroke. The cohort random sample was selected using stratified sampling to ensure sufficient representation of high-risk groups. Participants were given a random number and divided into 20 strata based on age (45–54, 55–64, 65–74, 75–84, ≥ 85 years), race and sex. In each stratum, participants were randomly selected to fulfill the desired distribution: 50% black, 50% white, 50% women, 50% men, and age groups 20% 45–54, 20% 55–64, 25% 65–74, 25% 75–84, and 10% ≥85. Of 1104 selected participants we excluded 87 with prebaseline stroke.
Laboratory Methods
Fasting baseline blood samples were drawn and stored using standardized methods15. NT-proBNP was measured in the case-cohort sample in August 2012 using an electrochemiluminescence immunoassay (Roche Elecsys 2010; Roche Diagnostics, CV <5%). There were 88 participants with missing NT-proBNP, leaving 546 stroke cases and 956 in the cohort random sample.
Statistical Methods
Analysis used SAS 9.3 (Cary, NC). Levels of stroke risk factors were displayed as means or proportions in quartiles of NT-proBNP in the cohort random sample. Differences among quartiles were compared by Chi Square tests or ANOVA using sampling weights. Independent associations of NT-proBNP with race were evaluated in a multivariable linear regression model including only factors significantly associated with NT-proBNP at p <0.05.
HRs of stroke by NT-proBNP quartiles were calculated using Cox proportional hazards models for case-cohort studies16, with weighting to account for the sampling design. Participants without incident stroke were censored at death or last follow-up. The first model included age, sex, race and an age*race interaction term because associations of race with stroke are larger at young ages8. Model 2 added income, education and Framingham stroke risk factors (anti-hypertensive medication use, systolic blood pressure, diabetes, LVH, history of heart disease, atrial fibrillation, smoking). Model 3 added heart failure status, eGFR and albuminuria to Model 2. Interactions of NT-proBNP with age, sex and race were modeled using cross product terms with statistical significance for interaction of p <0.10. Associations of NT-proBNP with each ischemic stroke subtype were estimated using the Model 2, censoring participants when they developed ischemic stroke of another subtype.
To assess robustness of findings we conducted sensitivity analyses using Model 2. First, we excluded participants with NT-proBNP above the manufacturer’s cutoff for heart failure diagnosis (>125 pg/mL if <75 years old and >450 pg/mL if ≥75). Second, we excluded participants with heart failure, LVH, eGFR <60 ml/min/1.73m2 or atrial fibrillation.
Using Framingham stroke risk variables, we calculated the category free net reclassification improvement for stroke by measurement of NT-proBNP in addition to Framingham stroke risk variables17.
Results
Values of NT-proBNP were 5–29,284 pg/ml in the cohort random sample. Table 1 shows risk factor levels by NT-proBNP quartile in the cohort sample. In the multivariable model there was no association of NT-proBNP with race, and factors significantly associated with NT-proBNP are shown in table 2. The strongest correlates were older age, atrial fibrillation, heart disease and kidney measures. Lower LDL cholesterol was also associated with higher NT-proBNP. Statin use was not associated with NT-proBNP but was retained in the model to ensure the association of NT-proBNP with LDL cholesterol was not due to statin use. There were not independent associations of NT-proBNP with other factors listed in table 1, and inclusion of these variables in the multivariable model did not change interpretations of the findings.
Table 1.
