Abstract
Eleven fatty acid analogs incorporating four-membered carbocycles (cyclobutenes, cyclobutanes, cyclobutanones, and cyclobutanols) were investigated for the ability to inhibit growth of Mycobacterium smegmatis (Msm) and Mycobacterium tuberculosis (Mtb). A number of the analogs displayed inhibitory activity against both mycobacterial species in minimal media. Several of the molecules displayed potent levels of inhibition against Mtb with MIC values equal to or below those obtained with the anti-tuberculosis drugs D-cycloserine and isoniazid. In contrast, two of the analogs displaying the greatest activity against Mtb failed to inhibit E. coli growth under either set of conditions. Thus, the active molecules identified here (1, 2, 6, and 8) may provide the basis for development of anti-mycobacterial agents against Mtb.
Keywords: cyclobutane, cyclobutene, fatty acid, mycobacteria, tuberculosis
Introduction
Tuberculosis resulting from Mycobacterium tuberculosis (Mtb) infection remains one of mankind’s most significant disease threats. Based on skin test reactivity, it is estimated that one-third of the world’s population has been exposed, resulting annually in approximately nine million incident cases and 1.4 million deaths (2010 data).[1] A number of therapeutic agents have been developed, but current treatment regimens require patients to take multiple drugs over a period of months and are associated with significant side effects.[2] The result is frequent patient noncompliance, leading to relapses and the emergence of drug resistance, with a high fraction of active cases now involving multi-drug resistant (MDR, XDR) strains.[1] There is, therefore, a need for new classes of therapeutics for tuberculosis.[3] Importantly, the lethal target and/or the mechanism of action should also be novel to avoid established mechanisms of resistance and provide synergy with existing treatments.
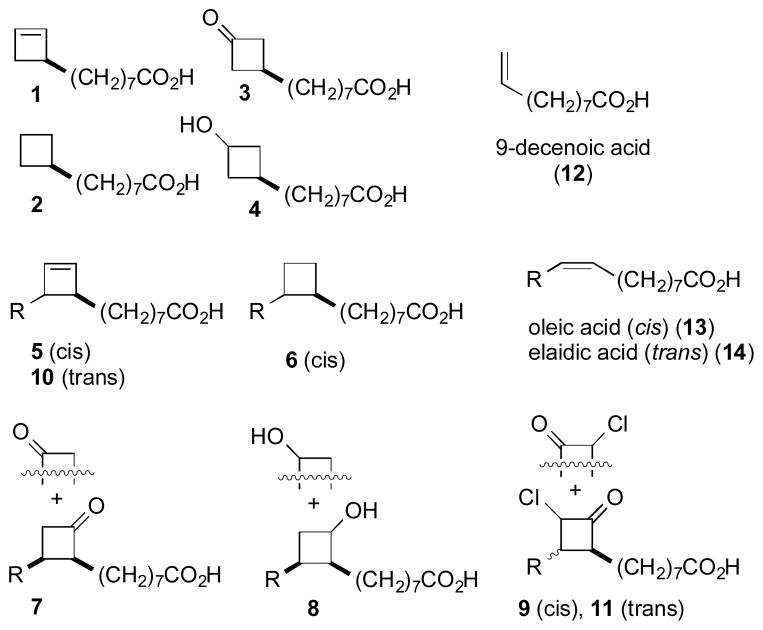
Much of the hardiness and drug resistance of mycobacteria is due to an unusually thick lipid cell wall containing a significant proportion of mycolic acids, a unique class of C54–C63 branched-chain fatty acids.[4] A number of existing treatments for Mtb, exemplified by isoniazid and ethionamide, disrupt mycolic acid biosynthesis by inhibiting important biosynthetic enzymes.[5] Mycobacteria incorporate intact C16 and C18 fatty acid skeletons as biosynthetic feed stocks and given the prior evidence for the incorporation of modified fatty acids into mycolic acid biosynthesis,[6] we hypothesized that hijacking this pathway could provide a unique approach for the development of potential therapeutics.[4] Towards this end, we proposed to investigate specifically functionalized fatty acids for their ability to limit mycobacterial growth.[7] As our first steps towards validating this hypothesis, we investigated two series of fatty acid analogs (Figure 1). One series (1–4) was based upon a decenoic acid (10:1) framework and a second series (5–11) based upon the frameworks of oleic or elaidic acids (18:1). The analogs preserve the approximate lengths and cross-sections of the “parent” fatty acids while incorporating four-membered carbocycles into the backbone. We now report that several of these analogs display significant anti-mycobacterial activity against Mtb.
Figure 1.
Analogs investigated, along with structures of oleic (cis-18:1), elaidic (trans-18:1) and decenoic (10:1) acids. R = octyl.
Results and Discussion
Substrate Preparation
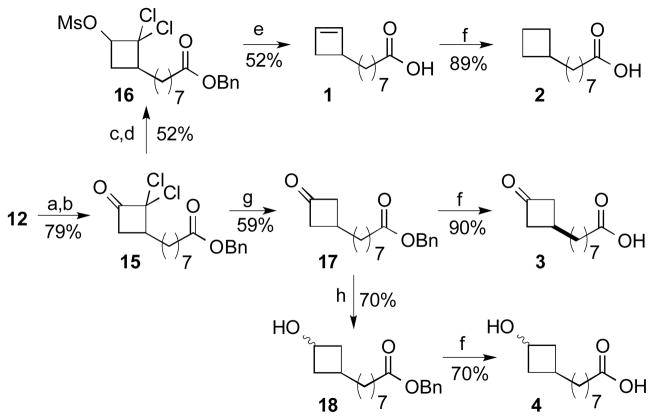
The only previous description of molecules included in this study was the preparation of a mixture of the methyl esters of 5 and 10 via a nonselective radical decarboxylation.[8] In search of an efficient, general, and stereospecific approach to incorporation of four-membered rings onto a fatty acid backbone, we were drawn to methodology for modification of dichlorocyclobutanones.[9] Our approach is illustrated in detail for the preparation of analogs 1–4 (Scheme 1). The benzyl ester of 9-decenoic acid underwent cycloaddition with dichloroketene to afford one major dichlorobutanone (15),[10] which was only moderately stable towards purification, and was therefore directly reduced with sodium borohydride to furnish a mixture of stereoisomeric 2,2-dichloro-1-cyclobutanols. The choice of sodium borohydride was for convenience as a similar product mixture is available using other reducing reagents such as BH3·THF and Na(OAc)3BH. The alcohols were converted to the corresponding methanesulfonate (mesylate) esters (16). Reaction with sodium in ammonia resulted in simultaneous reductive cleavage of the benzyl ester and fragmentation/reduction of the β-chloro methanesulfonate to furnish cyclobutene 1. Hydrogenation of 1 cleanly generated cyclobutane 2. Alternatively, dehalogenation of initial cycloadduct 15 with zinc in acetic acid, followed by deprotection of the benzyl ester with Pd(C)/H2, furnished cyclobutanone 3. Hydride reduction of the intermediate ketone with hydride prior to hydrogenolysis generated cyclobutanol 4 as a mixture of diastereomers at the newly created alcohol stereocenter.
