Abstract
Dengue vaccine development efforts have focused on the development of tetravalent vaccines. However, a recent Phase IIb trial of a tetravalent vaccine indicates a protective effect against only 3 of the 4 serotypes. While vaccines effective against a subset of serotypes may reduce morbidity and mortality, particular profiles could result in an increased number of cases due to immune enhancement and other peculiarities of dengue epidemiology. Here, we use a compartmental transmission model to assess the impact of partially effective vaccines in a hyperendemic Thai population. Crucially, we evaluate the effects that certain serotype heterogeneities may have in the presence of mass-vaccination campaigns.
In the majority of scenarios explored, partially effective vaccines lead to 50% or greater reductions in the number of cases. This is true even of vaccines that we would not expect to proceed to licensure due to poor or incomplete immune responses. Our results show that a partially effective vaccine can have significant impacts on serotype distribution and mean age of cases.
Introduction
Due to the possibility of severe disease arising from vaccine-induced immunity, the ideal dengue vaccine is one that has high and equal efficacy against all four serotypes. However, this ideal may be difficult to attain. The results of a recent Phase IIb trial indicate that the vaccine candidate furthest along in development protects against serotypes 1, 3 and 4 but not serotype 2 [1]. Though several statements of vaccine requirements have said that vaccines must protect against all four serotypes, partially effective vaccines may reduce morbidity and mortality [2,3]. Conversely, specific partially effective vaccines may result in increased clinical disease due to inducing immunity that pre-disposes individuals to more severe disease [4]. The potential population-level impacts of a partially effective vaccine have not been explored [5].
The dengue viruses exist as four antigenically distinct serotypes. Infection with one strain is thought to induce a life-long protective immune response to other viruses of the same serotype (homotypic immunity) and a short-term cross-protective response against other serotypes (heterotypic immunity), but waning heterotypic immunity has been associated with more severe illness upon secondary infection [6,7]. After secondary infection individuals generate a strong serological response that is broadly cross-reactive and, despite some evidence of tertiary and quaternary infections, it is generally assumed that most individuals can only undergo up to two infections [8].
While the target of dengue vaccine design has been to generate a balanced protective serological response to all four serotypes, vaccines targeting other antigenically diverse pathogens have shown a substantial public health impact even when inducing immunity to a subset of types of pathogen. Examples include pneumococcal conjugate vaccines[9] Human Papillomavirus (HPV) [10,11] and Haemophilus influenza B vaccines. [12,13] While dengue is unique due to the association that exists between secondary exposure and more severe forms of the disease, it is not clear that this difference needs to fundamentally change our approach to controlling dengue compared to other pathogens.
Evaluation of the potential impact of partially effective vaccines through simulation requires consideration of scenarios with heterogeneities between serotypes like those that are likely to exist in endemic/hyperendemic settings. Estimates of the force of infection derived from age-stratified seroprevalence studies conducted in Rayong, Thailand in 1980/81 and 2010 suggest that the average transmission intensity (and R0) of DENV-2 is higher than that of other serotypes [14,15]. Heterogeneity in the propensity to develop severe disease following infection with different serotypes has also been documented in multiple studies in Thailand and Nicaragua [16–19]. While the extent of immune enhancement of suceptibility/infectiousness by different infection sequences has been more difficult to estimate, there is some evidence to suggest that it might also vary between serotypes [14]. Furthermore, recent work suggests that such immune enhancement is important for serotype persistence in the presence of transmission heterogeneity[20].
The potential impact of vaccination on dengue transmission dynamics in Thailand and Vietnam has been explored in two recent publications by Chao et al. [21] and Coudeville et al. [22] using an agent-based model and an age-specific compartmental model, respectively. Both of these studies found that vaccines with efficacy of 70–90% against all serotypes have the potential to significantly reduce the frequency and magnitude of epidemics on a short to medium term However, while both of these models do account for some sources of heterogeneity between serotypes, for example, differences between the serotypes in transmission intensity, they do not systematically examine the potential impact of these heterogeneities in the context of partially effective vaccines.
Here, we use an age-stratified dengue transmission model to assess the potential impact of vaccines with high efficacy against dengue serotypes 1, 3 and 4 and low efficacy against dengue serotype 2 in a hyperendemic Thai population. We explore multiple disease/transmission scenarios to identify those that might lead to increases in clinically apparent cases and to identify the potential reductions in disease. Crucially, we evaluate the effects that certain serotype heterogeneities may have in the presence of mass-vaccination campaigns. We also explore overall, direct and indirect effects of reducing (or in some cases increasing) infection and disease in vaccinated individuals vs. reductions in transmission population wide.