Participant Characteristics by Baseline NT-proBNP Category in the Cohort Random Sample*
| Quartile of NT-proBNP, pg/ml† | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age, years | 60 | 63 | 66 | 70 |
| White, % | 43 | 50 | 59 | 51 |
| Male Sex, % | 62 | 48 | 42 | 47 |
| Non stroke belt, % | 48 | 47 | 44 | 50 |
| Income less than $20,000, % | 12 | 19 | 16 | 24 |
| Education <high school, % | 10 | 13 | 15 | 20 |
| Smoking Status, % | ||||
| Current | 17 | 18 | 12 | 11 |
| Never | 50 | 47 | 49 | 48 |
| Past | 33 | 35 | 39 | 41 |
| Hypertension Medication, % | 42 | 45 | 49 | 64 |
| Systolic blood pressure, mmHg | 138 | 127 | 128 | 130 |
| Diabetes, % | 21 | 17 | 17 | 27 |
| Heart Disease, % | 8 | 10 | 14 | 30 |
| Left Ventricular Hypertrophy, % | 7 | 8 | 8 | 11 |
| Atrial Fibrillation, % | 3 | 7 | 12 | 13 |
| Heart Failure, % | 15 | 19 | 14 | 14 |
| Statin Use, % | 25 | 26 | 30 | 38 |
| Body-mass index, kg/m2 | 30.7 | 29.6 | 28.6 | 28.0 |
| HDL cholesterol (mmol/L) | 1.27 | 1.32 | 1.40 | 1.32 |
| LDL cholesterol (mg/dL) | 3.08 | 3.11 | 2.85 | 2.64 |
| Triglycerides | 1.56 | 1.48 | 1.42 | 1.42 |
| eGFR <60 ml/min/1.73m2, % | 3 | 8 | 9 | 28 |
| Albuminuria, % | 7 | 8 | 11 | 28 |
SI conversion factors: to convert HDL and LDL to mg/dL, divide by 0.0259, and triglycerides to mg/dL, divide by 0.0113.
Analysis weighted to the full cohort. All P <0.001.
NT-proBNP quartile cutpoints 5–34 pg/ml, 34–67 pg/ml, 67–137 pg/ml, 137–29,284 pg/ml
Table 2.
Multivariable Model for Correlates of NT-proBNP in the Cohort Random Sample*
| Characteristic | Difference in NT-proBNP, pg/ml |
95% CI for Difference |
p value |
|---|---|---|---|
| Age, per 12 years | 47 | (17–77) | 0.002 |
| Race, Black | 9 | (−36–54) | 0.53 |
| Heart Disease, yes | 133 | (67–200) | <0.001 |
| Atrial Fibrillation, yes | 135 | (58–211) | <0.001 |
| LDL cholesterol, per 0.88 mmol/L | −27 | (−51– −4) | 0.02 |
| Statin use, yes | −15 | (−67–37) | 0.56 |
| eGFR <60 ml/min/1.73m2, yes | 227 | (147–307) | <0.001 |
| Albuminuria, yes | 223 | (156–289) | <0.001 |
Variables from table 1 were retained in the model if they were significantly associated with NT-proBNP, along with race as the main independent variable of interest and statin use. NT-proBNP differences are for presence vs absence of each categorical factor, or per standard deviation increment of continuous variables.
In Model 1 the HR of incident stroke increased with each increasing quartile of baseline NT-proBNP (Table 3); subjects in the 4th versus 1st quartile had a 3.9-fold increased risk. Adjustment for income, education and stroke risk factors decreased this HR to 2.9 (Model 2). None of the individual covariates explained the majority of this decline in HR. Added adjustment for eGFR, albuminuria and heart failure did not alter the HR (Model 3). Associations did not differ by age, sex or race (interaction p values with NT-proBNP 0.14, 0.31 and 0.38, respectively). Since NT-proBNP was not higher in blacks than whites, mediation analysis of the racial disparity in stroke by NT-proBNP was not pursued. Analysis by stroke etiology revealed similar associations as for overall stroke except the HR for cardioembolic stroke for NT-proBNP in the top quartile was higher at 9.1, and for unclassified stroke slightly lower at 2.1.
Table 3.