Scheme 1.
Preparation of analogs in the C10 series (1–4): a) DMAP, DCC, BnOH; b) Zn dust (5 equiv), Cl3CCOCl (2.5 equiv) rt, Et2O; c) NaBH4, 2-propanol; d) MsCl, Et3N; e) Na/NH3, −78 to −33 °C; f) H2, Pd/C; g) Zn (5 equiv), AcOH; h) NaBH4, MeOH.
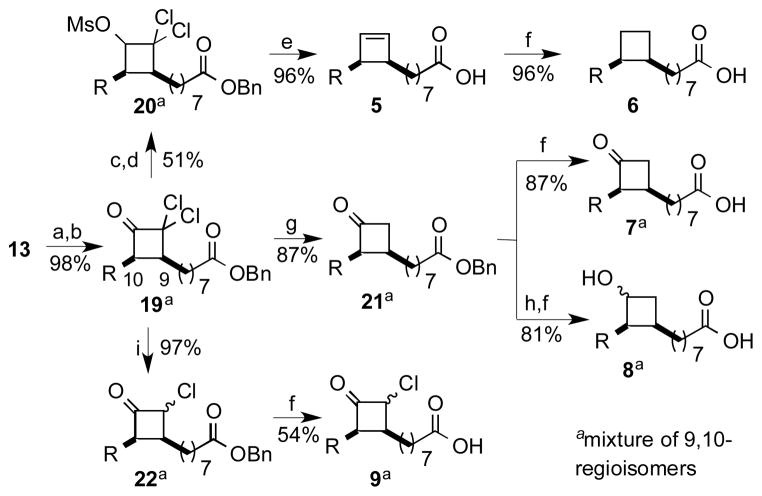
The preparation of analogs incorporating the four-membered ring carbocycles onto a cis C18 framework is illustrated in Scheme 2. In this case, the initial cycloaddition with benzyl oleate produced an inseparable mixture of regioisomeric dichlorocyclobutanones (19). The cycloadducts, which were prone to decomposition during purification, were directly reduced to furnish a mixture of regioisomeric 2,2-dichloro-1-cyclobutanols. The corresponding methanesulfonate (mesylate) esters (20) underwent reduction/fragmentation as described above to furnish the cyclobutene 5,[8] which could be hydrogenated to cyclobutane 6. The related cyclobutanone (7) and cyclobutanol (8) were prepared from the dichlorocyclobutanone in a similar manner as described earlier for the C10 series. Alternatively, controlled dehalogenation of the dichlorocyclobutanone cycloadduct with stoichometric Zn or Zn(Cu) in acetic acid furnished a mixture of regioisomeric monochlorocyclobutanones (22) contaminated by small amounts of dichloroketone (starting material) and cyclobutanone 3 (overreduction).[11] Hydrogenolysis of the monochlorocyclo butanone as before furnished analog 9.
Scheme 2.
Synthesis of analogs 5–9: a) DMAP, DCC, BnOH; b) Zn dust (5 equiv), Cl3CCOCl (2.5 equiv), ether; c) NaBH4, 2-propanol; d) MsCl, Et3N; e) Na/NH3, −78 to −33 °C; f) H2, Pd/C EtOAc; g) Zn (5 equiv), AcOH; h) NaBH4, MeOH, −10 °C; i) Zn (1.1 equiv), AcOH. R = octyl.
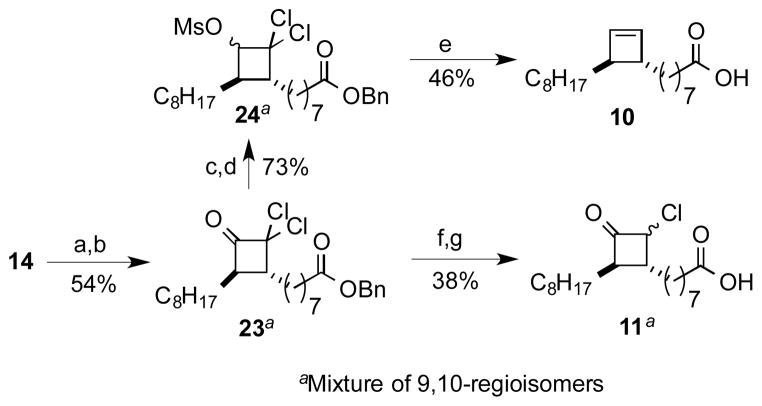
The ready availability of elaidic acid, a C18 fatty acid containing a trans-9,10 alkene, made it possible to investigate the influence of stereoisomers (Scheme 3). Beginning with elaidic acid (14) we were able to synthesize cyclobutene 10 and monochlorocyclobutanone 11 in a manner analogous to that described previously for oleate-derived substrates 5 and 9. The only major difference from the route employed in Scheme 2 was the need to employ activated Zn or Zn/Cu for the initial ketene generation/cycloaddition.[12]
Scheme 3.
Synthesis of analogs 10 and 11: a) DMAP, DCC, BnOH; b) Zn(Cu) (5 equiv), Cl3CCOCl (2.5 equiv), rt, ether; c) NaBH4, 2-propanol; d) MsCl, Et3N; e) Na/NH3, −78 to −33 °C; f) Zn (1.1 equiv), AcOH; g) H2, Pd/C EtOAc.
Inhibitor stability and solubility
Analogs 1 – 11 were each tested for thermal stability using differential scanning calorimetry (DSC) in tandem with thermal gravimetric analysis (TGA); details are provided in the Supporting Information. Although we originally had concerns for the stability of the cyclobutene-containing analogs (1, 5, and 10) towards thermally induced cycloreversion,[13] we observed no decomposition of any analog below 100 °C; most of the new analogs were stable to ≥ 150 °C.