Materials and methods
Mathematical Model
We formulated a deterministic, age-stratified compartmental dengue transmission model that includes explicit vector dynamics as well as cross-protection and infectiousness enhancement between dengue serotypes. Humans are assumed to be born susceptible and can undergo up to two infections by heterologous serotypes. Mosquito vectors are classified as susceptible or infected by each of the circulating serotypes.
We focus on the dengue vaccine being developed by Sanofi-Pasteur that requires three doses to achieve high protection. Vaccination reduces the susceptibility of vaccinated humans to dengue infection. We also allow for immune mediated vaccine induced enhancement in transmissibility.
Since the main objective of our study was to explore changes in the number of clinically apparent dengue cases, upon mass-vaccination, we made assumptions about the probability of developing clinically apparent disease following infection. These assumptions also allowed us to calibrate our model with data from surveillance systems. We assumed that: i) in unvaccinated individuals, clinical cases arise mostly from secondary infections; ii) In vaccinated individuals, clinical cases arise majorly from primary infection, but can arise from secondary infections depending on the vaccine’s efficacy against heterologous serotypes. Although we conservatively assumed the probability of clinical infection to be independent of age, we performed sensitivity analyses to consider age dependence as has been previously considered.
We discuss our mathematical model and related assumptions in more detail in the supplementary material. (Supplementary material S1)
Vaccination Campaign Parameters
For all simulations, we assumed that that the vaccine was equally effective against serotypes DENV-1, DENV-3 and DENV-4 (Vaccine efficacy=0.8, after 3 doses) but only partially effective against DENV-2. We also assumed that vaccine-derived immunity does not wane. Rollout of the vaccine consisted of 3 years of catch-up targeting children 2–15 years of age, followed by regular vaccination of 2–5 year olds. The vaccine was administered in up to three doses that were given on average every six months apart. Vaccination rates in catch-up and routine programs were constant over time and set so that vaccination coverage would reach 89% among 2–5 year olds and 69% in 2–15 year olds after 5 years. These vaccination rates were chosen to roughly correspond with the rate of vaccination achieved in Thailand with the Japanese Encephalitis three-dose vaccination using a combination of catch-up and routine immunization campaigns.
Vaccine effects
To explore the effects of vaccination at the population level, we compared the cumulative number of clinically apparent dengue cases in the 10 years after vaccine introduction, to the cumulative number of cases over the same period in the counterfactual population (i.e. same population had the vaccine not been introduced).
We also isolated overall, direct and indirect vaccine effects as proposed by Halloran, Longini and Struchiner [23]. In addition, we defined a counterfactual vaccine effect, comparing the cumulative incidence in vaccinated individuals of the vaccinated population to the cumulative incidence in “vaccinated” individuals of the counterfactual population (Supplementary material S1).
Since timing of vaccine introduction may impact the short and medium term effects of vaccination, we performed simulations introducing the vaccine at different points in the multiannual dengue cycle. We present vaccine effects that are averages over eight possible introduction years.
Results
Calibration and fit
We calibrated the model, at steady state, to the transmission dynamics of dengue in Rayong, Thailand, a traditionally hyperendemic setting (Figure 1). To fit the model to the demography of Rayong, we used data from the 2010 Thai Census. [24] (Supplementary figure S2.1). To estimate transmission parameters, we used age-specific incidence data from the Ministry of Public of Public Health (2002–2010) and age-stratified serological data from a seroprevalence study conducted among school-children in Rayong in 2010 [15,25].
Figure 1.
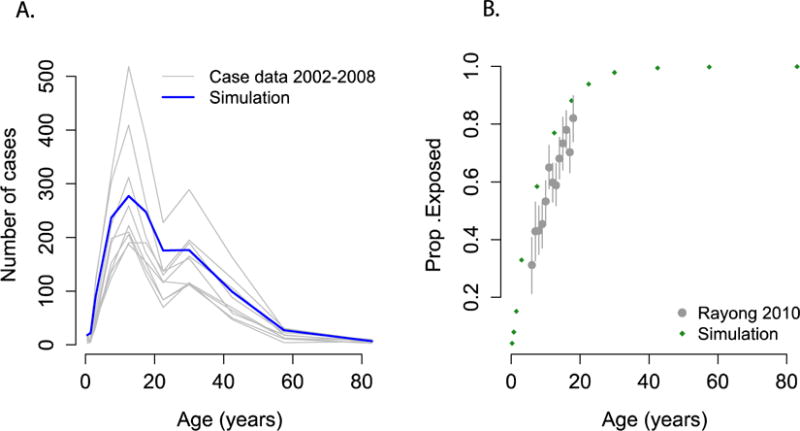
Output from the model compared to A) age-specific incidence from Rayong reported to the Ministry of Public Health, Thailand, 2002–2008. B)Results from an age-stratified serological study conducted in Rayong district, 2010.