Hazard Ratio (95% Confidence Interval) of Ischemic Stroke by Baseline NT-ProBNP*
| NT-ProBNP Quartiles† | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | p for trend |
|
| All Ischemic Stroke, n | 60 | 82 | 110 | 294 | |
| Model 1 | Reference | 1.3 (0.9–1.9) | 1.7 (1.1–2.5) | 3.9 (2.6–5.8) | <0.001 |
| Model 2 | Reference | 1.1 (0.7–1.7) | 1.3 (0.8–2.0) | 2.9 (1.9–4.5) | <0.001 |
| Model 3 | Reference | 1.1 (0.7–1.9) | 1.2 (0.8–1.9) | 2.9 (1.8–4.5) | <0.001 |
| Small Vessel Stroke, n | 8 | 10 | 2 | 47 | |
| Model 2 | Reference | 2.2 (0.97–5.1) | 1.7 (0.7–5.3) | 2.8 (0.99–5.3) | 0.17 |
| Large Vessel Stroke, n | 12 | 7 | 24 | 47 | |
| Model 2 | Reference | 1.0 (0.3–3.0) | 1.5 (0.5–4.4) | 3.5 (1.3–9.0) | 0.02 |
| Cardioembolic Stroke, n | 5 | 9 | 18 | 110 | |
| Model 2 | Reference | 1.2 (0.3–4.7) | 1.8 (0.5–6.4) | 9.1 (2.9–28.2) | <0.001 |
| Unclassified Stroke, n | 34 | 43 | 53 | 124 | |
| Model 2 | Reference | 1.1 (0.6–1.9) | 1.0 (0.6, 1.8) | 2.1 (1.2, 3.8) | 0.01 |
Model 1 adjusted for age, race, age-race interaction, sex. Model 2 added income, education, smoking, hypertension medication use, systolic blood pressure, atrial fibrillation, LVH, diabetes, prevalent heart disease. Model 3 added estimated glomerular filtration rate, albuminuria, heart failure.
Quartile cutpoints as in Table 1
In sensitivity analysis excluding participants with NT-proBNP above the cutpoint for heart failure diagnosis (leaving 335 strokes, 988 cohort random sample), the 4th quartile HR was 2.0 (95% CI 1.1–3.7). Individual exclusion of participants with baseline heart failure, LVH, eGFR <60 ml/min/1.73m2, or atrial fibrillation had no material influence on associations (data not shown).
Since they were strongly associated with NT-proBNP, we assessed whether NT-proBNP explained the associations of heart disease and albuminuria with stroke risk. Other NT-proBNP correlates, heart failure and eGFR <60 ml/min/1.73m2, could not be assessed since they were not stroke risk factors in this study. In Model 2, the beta coefficients for heart disease before and after adjustment for NT-proBNP were 0.57 and 0.38 indicating 38% reduction. The coefficients for albuminuria were 0.37 and 0.17 indicating 54% reduction.
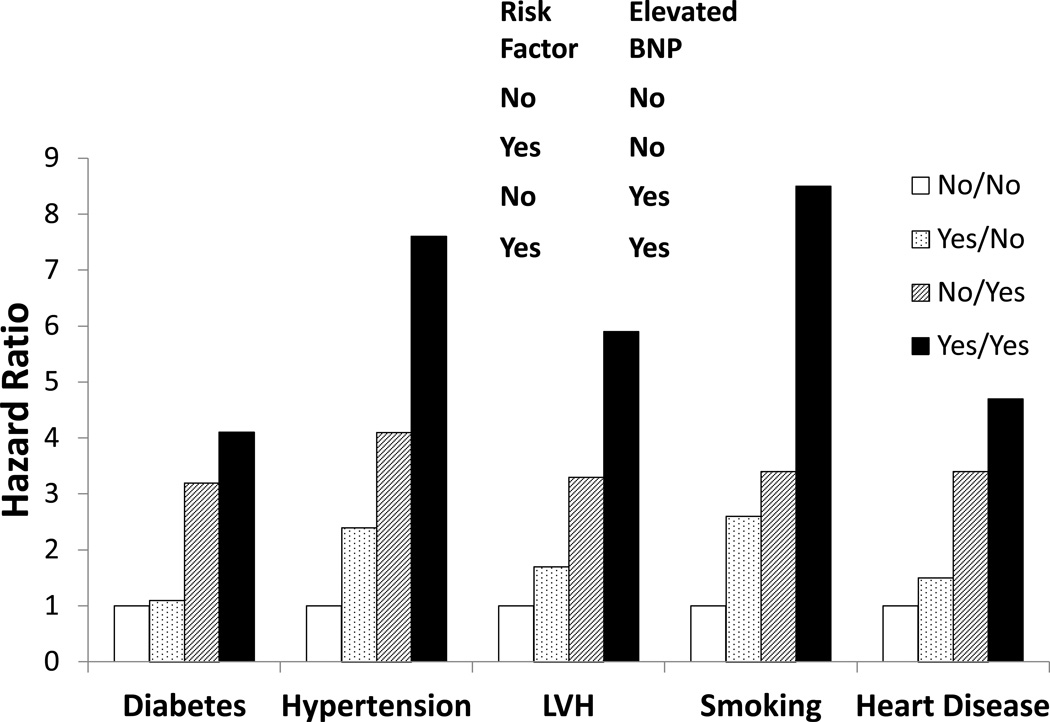
Figure 1 shows joint associations of NT-proBNP and other stroke risk factors with stroke risk. Participants with NT-proBNP in the top quartile plus either LVH, hypertension or smoking had stroke HRs of 5.9 (95% CI 2.2–12.8), 7.6 (95% CI 3.4–16.4) and 8.5 (95% CI 3.9–18.7), respectively. In those without higher NT-proBNP, diabetes was not associated with stroke (HR 1.1; 95% CI 0.5–2.3). These analyses could not be done for atrial fibrillation since nearly all participants with atrial fibrillation had NT-proBNP in the top quartile.