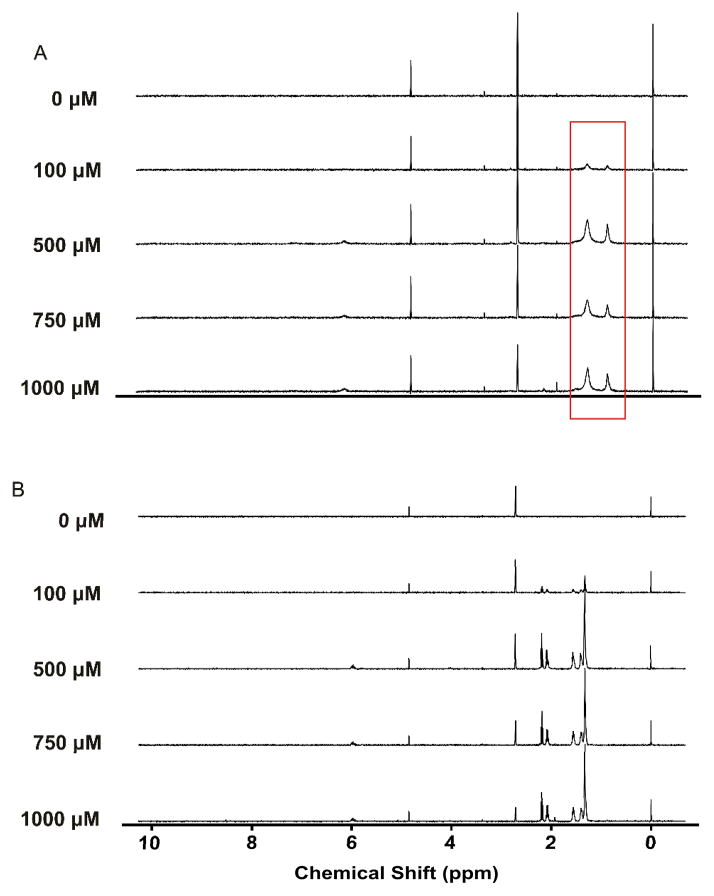
One-dimensional (1D) 1H NMR spectroscopy was used to verify the aqueous stability and solubility for each of the eleven analogs. An example series is illustrated in Figure 2; more detailed data is provided in Supporting Information. The results demonstrate the analogs to be stable in aqueous buffer. All of the analogs based upon a C18 scaffold (5–11) display evidence of aggregation at all concentrations tested (down to 100 mM). In contrast, the analogs based upon the C10 backbone (1–4) were not observed to aggregate at even mM concentrations (Table 1).
Figure 2.
1D 1H NMR spectra of compounds 5 (Figure 2a) and 1 (Figure 2b) were inspected for evidence of micelle formation (peak broadening) by comparing peak widths relative to TMSP (standard). Peak widths for 5 demonstrated potential micelle formation or aggregation over the range of tested concentrations (highlighted region). No micelle formation was observed for compounds 1–4 between 100 – 1000 μM.
Table 1.
Critical micellar coefficient (CMC) of analogs under assay conditionsa
| CMC | ≤ 100 μM | ≥ 1000 μM |
|---|---|---|
| Analogs | 5–11 | 1–4 |
Designated concentration or range indicates onset of aggregation observed by 1H NMR of buffer solutions; see Experimental Section.
MIC determination
MICs were determined against the following bacteria: Escherichia coli (E. coli) wild type strain G58-1; M. smegmatis (Msm) strain mc2155, a non-pathogenic mycobacteria used as a model for Mtb to analyze processes that are likely to be conserved in the genus; and two Mtb strains (CDC1551 and H37Rv). Kanamycin (Kan) was used as a positive inhibition control for E. coli. Isoniazid (Inh), one of the main first-line drugs used to treat TB, and D-cycloserine (DCS), a second line clinically used TB drug, were employed as controls that would allow benchmarking against other inhibitors. Results for the fatty acid analogs are shown in Table 2.
Table 2.
MIC values against E. coli, Msm and Mtb[a]
| Compound | Scaffold | Functionality |
E.Coli (G58-1) Day 2 ug/mL (uM) |
Msm mc2155 Day 4 ug/mL (uM) |
Mtb | ||
|---|---|---|---|---|---|---|---|
| CDC1551 ug/mL (uM) | H37Rv Week 7 ug/mL (uM) | ||||||
|
| |||||||
| LB | Minimal Media | ||||||
| 1 | C10 | alkene | >512 (>2608) | >512 (2608) | 512 (2608) | 16 (82) | 16 (82) |
| 2 | C10 | alkane | - | - | 512 (2582) | 4 (20) | 4 (20) |
| 3 | C10 | ketone | - | - | 256 (1206) | 128 (603) | 256 (1206) |
| 4 | C10 | alcohol | - | - | 128 (597) | 64 (299) | 128 (597) |
| 5 | cis-C18 | alkene | - | - | 128 (415) | 128 (415) | 16 (52) |
| 6 | cis-C18 | alkane | >512 (>1649) | >512 (1649) | 256 (824) | 8 (26) | 16 (52) |
| 7 | cis-C18 | ketone | - | - | 128 (394) | 64 (197) | 32 (99) |
| 8 | cis-C18 | alcohol | - | - | 256 (784) | 8 (24) | 2 (6) |
| 9 | cis-C18 | chloroketone | - | - | 256 (713) | 256 (713) | 16 (45) |
| 10 | trans-C18 | alkene | - | - | 256 (830) | 128 (415) | 16 (52) |
| 11 | trans-C18 | chloroketone | - | - | 128 (357) | 256 (713) | 256 (713) |
| 12 | C10 | alkene(acyclic) | - | - | 512 (3007) | 32 (188) | 32 (188) |
| DCS | 25 (2508) | 16 (157) | 64 (627) | 4 (39) | 8 (78) | ||
| Isoniazid | >512 (>3733) | 16 (117) | 32 (233) | 4 (29) | 8 (58) | ||
| Kanamycin | 64 (110) | < 8 (14) | - | - | - | ||
MIC’s were determined as described in the experimental section.