Simulated Scenarios
Since a major objective of this study was to explore heterogeneities that could potentially lead to adverse results upon mass vaccination (i.e. that could lead to increases in the number of cases), we focused on scenarios that would favor the transmission of the serotype with lowest vaccine efficacy, i.e. DENV-2. Thus, the three main scenarios explored were: A) Risk of clinically apparent disease after infection by DENV-2 is greater than risk for other serotypes, B) Transmission intensity of DENV-2 is greater than transmission intensity of other serotypes, C) Enhancement of infectiousness upon secondary infection with DENV-2 is greater than enhancement by other serotypes. Example output of the simulated annual incidence of clinically apparent dengue and seroprevalence under the three scenarios explored is shown in the supplementary material (Supplementary figures S2.2 and S2.3).
Effects of partially effective vaccine on serotype distribution
Figure 2 shows example output from simulations under the “base case”, where all serotypes are equally transmissible, have an equal probability of leading to clinical disease, and do not interact. As expected, a vaccine that is equally effective against all serotypes leads to a symmetric decline in the serotype specific incidence (Figure 2A). In contrast, if the vaccine is only effective against 3 out of 4 circulating serotypes, reductions in the incidence of some serotypes are accompanied by an absolute increase in the incidence from serotypes with lower efficacy (Figure 2B). Since this model assumes that individuals can only suffer up to two infections, there is intrinsic competition between the dengue serotypes. Vaccine induced reductions in the incidence of some serotypes reduces this competition and favors the serotype with lower vaccine efficacy.
Figure 2.
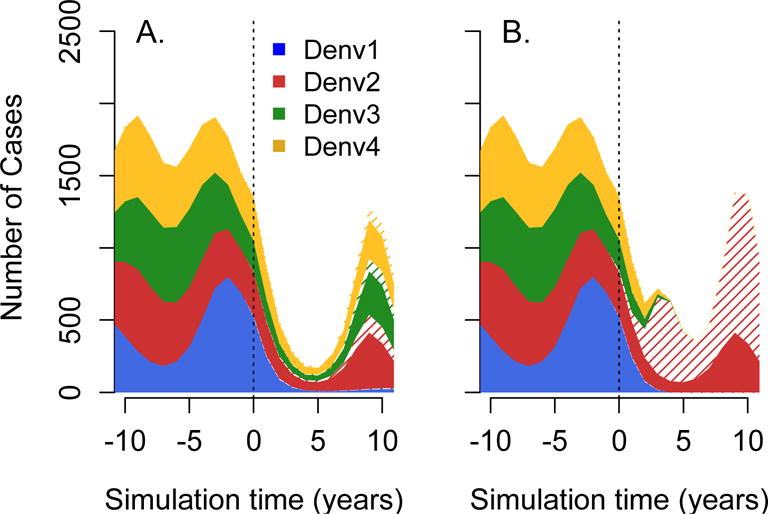
Effects of vaccination on the serotype specific incidence of dengue. Figure showing example output of the “base case” simulation, where all serotypes are equally transmissible, have an equal probability of leading to clinical disease and do not interact. In Panel A, vaccine efficacy against dengue 1–4 was assumed to be 0.8. In panel B, vaccine efficacy against DENV- 2 was reduced to 0.05. Solid regions indicate cases occurring in unvaccinated population and hatched region indicates those occurring in people who have received at least one vaccine dose.
Vaccine effects over a ten-year period
Figure 3 summarizes the results obtained after performing simulations over a wide range of vaccine efficacies for the three scenarios. In a large proportion of scenarios explored, partially effective vaccines result in a 50% or greater reduction in the cumulative number of clinical cases over 10 years. This is the case even for scenarios that included large heterogeneities in the probability of infections being clinically apparent (Figure 3A), transmission intensity (Figure 3B) and infectiousness enhancement (Figure 3C). Decreases in the cumulative number of cases were even more dramatic in simulations that considered low-transmission settings (see Supplementary materials S3).
Figure 3.