Figure 1.
Joint Associations of NT-proBNP in the Fourth Quartile and Other Risk Factors with Ischemic Stroke.
Abbreviations: NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVH, left ventricular hypertrophy
Based on net reclassification improvement, predicted ischemic stroke risk was more accurate in 27% of participants if NT-proBNP was measured with traditional stroke risk factors (95% CI 26–28%, p<0.001).
Discussion
In this population-based study of black and white Americans NT-proBNP was a major risk marker for ischemic stroke (especially cardioembolic stroke) and improved risk classification by traditional stroke risk factors for 27% of participants. Adjustment for NT-proBNP substantially reduced the associations of heart disease and albuminuria with stroke risk.
REGARDS is investigating reasons for racial and geographic disparities in stroke incidence and mortality. NT-proBNP levels were similar in blacks and whites, and did not differ by region, so cannot explain these disparities. Associations of NT-proBNP with stroke also did not differ comparing blacks to whites. Unlike here, two studies reported NT-proBNP was lower in blacks than whites, but multivariable analyses were not undertaken7, 18.
To provide perspective on NT-proBNP and stroke prediction, in this cohort the HRs of stroke were 1.5 for atrial fibrillation19 and 1.8 for hypertension20. Based on their prevalences in the cohort, the population attributable risk percentages for these three risk factors are 33% for NT-proBNP in the 4th quartile, 4% for atrial fibrillation and 29% for hypertension.
Results in this contemporary cohort were almost identical to an ARIC report7, most notably for cardioembolic stroke. ARIC enrolled a younger cohort in 1987–9; among 10,902 stroke-free participants, with 444 ischemic stroke outcomes, NT-proBNP in the top quintile was associated with a 2.9-fold increased risk of ischemic stroke in analyses adjusting for risk factors including atrial fibrillation and coronary disease, but not kidney disease7. Our observed associations were larger than some other studies, even after controlling for a larger number of covariates (kidney function, atrial fibrillation, LVH). The Rotterdam Study demonstrated a 2-fold increased stroke risk in elderly men and women for BNP in the top tertile6. The Framingham Offspring Study assessed only 53 outcomes of combined stroke plus transient ischemic attack, and reported a 2.1-fold increased risk for B-type natriuretic peptide above the 80th percentile. In a Japanese study associations were present for men but not women5, a finding we did not confirm with our much larger sample size. Only ARIC included substantial numbers of black participants, and they also found no racial difference in the association of NT-proBNP with stroke.
An association of NT-proBNP with risk of cardioembolic stroke may not be surprising given the correlation of NT-proBNP with cardiac function. The association of NT-proBNP with other types of stroke may be due to shared risk factors, unknown mechanisms for these strokes, or unknown effects of NT-proBNP. Increasing evidence suggests causal relationships of natriuretic peptides to endothelial permeability, which might predispose to atherosclerosis, for example21.