Neither of the two analogs (1, a C10 cyclobutene and 6, a C18 cyclobutane) tested against E. coli were inhibitory in rich (LB broth) or minimal media. Interestingly, the susceptibility of E. coli towards the control drugs (DCS, Inh and Kan) was greater in minimal media as indicated by the significantly lower MIC values. Preliminary studies indicated that Msm was not susceptible to compounds 7 and 10 in complete Middlebrook 7H9 broth supplemented with either Tween or Tyloxapol (data not shown). In this context, a potential interfering compound is bovine serum albumin that has been shown to bind to fatty acids.[14] Indeed, preliminary tests using Msm and compound 7 indicate that increasing concentrations of BSA significantly increase the MIC value (unpublished results). Thus, the entire set of compounds synthesized was tested in minimal media against Msm and the two Mtb strains. Under these conditions, none of the compounds demonstrated significant activity against Msm. However, four (1, 2, 6, and 8) of the eleven compounds yielded MIC values < 100 μM for both Mtb strains, with two of the compounds (2 and 8) proving superior to DCS on a molar basis. Importantly, compound 2 yielded MIC values equal (CDC1551 strain) or superior (H37Rv strain) to those obtained with Inh. In summary, these compounds were poor inhibitors of Msm but quite effective against Mtb grown in minimal media.
Nonspecific toxicity
A sampling of six analogs, along with decanoic acid (12) and the Mtb therapeutics isoniazid and DCS, were investigated for toxicity in RAW 264.7 macrophages. SDS was employed as a positive control for maximum toxic effect. As illustrated in Table S1 (see Supporting Information), three of the tested analogs (4, 5, and 6), as well as DCS and isoniazid showed little or no toxicity at concentrations below 250 μM;[15] toxicity for 12 was only slightly greater. Modest or significant toxicity was observed for analogs 1, 2, and 8
Discussion
The MIC results demonstrate promising levels of inhibition with a variety of four-membered ring carbocycles (cyclobutanol, cyclobutanone, cyclobutene, and cyclobutane). A critically important component of evaluating new and potentially promising therapeutic entities is validation of a drug-like mode of action. Unfortunately, there are numerous undesirable mechanisms resulting from poor physical behavior of a compound that will result in a false positive in a biological assay. These poor properties may include insolubility, reactivity, micelle formation, impurities, aggregation, instability and non-specific binding. This has resulted in numerous compounds erroneously reported as chemical leads that are not acceptable drug candidates because of these undesirable physiochemical properties.[16] The fatty acid analogs discussed here are thermally stable. All of the analogs based upon a C18 backbone aggregate at concentrations overlapping the MIC values observed for Mtb or Msm. Correspondingly, the biological mechanism of action for analogs 5 to 11 is suspect and these analogs are unlikely to be viable drug-leads. The propensity for these compounds to form micelles or aggregate at concentrations lower than their MIC values must also be considered in any attempt to extract structure-activity relationships. In contrast, the analogs based upon a C10 backbone (1–4) displayed no sign of aggregation at concentrations up to 1000 μM, levels well above the measured MICs (Figure 3). The observation of significant and superior inhibition of Mtb with compounds from the shorter chain series provides strong evidence that the inhibition is specific and not simply due to the general toxicity often observed with very hydrophobic substrates.[16] Although some short-chain fatty acids have also demonstrated limited antimicrobial activity,[7] Mtb is also reported to use decanoic acid (10:0) as a carbon source[17] Moreover, decenoic acid (12), the parent 10:1 framework of our shorter chain analogs, demonstrated very modest levels of inhibition in Mtb. Finally, we note that the extremely hydrophobic oleic acid (13), the parent 18:1 framework of analogs 5–9, is used as a standard nutrient for Mtb at 0.05 g/L (177 μM), a concentration higher than the MICs of the best inhibitors. Nonetheless, oleic acid is always used in media containing bovine serum albumin that may ameliorate its toxicity.[14]
The nature of the functionality has a clear impact on activity. Within the less hydrophobic short-chain series (compounds 1–4), the measured MICs vary by more than sixty fold against Mtb H37Rv and more than thirty fold against strain CDC1551. In contrast, less than a ten-fold difference is observed for MICs measured against Msm. Although the potential for micelle formation is cause for caution in making structure-activity comparisons involving the long chain series, the different influences of a given functional group within the C18 and C10 series are interesting. For example, the short-chain cyclobutanone (3) and cyclobutanol (4) are much less potent toward Mtb than the corresponding long-chain analogs (7 and 8), while the values for the cyclobutenes (1 vs. 5 or 10) are similar. The influence of stereochemistry varies by analog. The MICs for the cis and trans isomers of the C18 monochloroketones (9 and 11) differ by more than ten-fold for Mtb (H37Rv); in contrast, no difference is seen between the cis- and trans-cyclobutenes (5 and 10) with either Mtb strain. Finally, we note that in both the C18 and C10 series, the saturated analogs (cyclobutanes 6 and 2) are considerably more potent than the corresponding cyclobutenes (5 and 1).
Comparing MIC values obtained against Mtb vs. Msm, ten of eleven analogs demonstrate lower MIC values in at least one strain of Mtb compared with Msm; for six of the eleven, lower MIC values are obtained against both strains of Mtb. The ratio of MIC values for Mtb vs. Msm ranges from less than one to over six hundred; viewed across the eleven analogs, the average ratio of MICs for Mtb vs Msm is sixty eight (CDC1551) and nearly ninety (H37Rv). The different levels of inhibition produced in Msm and Mtb may result from a mechanism of action specific to Mtb. Isoniazid, for example, demonstrates 100-fold more potent inhibition of Mtb compared with Msm and possesses low toxicity toward other mycobacteria and prokaryotic pathogens.[21] The lack of inhibition observed in E. coli using two of the analogs (1 and 6) most potent against Mtb could indicate specific inhibition of a metabolic pathway unique to the latter.
The initial hypothesis behind the design of the molecules reported here was that uptake of a fatty acid analog bearing a reactive functional group (for example, cyclobutene, cyclobutanone, chloroketone) might disrupt an enzymatic process related to cell wall synthesis. We imagined that one such target might be methyl transferases, which are common in mycobacteria, but relatively uncommon in other organisms.[18] In an earlier example of a related approach, the inhibition of growth in Mtb observed in the presence of alkyne-containing fatty acids (MICs of 20–25 μM) was attributed to irreversible modification of an enoyl reductase within the mycobacterial FASII system.[4,7] However, the significant inhibition produced by cyclobutanes 2 and 6, which lack a chemically reactive group, is more suggestive of a noncovalent mode of inhibition. The relatively low MIC values indicate our fatty acid analogs have promise as anti-mycobacterial agents, but there is still a need to confirm cellular uptake and to establish the in vivo target.