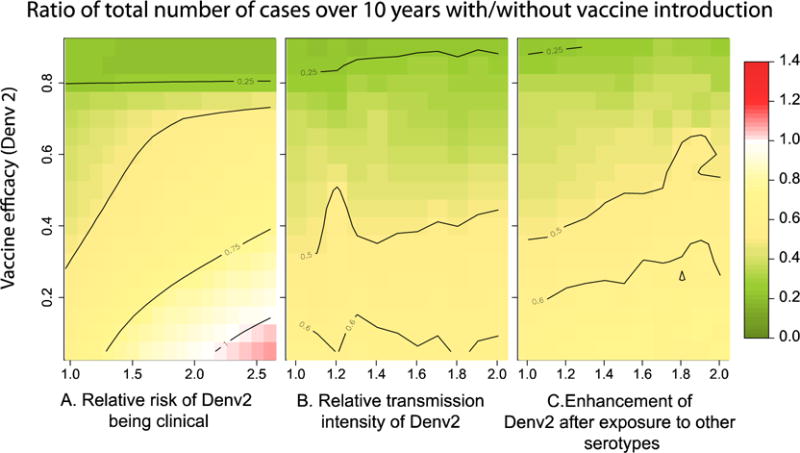
Figure summarizing the expected 10-year impact of vaccination with a partially effective vaccine under different scenarios. Each grid cell represents the ratio of the cumulative number of dengue cases 10 years after vaccine introduction, vs. the cumulative number of cases in the same 10 years, had the vaccine not been introduced. We performed simulations over a wide range of vaccine efficacies (for DENV-2) and A) relative risk of DENV-2 being clinical, B) relative transmission intensity of DENV-2, C) enhancement/inhibition of transmission intensity of secondary infections by DENV-2 (after prior primary exposure by any of the other serotypes). For all simulations we assumed the efficacy of the vaccine against other circulating serotypes to be 0.8.
Our results also show that, even in the presence of high efficacy against 3/4 serotypes (leading to near elimination of them, Supplementary figure S2.5) vaccination can lead to non-significant reductions or even increases in the incidence of dengue under certain scenarios. Increases in the 10-year cumulative number of cases were only observed for scenarios in which DENV-2 had a relative risk of clinically apparent disease greater than two.
We isolated the direct and indirect effects of vaccination at the population level 10 years after vaccine introduction, by comparing the overall, direct and indirect vaccine effects. While in the vast majority of scenarios explored vaccination reduced the risk of unvaccinated individuals by 50–80% (due to indirect effects), direct effects of vaccination (i.e. reductions in the number of cases in vaccinated individuals as compared to unvaccinated individuals) were smaller (Figure 4). Interestingly, in scenarios that included high heterogeneity in the transmission intensity and very low vaccine efficacy against DENV-2, direct effects of vaccination were negative. However, even under these scenarios, there was an absolute reduction in the cumulative incidence among vaccinated individuals, as compared to themselves had no vaccination program been implemented (counterfactual effect). This reduction reflects the cumulative effects of both direct and indirect protection that vaccinees experience.
Figure 4.
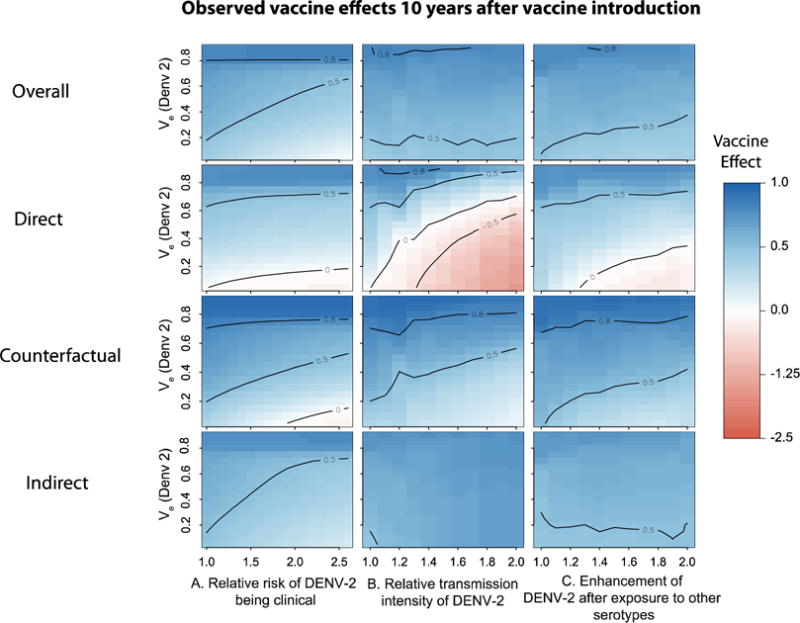
Figure summarizing the expected 10-year vaccine effects of a partially effective vaccine under different scenarios. We performed simulations over a wide range of vaccine efficacies (for DENV-2) and sources of heterogeneity in A) relative risk of DENV-2 being clinically apparent, B) relative transmission intensity of DENV-2, C) enhancement/inhibition of transmission intensity of secondary infections by DENV-2 (after prior primary exposure to any of the other serotypes). See methods for description of effects. Each grid cell represents the 10-year population vaccine effect for that particular scenario. For all simulations we assumed the efficacy of the vaccine against other circulating serotypes to be 0.8.