There may be applications for NT-proBNP measurement in stroke prediction in selected populations such as those with atrial fibrillation. In RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy), among 6,189 patients with atrial fibrillation, 183 had stroke or systemic embolism during follow up and those with baseline NT-proBNP levels in the top quartile had a 2.4-fold increased risk of this outcome22. While we had few stroke events among those with baseline atrial fibrillation, the HR of cardioembolic stroke for NT-proBNP in the top quartile was substantial at 9.1, and 77% of cardioembolic stroke cases were in the top quartile of NT-proBNP. Together with RE-LY and ARIC, findings suggest that NT-proBNP could be used in cardioembolic stroke prediction, even in the absence of atrial fibrillation23. Other data support clinical uses of NT-proBNP. After acute stroke higher NT-proBNP is independently associated with mortality24. In the Warfarin-Aspirin Recurrent Stroke Study, among patients with stroke that was not cardioembolic, those with NT-proBNP >750 pg/ml benefitted from warfarin as compared to aspirin to prevent recurrent stroke25.
In this study NT-proBNP was higher with baseline atrial fibrillation, heart disease, eGFR <60 ml/min/1.73m2, and albuminuria. One other study recently reported higher NT-proBNP with both albuminuria and lower eGFR, independent of each other26. In that study, NT-proBNP was also associated with risk of future coronary events, independent of albuminuria or kidney function. Authors speculated that renal function impairment causes cardiac dysfunction and higher NT-proBNP. We observed no impact of adjustment for kidney function markers on the association of NT-proBNP with stroke, suggesting separate causal pathways. Adjustment for NT-proBNP reduced the risk estimate for albuminuria and stroke risk by 54%, suggesting that both biomarkers reflect vascular dysfunction but that NT-proBNP more likely represents a factor involved in the causal path biology for stroke. This causal pathway may be related to clinical or subclinical atherosclerosis, not kidney disease, as supported by the reduction in the risk of stroke related to heart disease when NT-proBNP was accounted for. More research is needed.
Strengths of this study include the large geographically dispersed cohort of blacks and whites followed prospectively after extensive baseline data collection. Events were carefully adjudicated using medical records, minimizing misclassification bias and allowing accurate etiologic subtyping. Cohort retention was high with only 12.7% cumulative dropout as of January 2011. We used measured levels of potential confounders. Limitations must be considered. Results only generalize to black and white Americans. NT-proBNP was measured once so we could not control for regression dilution bias, although this would only bias results to the null hypothesis, making observed associations underestimates. Prevalent heart failure was not associated with higher NT-proBNP, possibly because of the proxy definition used. We could not account for post-baseline atrial fibrillation to determine whether this was the cause of the large association between NT-proBNP and cardioembolic stroke, although this only partly explained the large association observed by the ARIC investigators7.
Summary
There was a substantial association of higher NT-proBNP with risk of ischemic stroke in this study. The attributable risk for NT-proBNP in the 4th quartile was similar to that of hypertension. Along with other findings4–7, 22, 25, 26, and data on risk prediction of other cardiovascular diseases2, 3, 27–30, there is potential for wide clinical application of NT-proBNP testing.
Acknowledgments
The authors thank the investigators, staff and participants of the REGARDS study for their valuable contributions. A full list of investigators and institutions can be found at http://www.regardsstudy.org.
Source of Funding
U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the National Institutes of Health. Additional funding for urinary albumin assays was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen and NINDS did not have any role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures
OMG: research funding from Amgen. BK: consultancy, Allergan.