A variety of carbocyclic fatty acids including cyclopropyl, cyclopentyl, cyclohexyl and cycloheptyl fatty acids are found in bacteria and plants.[19] Their roles, while not always completely understood, may be related to control of membrane fluidity.[20] In the case of mycobacteria, the presence of cyclopropane subunits in mycolic acids is known to be associated with the structural integrity of the cell wall and the ability of the tubercle bacillus to resist oxidative stress inside macrophages. In contrast, cyclobutane fatty acids are rare. An unusual fatty acid incorporating a ladder-type structure of fused cyclobutanes, recently isolated from anammox bacteria found near undersea vents, is believed to contribute to the formation of dense and impermeable lipid membranes that protect the bacteria from harsh chemical environments.[19] Thus, as an alternative to disrupting mycolic acids biosynthesis, it is also plausible that our four-membered ring analogs are converted into analogs of mycolic acids and that the physical properties of these unnatural cell wall constituents results in cell death. However, it must be noted that while mycobacteria are known to directly incorporate structurally modified fatty acid feedstocks into mycolic acid biosynthesis,[6] there is as yet no evidence that the same is true for the specific fatty acid analogs described here.[7] Indeed, further studies will be required in order to determine an in vivo mechanism of inhibition for our compounds.
In this context, it is worth noting that our initial choice of molecular scaffold was based upon the availability of low-cost precursors. If the modified fatty acid chains are indeed taken up into the mycobacterial biosynthetic pathways, greater activity or different specificities might be identified through the use of different molecular backbones or alternative positioning of the four-membered ring carbocycles.
Conclusion
Analogs of fatty acids incorporating four-membered ring carbocycles may provide a starting point for the development of new antimycobacterials. Four of eleven analogs tested yielded MIC values < 100 μM for both Mtb strains, with two of the analogs proving more active than DCS and one analog proving equally potent or superior to Inh, one of the main first-line drug used for treatment of tuberculosis.
Experimental Section
General procedures
All reagents and solvents were used as purchased except for pyridine and CH2Cl2 (distilled from CaH2 and kept under N2) and THF (distilled from Na/benzophenone under N2). Reactions were performed under a N2 atmosphere unless stated otherwise. Thin-layer chromatography (TLC) was performed on 0.25 mm hard-layer silica G plates; developed plates were visualized with a hand-held UV lamp or by staining: 1% Ce(SO4)2 and 10% (NH4)2MoO4 in 10% aq. H2SO4 (general stain, after heating); 1% aq. KMnO4 (for unsaturated compounds); 3% vanillin in 3% H2SO4 in EtOH (general stain, after heating). Unless otherwise noted, NMR spectra were recorded at 400 MHz (1H) or 100 MHz (13C) in CDCl3; peaks are reported as: chemical shift (multiplicity, J couplings in Hz, number of protons); “app” and “br” refer to apparent and broad signals, respectively. IR spectra were recorded as neat films (ZnSe, ATR mode) with selected absorbances reported in wavenumbers (cm−1). Flash chromatography was performed on 32–60 μm silica gel. Preparative HPLC was performed on a 21 × 250 mm normal phase Si (8 micron) column at 10 mL/min of the indicated solvent unless otherwise noted. Analytical purity of compounds was checked using an analytical column (250 mm × 4.6 mm; Microsorb) at 1 mL/min of 20% EtOAc/Hexane; detection was accomplished using a differential refractometer interfaced with a data module. All compounds tested for biological activity showed >97% purity by HPLC analysis except for 5 and 7. Melting points are uncorrected.
3-(Octanoic acid, 8-yl, benzyl ester) 2,2-dichlorocyclobutanone (15) was prepared similarly to compound 23 and afforded (13.8 g, 80%) as a brown oil from benzyl 9-decenoate (12.4 g, 48 mmol), trichloroacetyl chloride (10.7 mL, 95 mmol), and Zn(Cu) (15.55 g, 238 mmol) in ether 0.1 M (340 mL). Consistent with a previous report, only one isomer was observed.[22] The assignment was confirmed by observation of the pair of geminal hydrogens adjacent to the ketone at δ 3.33 and 2.94 by 1H-NMR and by the observation of eleven rather than twelve carbons (symmetry) upon dechlorination to cyclobutanone 3: Rf = 0.34 (10% EtOAc/Hex); IR: 2935, 2852, 1814, 1730, 905, 725 cm−1; 1H NMR: δ 7.39-7.29 (m 5H), 5.12 (s, 2H), 3.33 (dd, J = 9.4, 17.2, Hz, 1H), 2.94 (dd, J = 9.0, 17.2 Hz, 1H), 2.88-2.81 (m, 1H), 2.37/2.36 (overlapping t, J = 7.5 Hz, 2H), 1.94-1.84 (m, 1H), 1.70-1.72 (m, 3H), 1.46-1.28 (m, 8H); 13C NMR: δ 192.8,173.4, 136.0, 128.4, 128.0, 88.8, 65.9, 47.7, 45.8, 34.1, 31.2, 29.0, 28.9, 28.8, 27.2, 24.7; HREI-MS calcd. for C19H25O3Cl2: [M+H]+ 371.1181; found: 371.1188.
3-(Octanoic acid, 8-yl, benzyl ester) 2,2-dichlorocyclobutanol, methanesulfonate ester (16) was prepared similarly to compound 20 from the previous dichloroketone 15 (10.0 g, 26.9 mmol), NaBH4 (1.53 g, 40.4 mmol), and iPrOH (350 mL). The crude product was purified by flash column chromatography with 10% EtOAc/Hex to furnish the dichlorobutanol (5.76 g, 57%) as a colorless oil: Rf = 0.39 (20% EtOAc/Hex); IR: 3440 (br), 2927, 2854, 1733, 1164, 960, 697 cm−1; 1H NMR: δ 7.40-7.30 (m 5H), 5.12 (s, 2H), 4.73-4.64 (m, 0.1H) 4.34-4.27 (m, 0.9H), 2.97 (d, J = 8.2 Hz, 0.1H, OH is trans to backbone), 2.87 (d, J = 10.3 Hz, 0.9H, OH is cis to backbone), 2.48-2.34 (m, 1H), 2.36 (t, J = 7.6 Hz, 2H), 1.78-1.60 (m, 3H), 1.53-1.28 (m, 11H); 13C NMR: δ 173.7, 136.0, 128.5, 128.1, 92.9, 75.4, 66.1, 45.6, 34.5, 34.2, 29.8, 29.2, 29.0, 28.9, 26.5, 24.8; HRESI-MS calcd. for C19H23O3Cl2 [M+Na]+: 395.1157; found: 395.1151.