Impact of vaccination on temporal dynamics and age of infection
We assessed the impact of vaccination on the yearly incidence of clinically apparent dengue, across all serotypes, for 50 years after vaccine introduction (Figure 5). While significant decreases were observed in all scenarios (relative to the average incidence prior to vaccination), several short-term increases over pre-vaccine levels occur within thirty years of vaccine introduction. These increases result from the build up of susceptible individuals in certain age groups and, as expected, are less frequent in scenarios with higher efficacy against DENV-2. Despite these periodic increases, the expected cumulative incidence of clinically apparent dengue was significantly lower than the cumulative incidence without vaccine for the great majority of scenarios explored (Figure 5, right panel).
Figure 5.
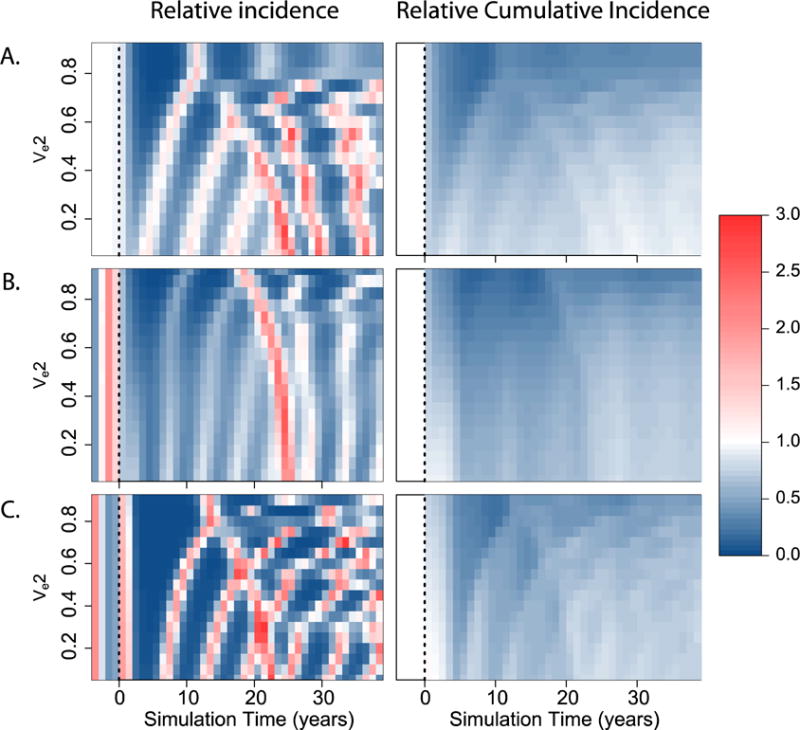
Incidence of clinical dengue over time after vaccine introduction. Figure showing changes in the incidence of clinical dengue during the years following vaccine introduction (time=0, dashed line). Left: Relative yearly incidence of clinical dengue as compared to the average yearly incidence before vaccine introduction. Right: Reductions in the cumulative incidence of clinical dengue at different times after vaccine introduction, relative to the cumulative incidence had the vaccine not been introduced. We present results for three different scenarios. A) Risk of developing clinical disease after DENV-2 infection is 1.5 times greater than after infection by any other serotype. B)Transmission intensity of DENV-2 is 1.5 times higher than the transmission intensity of any other serotype. C) Secondary infection by DENV-2 results in infectiousness enhancement by a factor of 1.5. In all cases, the vaccine efficacy against dengue 1, 3, and 4 was assumed to be 0.8.
We also explored the impact of vaccination on the mean-age of clinical cases (Figure 6). While vaccination with high efficacy across all serotypes led to an increase in the mean age of cases, in certain instances of low vaccine efficacy against DENV-2 we observed decreases in the mean-age. The largest decreases were observed in scenarios that included heterogeneity in transmission intensity (Figure 6B), and result mostly from breakthrough infections by DENV-2 in vaccinated children. Sudden increases in the mean-age of cases were also observed at varying times after vaccine introduction and result from susceptibility accumulating in certain age-classes.