References
- 1.Silvet H, Young-Xu Y, Walleigh D, Ravid S. Brain natriuretic peptide is elevated in outpatients with atrial fibrillation. Am J Cardiol. 2003;92:1124–1127. doi: 10.1016/j.amjcard.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, et al. N-terminal pro-b-type natriuretic peptide is a major predictor of the development of atrial fibrillation: The Cardiovascular Health Study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Nakamura M, Onoda T, Ohsawa M, Tanno K, Itai K, et al. Predictive value of plasma b-type natriuretic peptide for ischemic stroke: A community-based longitudinal study. Atherosclerosis. 2009;207:298–303. doi: 10.1016/j.atherosclerosis.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Rutten JH, Mattace-Raso FU, Steyerberg EW, Lindemans J, Hofman A, Wieberdink RG, et al. Amino-terminal pro-b-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: The Rotterdam Study. Hypertension. 2010;55:785–791. doi: 10.1161/HYPERTENSIONAHA.109.143313. [DOI] [PubMed] [Google Scholar]
- 7.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin t, n-terminal pro-b-type natriuretic peptide, and incidence of stroke: The Atherosclerosis Risk in Communities study. Stroke. 2013;44:961–967. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The REasons for Geographic and Racial Differences in Stroke study: Objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 10.Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: A survey of practice. Am J Epidemiol. 2006;163:197–203. doi: 10.1093/aje/kwj036. [DOI] [PubMed] [Google Scholar]
- 11.Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating cornell voltage from nonstandard chest electrode recording site in the Reasons for Geographic and Racial Differences in Stroke study. J Electrocardiol. 2010;43:209–214. doi: 10.1016/j.jelectrocard.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pullicino PM, McClure LA, Wadley VG, Ahmed A, Howard VJ, Howard G, et al. Blood pressure and stroke in heart failure in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2009;40:3706–3710. doi: 10.1161/STROKEAHA.109.561670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones WJ, Williams LS, Meschia JF. Validating the questionnaire for verifying stroke-free status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 14.Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, et al. Ischemic stroke subtypes: A population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem. 2009;55:1627–1636. doi: 10.1373/clinchem.2008.122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onland-Moret NC, van der AD, van der Schouw YT, Buschers W, Elias SG, van Gils CH, et al. Analysis of case-cohort data: A comparison of different methods. J Clin Epidemiol. 2007;60:350–355. doi: 10.1016/j.jclinepi.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the roc curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, et al. N-terminal pro-b-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–734. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soliman EZ, Howard G, Meschia JF, Cushman M, Muntner P, Pullicino PM, et al. Self-reported atrial fibrillation and risk of stroke in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2011;42:2590–2593. doi: 10.1161/STROKEAHA.111.621367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: Lessons from the half-full (empty?) glass. Stroke. 2011;42:3369–3375. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannone V, Huntley BK, Olson TM, Heublein DM, Scott CG, Bailey KR, et al. Atrial natriuretic peptide genetic variant rs5065 and risk for cardiovascular disease in the general community: A 9-year follow-up study. Hypertension. 2013;62:860–865. doi: 10.1161/HYPERTENSIONAHA.113.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: A randomized evaluation of long-term anticoagulation therapy (RE-LY) substudy. Circulation. 2012;125:1605–1616. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]
- 23.Montaner J, Perea-Gainza M, Delgado P, Ribo M, Chacon P, Rosell A, et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280–2287. doi: 10.1161/STROKEAHA.107.505354. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Berrocoso T, Giralt D, Bustamante A, Etgen T, Jensen JK, Sharma JC, et al. B-type natriuretic peptides and mortality after stroke: A systematic review and meta-analysis. Neurology. 2013;81:1976–1985. doi: 10.1212/01.wnl.0000436937.32410.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longstreth WT, Jr, Kronmal RA, Thompson JL, Christenson RH, Levine SR, Gross R, et al. Amino terminal pro-b-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheven L, de Jong PE, Hillege HL, Lambers Heerspink HJ, van Pelt LJ, Kootstra JE, et al. High-sensitive troponin t and n-terminal pro-b type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. Eur Heart J. 2012;33:2272–2281. doi: 10.1093/eurheartj/ehs163. [DOI] [PubMed] [Google Scholar]
- 27.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 28.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. Cardiovascular risk prediction by n-terminal pro brain natriuretic peptide and high sensitivity C-reactive protein is affected by age and sex. J Hyperten. 2008;26:26–34. doi: 10.1097/HJH.0b013e3282f18301. [DOI] [PubMed] [Google Scholar]
- 29.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw LJ, Polk DM, Kahute TA, Wong ND, Moon J, Miranda-Peats R, et al. Prognostic accuracy of b-natriuretic peptide measurements and coronary artery calcium in asymptomatic subjects (from the Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research [EISNER] study) Am J Cardiol. 2009;104:1245–1250. doi: 10.1016/j.amjcard.2009.06.041. [DOI] [PubMed] [Google Scholar]