Conversion to the methanesulfonate was performed as described for compound 20, using methanesulfonyl chloride (20 mL, 29.4 mmol), the dichlorobutanol (5.50 g, 14.7 mmol), and triethylamine (9.20 mL, 66.3 mmol) in CH2Cl2 (70 mL). The crude product was purified by flash chromatography (10% EtOAc/Hex) to afford 16 as a light yellow oil (6.13 g, 92%): Rf = 0.36 (20% EtOAc/Hex); IR: 2927, 2858, 1731, 1364, 1179, 952 cm−1; 1H NMR: δ 7.36-7.31 (m 5H), 5.11 (s, 2H), 5.13-5.09 (m, 1H), 3.18 (s, 3H), 2.56-2.50 (m, 2H), 2.35 (t, J = 7.6 Hz, 2H), 1.93-1.28 (m, 13H); 13C NMR: δ 173.5, 136.0, 128.4, 128.1, 88.4, 78.3, 66.0, 45.8, 39.3, 34.1, 31.6, 29.8, 29.1, 28.87, 28.85, 26.2, 24.8; HRESI-MS calcd. for C20H28O5Cl2SNa [M+Na]+: 473.0932; found: 473.0930.
8-(2-Cyclobuten-1-yl)-octanoic acid (1) was prepared by a similar procedure as employed for 5 via reaction of Na metal (2.80 g, 120 mmol) with a solution of 5.40 g (12.0 mmol) of the mixture of methanesulfonate 16 in THF (30 mL) and NH3 (~ 250 mL). The crude product was purified by flash column chromatography (2% EtOAc/Hex) to afford 9,10-ethenooctadecanoic acid 1 (1.64 g, 52 %) as a low-melting white solid: Rf = 0.47 (6:4:0.05 of Hex:EtOAc:AcOH); m.p. = 20–21 °C; IR: 3112 (br), 3043, 2920, 2853, 1705, 697 cm−1; 1H NMR: δ 10.27 (br s,1H, COOH), 6.11 (br d, J = 2.4 Hz, 1H), 6.04 (br dd, J = 0.8, 2.0 Hz, 1H), 2.77 (m, 1H), 2.66 (ddd, J = 0.8, 4, 13.2 Hz, 1H), 2.35 (t, J = 7.2 Hz, 2H), 2.04 (d, J = 13.2 Hz, 1H), 1.64 (quintet, J = 7.2 Hz, 2H), 1.51-1.38 (br m, 2H), 1.38-1.23 (br m, 8H); 13C NMR: δ 180.0, 141.1, 135.1, 44.2, 36.9, 34.6, 34.0, 29.5, 29.2, 29.0, 27.9, 24.7; HRESI-MS calcd. for C12H20O2Na [M+Na]+: 219.1361; found: 219.1355. Purity 100% by HPLC (retention time, 5.01 min).
8-Cyclobutyloctanoic acid (2) was prepared by a similar procedure as for 6 from the hydrogenation of cyclobutene 1 (0.034, 0.170 mmol), over 10% Pd/C (0.034 g, 0.030 mmol) in EtOAc (1.7 mL) to afford the unprotected acid 2 (0.030 g, 89%) as a low-melting white solid: Rf = 0.47 (6:4:0.05 of Hex:EtOAc:AcOH); m.p. = 28–30 °C; IR: 3035 (br), 2916, 2849, 1695, 909, 733 cm−1; 1H NMR: δ 11.34 (br s, 1H, COOH), 2.37 (t, J = 7.2, 2H), 2.25 (septet, J = 7.2, 1H), 2.10-1.98 (m, 2H), 1.90-1.75 (m, 2H), 1.70-1.52 (m, 2H), 1.40-1.12 (br m, 10H); 13C NMR: δ 180.3, 37.0, 36.2, 34.1, 29.4, 29.2, 29.0, 28.4, 27.1, 24.7, 18.5; HRCI-MS calcd. for C12H22O2: [M+H]+ 199.1698; found: 199.1695. Purity > 99% by HPLC (retention time, 4.92 min).
3-(Octanoic acid benzyl ester, 8-yl) cyclobutanone (17) was prepared in a similar manner as 21, via reaction of dichloroketone 15 (1.05 g, 2.69 mmol), 6 mL of glacial acetic acid, and Zn(Cu) (1.76 g, 26.9 mmol). The product was purified by flash chromatography (10% EtOAc/Hex) to afford a colorless oil (0.4859 g, 59%) which was a mixture of regioisomers: Rf = 0.55 (20% EtOAc/Hex). IR: 2923, 2854, 2780, 2732, 1163, 730, 697 cm−1; 1H NMR: δ 7.35-7.28 (br m, 5H), 5.09 (s, 2H), 3.13-3.03 (m, 2H), 2.65-2.57 (m, 2H), 2.33 (t, J = 7.2, 2H), 2.33-2.26 (m, 1H), 1.68-1.58 (br quintet, J = 6.4, 2H), 1.58-1.48 (br q, J = 7.2, 2H), 1.29 (br s, 8H); 13C NMR: δ 208.2, 173.3, 134.0, 128.3, 127.9, 65.8, 52.3, 36.1, 34.0, 28.9, 28.8, 28.0, 24.7, 23.6; HRESI-MS calcd. for C19H26O3Na [M+Na]+: 325.1780; found: 325.1775.
3-(Octanoic acid, 8-yl) cyclobutanone (3) was prepared by a similar procedure as for 7 through reaction of benzyl ester 17 (0.0346, 0.1 mmol), 10% Pd/C (0.020 g, 0.02 mmol) and EtOAc (1 mL). The product was purified by flash column chromatography on silica gel (step gradient of 20%/40% EtOAc/Hex) to afford acid 3 (0.0189 g, 90%) as a low-melting white solid: Rf = 0.3 (6:4:0.05 of Hex:EtOAc:AcOH); m.p. = 35–37 °C; IR: 3094 (br), 2923, 2853, 1780, 1705 cm−1; 1H NMR: δ 10.61 (br s, 1H, COOH), 3.17-3.08 (m, 2H), 2.69-2.61 (m, 2H), 2.35 (br t, J = 7.6, 2H), 2.39-2.31 (br, 1H), 1.69-1.53 (br m, 4H), 1.32 (br s, 8H); 13C NMR: δ 208.8, 179.9, 52.5, 36.3, 34.0, 29.2, 29.1, 28.9, 28.2, 24.6, 23.8; HRCI-MS calcd. for C12H20O3Na [M+Na]+: 213.1491; found: 213.1490. Purity > 97% by HPLC (retention time, 10.75 min).