Figure 6.
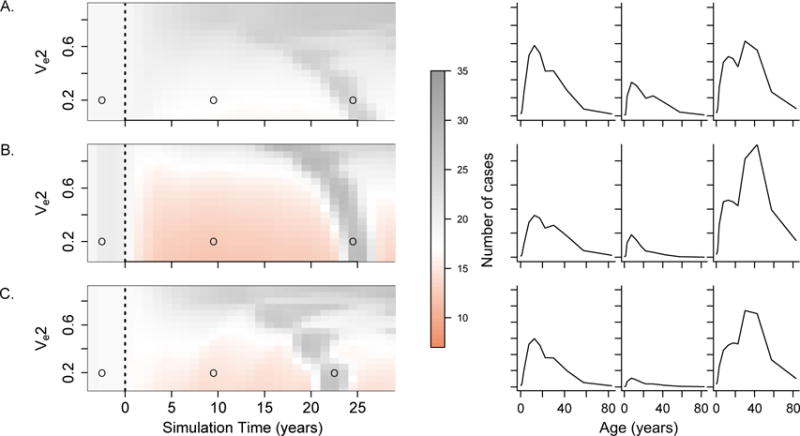
Age distribution of clinical cases over time after vaccine introduction. Left panels show changes in the mean age of dengue cases during the years following vaccine introduction (dashed line) as compared to the years before vaccine introduction. Right panels show changes in the age distribution of clinical cases for the three time points marked with open circles in the corresponding left panel. We present results for three different scenarios. A) Risk of developing clinical disease after DENV-2 infection is 1.5 times greater than after infection by any other serotype. B) Transmission intensity of DENV-2 is 1.5 times higher than the transmission intensity of any other serotype. C) Secondary infection by DENV-2 results in infectiousness enhancement by a factor of 1.5. In all cases, the vaccine efficacy against dengue 1, 3, and 4 was assumed to be 0.8.
Discussion
The impact of any particular vaccine formulation depends on at least four separate effects: 1) direct protection of vaccinees against infection and/or disease 2) indirect protection of all members of vaccinated communities 3) an impact on serotype distribution and 4) the immunopathogenic effects of partial vaccine-induced immunity. Our results, from a four-serotype, age-specific compartmental dengue transmission model suggest that partially effective vaccines can have a significant positive impact, on average, in reducing dengue transmission and disease.
We evaluated the potential impact of mass vaccination with partially effective vaccines in the presence of certain serotype heterogeneities. Results from our simulations suggest that vaccines effective against only 3 out of 4 circulating serotypes can lead to reductions even in scenarios where the serotype with low or zero efficacy (in this case DENV-2) is more pathogenic, more transmissible or experiences greater infectiousness enhancement. These findings indicate that vaccines effective against only three serotypes may have positive impacts at the population level, even under some of the adverse scenarios that led to recommendations to focus on the development of tetravalent dengue vaccines[26].
These results provide insight into the impact that competition between serotypes may have on the overall efficacy of partially effective vaccines and are consistent with previously published work[27]. Assuming that individuals can only undergo up to two infections, in hyperendemic settings (where 2 or more serotypes circulate) partially effective vaccines can lead to a decrease in competition and increased transmission of serotypes for which the vaccine has low efficacy. The overall reduction in the number of clinical cases will depend on the pathogenicity of the serotypes that benefit from this reduced competition.
Our results also show that vaccination might lead to a shift in the mean-age of cases towards younger age groups. If vaccine induced immunity enhances severity of infections among those that experience infection, vaccinating young immunologically naive children might predispose them to clinically apparent disease earlier in life. This result might have important implications since severe dengue manifestations (dengue hemorrhagic fever and dengue shock syndrome) are thought to be more frequent and severe among infants and young children [28].
Finally, our results indicate that direct and indirect effects of a vaccine could differ, potentially resulting in non-vaccinees in a highly vaccinated population experiencing the greatest reductions in cumulative incidence of clinically apparent dengue. Much of this effect is dictated by the immunopathogenic effects of vaccine derived immunity that we assumed, and would not be observed if vaccine immunity conferred protection against clinical disease. While in all of these instances the cumulative incidence in vaccineees was lower than what it would have been in the absence of vaccine, and the overall population effects were positive, this finding raises issues about the relevance of individual versus population protection. The use of incentives to promote vaccination may be used to manage expectation regarding specific benefits of vaccination vs. non-vaccination under different vaccination coverages[29,30].
Two other efforts have recently estimated the potential impact of a dengue vaccine [21,22]. Neither of these papers address the potential impact of vaccines that differ in their efficacy by serotype, a key feature of the vaccine reported by Sabchaereon et al. [1]. A difference between our model and those by Chao et al. and Coudeville is that ours assumes that people can only undergo natural infection by up to two dengue serotypes while they assume that up to four infections are possible. Our assumption is supported by the low frequency of tertiary and quaternary infections among hospital cohorts [8,19] and by the broadly cross-reactive neutralizing antibody response that is maintained after secondary infection. However, whether tertiary and quaternary play some role in the transmission dynamics of dengue is still under debate. Relaxing this assumption would remove the competition between serotypes imposed by our model, and in general lead to greater reductions in cumulative incidence with the use of partially effective vaccines.
Our model makes the assumption that the probability of developing clinically apparent disease is higher in the presence of pre-existing immunity, regardless of whether this immunity is the result of natural infection or vaccination. A similar assumption is made in the model by Coudeville [22]. While in the context of natural infections it is well established that pre-existing immunity against a heterologous serotype is the main risk-factor for the development of severe disease [7], immunopathogenic effects of vaccine-induced immunity are yet to be elucidated. If heterologous vaccine induced immunity protects against infection or clinically apparent disease, the impact of partially effective vaccines will be greater than that estimated by our model.
While we calibrated our transmission parameters to fit the age distribution of seroprevalence and reported cases in Rayong, Thailand, current knowledge of dengue epidemiology can distinguish between many of the scenarios that we simulated. Multiple studies have found evidence of heterogeneity [14,31,32] but the extent to which heterogeneity in clinical expression, transmissibility or enhancement exists is not known. One of the main objectives of this research was to identify scenarios that could potentially result in adverse population effects after mass vaccination with partially effective vaccines, and therefore we deliberately chose to explore a wide parameter space, even if this resulted in unrealistic dynamics in some cases.
There are important gaps in our understanding of serotype dynamics, cross-protection[33], enhancement and pathogenicity[34–36]. Our results aim to represent hyperendemic areas generally, but predicting the potential impact of vaccination in any specific setting would require extensive serotype-specific longitudinal data that is only available from cohort studies. While our sensitivity analyses suggest that partially effective vaccines have the potential to be even more useful in settings with stable low transmission, better understanding of the changing epidemiology of dengue in settings of more recent re-emergence (e.g., South America and the Indian sub-continent) will be fundamental to properly model the impact of vaccines in these settings.
Dengue vaccine development efforts have been ongoing for several decades and have focused on the development of tetravalent vaccines. The realities of vaccine development and individual heterogeneity in vaccine responses indicate that vaccines might not invoke a strong protective response in all individuals to all serotypes. Our results suggest that despite the virologic and immunologic characteristics of dengue, partially effective vaccines have the potential to be important tools for dengue control. Consideration of imperfect vaccines will require careful measurement of the epidemiology of dengue in each place that vaccine might be evaluated and/or used, anticipation of negative outcomes that could occur and management of expectations for the public health impact of the vaccine.
Supplementary Material
Highlights.
-
–
We explore the population impact of partially effective dengue vaccines (PEV)
-
–
We consider scenarios of heterogeneity between serotypes
-
–
We use an age-stratified compartmental dengue transmission model
-
–
We show that PEV could be important tools for dengue control
-
–
We examine direct vs. indirect effects of vaccination
Acknowledgments
IRB, DSB and DATC received support from the Bill and Melinda Gates Foundations Vaccine Modeling Initiative and the National Institutes of Health (NIH) Grant 1U54GM088491-0109. LMTR, IBS and DATC received support from the National Institute Of General Medical Sciences (R01GM090204). DATC holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. IBS is also supported by the Office of Naval Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. The Lancet. doi: 10.1016/S0140-6736(12)61428-7. n.d. [DOI] [PubMed] [Google Scholar]
- 2.Hombach J. Guidelines for clinical trials of dengue vaccine in endemic areas. Journal of Clinical Virology. 2009;46:S7–S9. doi: 10.1016/S1386-6532(09)70287-2. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz J, Roehrig J, Barrett A, Hombach J. Next generation dengue vaccines: a review of candidates in preclinical development. Vaccine. 2011;29:7276–84. doi: 10.1016/j.vaccine.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson JR. Understanding dengue pathogenesis: implications for vaccine design. Bull World Health Organ. 2005;83:308–14. [PMC free article] [PubMed] [Google Scholar]