3-(Octanoic acid benzyl ester) cyclobutanol (18) was prepared by a similar procedure as compound 8 via reaction of cyclobutanone 17 (0.0509 g, 0.17 mmol) and NaBH4 (0.013 g, 0.34 mmol) in anhydrous MeOH (2.5 mL). 18 (after purification by flash column chromatography with 20% EtOAc/Hex) was isolated as a low-melting white solid (0.040 g, 76%). The product was a 9:1 mixture of cis/trans stereoisomers based on the previous assignments for cyclobutanol 8. Rf = 0.29 (20% EtOAc/Hex); m.p. = 30–31 °C; IR: 3356 (br), 2922, 1734, 1154, 735, 696 cm−1; 1H NMR: δ 7.40-7.30 (m, 5H), 5.11 (s, 2H), 4.42-4.34 (br m, 0.1H), 4.14-4.03 (br m, 0.9H), 2.48-2.40 (m, 0.18H), 2.35 (t, J = 7.2, 0.18H), 2.16-2.04 (m, 0.2H), 1.95 (t, J = 6.6, 0.2H), 1.70-1.56 (br m, 3H), 1.48-1.13 (br m, 12H); 13C NMR: δ 173.6, 136.0, 128.4, 128.1, 66.0, 63.7, 39.7, 37.0, 34.2, 29.2, 29.1, 29.0, 27.3, 25.4, 24.82; HRCI-MS calcd. for C19H29O3Na [M+Na]+: 305.2117; found: 305.2116.
3-(Octanoic acid) cyclobutanol (4) was prepared by a similar procedure as employed for 8 through reaction of benzyl ester 18 (0.034g, 0.110 mmol), 10% Pd/C (0.024 g, 0.022 mmol) and EtOAc (1 mL). The crude product was purified by flash column chromatography (40% EtOAc/Hex) to afford acid 4 (0.013 g, 54%) as a white solid: Rf = 0.24 (6:4:0.05 of Hex:EtOAc:AcOH); m.p. = 74-75 °C; IR: 3341 (br), 2916, 2849, 1695, 1064 cm−1; 1H NMR (MeOD): δ 4.30 (quintet, J = 6.8, 0.1H), 4.01 (quintet, J = 6.4, 0.9H), 2.45-2.35 (br m, 2H), 2.29 (t, J = 7.2, 2H), 1.75-1.55 (br m, 3H), 1.50-1.15 (br m, 12H); 13C NMR (MeOD): δ 176.3, 62.8, 38.9, 36.9, 36.8, 33.6, 29.1, 29.0, 28.8, 27.1, 25.4, 24.7; HRESI-MS calcd. for C12H22O3Na [M+Na]+: 237.1467 found: 237.1460. Purity > 97% by HPLC (retention time, 9.65 and 11.36 min).
2,2-Dichloro-4-(octanoic acid benzyl ester, 8-yl) 3-octylcyclobutanone-; 2,2-Dichloro-3-(octanoic acid benzyl ester, 8-yl) 4-octylcyclobutanone (19) was prepared by a modification of a known procedure.[22–24] To a mixture of zinc dust (0.879 g, 13.4 mmol) and benzyl oleate 19 (1.02 g, 2.73 mmol) in 20 mL of anhydrous ether was slowly added a solution of trichloroacetyl chloride (0.75 mL, 6.8 mmol) in 7 mL of diethyl ether via dropping funnel over a period of 45 min. The reaction was stirred until judged complete by the absence of starting material (4 h, TLC). The reaction mixture was filtered through a plug of Celite and the residue was washed with ether (2 × 75 mL). The organic solution was stirred a minimum with 50% aq. NaHCO3 and the separated aqueous layer back-extracted with ether (2 × 20 mL). The combined organic layers were dried over Na2SO4 and concentrated under vacuum to give g of the product (1.30 g, 99%) as a yellow oil. The unpurified material was generally used directly for the next reaction because of a tendency to decompose during chromatography. However, small quantities could be purified by rapid flash chromatography (5% EtOAc/Hex) to afford a 1:1 mixture of regioisomeric dichlorocyclobutanones 19 as a colorless oil: Rf = 0.53 (10% EtOAc/Hex); IR: 2926, 2855, 1800, 1735, 1456, 1162, 696 cm−1; 1H NMR: δ 7.36-7.32 (5H), 5.120/5.116 (overlapping s, 2H), 3.55-3.49 (m, 1H) 2.97-2.93 (m, 1H), 2.37/2.35 (overlapping t, J = 7.6 Hz, 2H), 1.74-1.62 (m, 4H), 1.66-1.48 (m, 4H), 1.45-1.27 (br m, 18H), 0.89/0.88 (overlapping triplets, J = 6.9 Hz, total 3H); 13C NMR: δ 197.5, 173.5, 136.1, 128.5, 128.1, 88.1, 66.0, 58.0, 57.9, 49.4, 49.4, 34.2, 31.8, 31.80, 29.60, 29.3, 29.3, 29.2, 29.18, 29.15, 29.12, 29.0, 28.9, 28.9, 28.04, 27.96, 26.72, 26.68, 26.3; HRFAB-MS (3-NBA+Li matrix: calcd. for C27H40O3Cl2Li [M+Li]+:489.2515; found: 489.2499.