- 5.Group W-VDVM. Assessing the Potential of a Candidate Dengue Vaccine with Mathematical Modeling. PLoS Negl Trop Dis. 2012;6:e1450. doi: 10.1371/journal.pntd.0001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halstead SB. Immune enhancement of viral infection. Prog Allergy. 1982;31:301–64. [PubMed] [Google Scholar]
- 7.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007;77:910–3. [PubMed] [Google Scholar]
- 9.Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. The Pediatric Infectious Disease Journal. 2012;31:501–8. doi: 10.1097/INF.0b013e31824de9f6. [DOI] [PubMed] [Google Scholar]
- 10.Hariri S, Markowitz L. Monitoring HPV vaccine impact: early results and ongoing challenges. J Infect Dis. 2012;206:1633–5. doi: 10.1093/infdis/jis593. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 12.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev. 2000;13:302–17. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Progress toward elimination of Haemophilus influenzae type b invasive disease among infants and children–United States, 1998–2000. MMWR Morb Mortal Wkly Rep. 2002;51:234–7. [PubMed] [Google Scholar]
- 14.Ferguson NM, Donnelly CA, Anderson RM. Transmission dynamics and epidemiology of dengue: insights from age-stratified sero-prevalence surveys. Philos Trans R Soc Lond B Biol Sci. 1999;354:757–68. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Barraquer I, Buathong R, Iamsirithaworn S, Nisalak A, Lessler J, Jarman RG, et al. Revisiting Rayong: Shifting seroprofiles of dengue in Thailand and their implications for transmission and control. American Journal of Epidemiology. doi: 10.1093/aje/kwt256. Accepted for publication. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balmaseda A, Hammond SN, Perez L, Tellez Y, Saborio SI, Mercado JC, et al. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74:449–56. [PubMed] [Google Scholar]
- 17.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–69. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 18.Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon I-K, et al. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis. 2010;4:e617. doi: 10.1371/journal.pntd.0000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 20.Mier-y-Teran-Romero L, Schwartz IB. Breaking the symmetry: Immune enhancement increases persistence of dengue viruses in the presence of asymmetric transmission rates. Journal of Theoretical …. 2013 doi: 10.1016/j.jtbi.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao DL, Halstead SB, Halloran ME, Longini IM., Jr Controlling dengue with vaccines in Thailand. PLoS Negl Trop Dis. 2012;6:e1876. doi: 10.1371/journal.pntd.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coudeville L, Garnett GP. Transmission Dynamics of the Four Dengue Serotypes in Southern Vietnam and the Potential Impact of Vaccination. PLoS ONE. 2012;7:e51244. doi: 10.1371/journal.pone.0051244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halloran ME, Jr, Longini IM, Struchiner CJ. Design and Analysis of Vaccine Studies. Springer; 2012. [Google Scholar]
- 24.National Statistical office, Thailand. Population and Housing Census, 2010. 2010 [Google Scholar]
- 25.Ministry of Public Health Thailand. Annual epidemiological surveillance report. Nonthaburi: n.d. [Google Scholar]
- 26.Thomas SJ, Endy TP. Critical issues in dengue vaccine development. Curr Opin Infect Dis. 2011;24:442–50. doi: 10.1097/QCO.0b013e32834a1b0b. [DOI] [PubMed] [Google Scholar]
- 27.McLean AR. Vaccination, evolution and changes in the efficacy of vaccines: a theoretical framework. Proc Biol Sci. 1995;261:389–93. doi: 10.1098/rspb.1995.0164. [DOI] [PubMed] [Google Scholar]
- 28.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6:118–24. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee AV, Duflo E, Glennerster R, Kothari D. Improving immunisation coverage in rural India: clustered randomised controlled evaluation of immunisation campaigns with and without incentives. Bmj. 2010;340:c2220. doi: 10.1136/bmj.c2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MORRIS S, FLORES R, OLINTO P, MEDINA J. Monetary incentives in primary health care and effects on use and coverage of preventive health care interventions in rural Honduras: cluster randomised trial. The Lancet. 2004;364:2030–7. doi: 10.1016/S0140-6736(04)17515-6. [DOI] [PubMed] [Google Scholar]
- 31.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 32.Rico-Hesse R. Dengue Virus. Vol. 338. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. Dengue Virus Virulence and Transmission Determinants; pp. 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SABIN AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 34.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–84. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- 35.Halstead SB, Chow JS, Marchette NJ. Immunological enhancement of dengue virus replication. Nature New Biol. 1973;243:24–6. [PubMed] [Google Scholar]
- 36.Halstead SB. Advances in Virus Research. Vol. 60. Elsevier; 2003. Neutralization and Antibody-Dependent Enhancement of Dengue Viruses; pp. 421–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