2,2-Dichloro-4-(octanoic acid benzyl ester, 8-yl) 3-octylcyclobutanol, methanesulfonate ester; 2,2-Dichloro-3-(octanoic acid benzyl ester, 8-yl) 4-octylcyclobutanol, methanesulfonate ester (20). Reduction of the dichlorocylobutanone 19 was achieved using a modification of a known procedure.[9,24] To a 0 °C solution of dichlorocyclobutanones 19 (1.5 g, 3.1 mmol) in iPrOH (45 mL) was added NaBH4 (0.176 g, 4.65 mmol). The reaction was allowed to slowly warm to rt and stirred until starting material had disappeared (TLC, ~ 2.5h; byproducts tended to accumulate upon prolonged reaction). The reaction was then cooled to 0 °C and quenched with 1N HCl (40 ml). The solution was stirred for an additional 30 min, concentration to one third the original volume, and then extracted with EtOAc (3 × 100 mL). The organic layer was washed sequentially with water/sat. NaHCO3/brine and then dried over Na2SO4. Evaporation of the organic solvent and flash chromatography of the residue on silica gel, using a step gradient of 5%/10% EtOAc/Hex, gave (0.821 g, 55% yield) of a 1:1 mixture of regioisomeric cyclobutanols as a colorless oil. The products were predominantly a regioisomeric mixture of the cis,cis diastereomers[22] based on 1H NMR, HSQC, 2D-COSY and 2D-NOESY analysis:[25] Rf (10% EtOAc/Hex) = 0.33 (major, OH is cis to backbone) 0.28 (minor, OH is trans to backbone), 0.2 (major, OH is cis to backbone) and 0.15 (minor, OH is trans to backbone); IR: 3473, 3036, 2924, 2854, 1733, 1456, 1168, 696 cm−1; 1H NMR: δ 7.36-7.32 (m, 5H), 5.116/5.112 (app s, total 2H), 4.55 (dd, J = 10.8, 8.8 Hz, 0.92H, CH-OH, cis to the backbone assigned based on 2D-NOESY analysis and 4Jdiagonal H = 0), 3.99 (br t, J = 10 Hz, 0.08H), 2.75-2.69 (m, 1H), 2.64/2.62 (overlapping d, J = 10.8 Hz, total 1H, -OH proton assigned based on the disappearance of this peak upon D2O addition), 2.60-2.55 (m, 1H), 2.36/2.35 (overlapping t, J = 7.6 Hz, total 2H), 1.67-1.62 (bm, 3H), 1.52-1.19 (24H), 0.89/0.88 (overlapping t, J = 6.8 Hz, total 3H). Small quantities of the individual isomers could be isolated by semipreparative HPLC (10% EtOAc/Hex, RI detection):
First-eluting cis
Rf = 0.33; IR: 3457, 2924, 2854, 1736, 1456, 1170, 697 cm−1; 1H NMR: δ 7.37-7.32 (m, 5H), 5.12 (s, 2H), 4.55 (dd, J = 10.8, 8.8 Hz, 1H, CH-OH, cis to the backbone assigned based on 2D-NOESY analysis and 4Jdiagonal H = 0), 2.72 (dt, J = 9.2, 5.6 Hz, 1H), 2.59 (d, J = 10.8 Hz, 1H, -OH), 2.55 (app dq, J = 9.2, 5.6 Hz, 1H), 2.36 (t, J = 7.6 Hz, 2H), 1.67-1.51 (br m, 3H), 1.52-1.13 (m, 24H), 0.88 (app t, J = 6.8 Hz, 3H); 13C NMR: δ 173.6, 136.1, 128.5 (two overlapping signals), 128.2 (two overlapping signals), 93.5, 77.2, 66.1, 49.5, 40.9, 34.3, 31.8, 29.9, 29.4, 29.4, 29.3, 29.2, 29.0, 27.0, 25.7, 24.9, 23.2, 22.7, 14.1; HRFAB-MS (3-NBA matrix): calcd. for C27H43O3Cl2 [M+H]+: 485.2589; found: 485.2572.
First-eluting trans
Rf = 0.28; IR: 3449, 3028, 2920, 2850, 1736, 1456, 1157, 696 cm−1; 1H NMR: δ 7.37-7.32 (5H), 5.12 (s, 2H), 3.99 (ddd, J = 10.0, 8.4, 1.2 Hz, 1H, CH-OH, trans to the backbone assigned based on 2D-NOESY analysis and 4Jdiagonal H = 1.2 Hz), 2.68-2.64 (m, 1H), 2.52 (d, J = 10.0 Hz, 1H, -OH), 2.34 (t, J = 7.6 Hz, 1H), 2.29-2.20 (m, 1H), 1.67-1.26 (br m, 27H), 0.88 (app t, J = 6.8 Hz, 3H); 13C NMR: δ 173.6, 136.1, 128.5 (two overlapping signals), 128.2 (two overlapping signals), 91.1, 82.6, 66.1, 50.3, 44.4, 34.3, 31.9, 29.7, 29.5, 29.2, 29.04, 29.02, 28.4, 28.23, 28.15, 27.7, 24.9, 22.7, 14.1; ESI-MS calcd. for C27H42O3Cl2Na [M+Na]+: 507.2409; found: 507.2422.
Second-eluting cis
Rf = 0.20; IR: 3464, 2924, 2854, 1736,1456, 1169, 696 cm−1; 1H NMR: δ 7.37-7.32 (m, 5H), 5.11 (s, 2H), 4.55 (dd, J = 10.8, 8.8 Hz, 1H CH-OH, cis to the backbone assigned based on 2D-NOESY analysis and 4Jdiagonal H = 0), 2.72 (dt, J = 9.2, 5.6 Hz, 1H), 2.65 (d, J = 10.8 H z, 1H, -OH), 2.54 (app dq, J = 9.2, 5.6 Hz, 1H), 2.35 (t, J = 7.2 Hz, 2H), 1.68-1.60 (bm, 3H), 1.52-1.13 (m, 24H), 0.89 (app t, J = 6.8 Hz, 3H); 13C NMR: δ 173.7, 136.1, 128.5 (two overlapping signals), 128.2 (two overlapping signals), 93.5, 77.2, 66.1, 49.5, 40.9, 34.3, 31.8, 29.6, 29.4, 29.2, 29.1, 29.04, 29.00, 27.0, 25.8, 24.8, 23.2, 22.7, 14.1; HRFAB-MS (3-NBA matrix): calcd. for C27H43O3Cl2 [M+H]+: 485.2589; found: 485.2581
Second-eluting trans
Rf = 0.15; IR: 3464, 3033, 2925, 2854, 1738, 1498, 1160, 697 cm−1; 1H NMR: δ 7.37-7.32 (m, 5H), 5.11 (s, 2H), 3.99 (ddd, J = 10.0, 8.4, 1.2 Hz, 1H, CH-OH, trans to the backbone assigned based on 2D-NOESY analysis and 4Jdiagonal H = 1.2 Hz), 2.69-2.64 (m, 1H), 2.56 (d, J = 10.0 Hz, 1H, -OH), 2.35 (t, J = 7.6 Hz, 2H), 2.26-2.22 (m, 1H), 1.66-1.26 (m, 27H), 0.88 (app t, J = 6.8 Hz, 3H); 13C NMR: δ 173.7, 136.1, 128.5 (two overlapping signals), 128.2 (two overlapping signals), 91.1, 82.6, 66.1, 50.3, 44.3, 34.3, 31.8, 29.7, 29.4, 29.2, 29.04, 28.97, 28.5, 28.21, 28.18, 27.5, 24.8, 22.7, 14.1; HRESI-MS calcd. for C27H42O3Cl2Na [M+Na]+: 507.2409; found: 507.2426.
The mixture of regioisomeric cyclobutanols converted to the methanesulfonate using a variant of published procedures.[9,24] Methanesulfonyl chloride (62 mL, 0.80 mmol) was slowly added to a stirred 0 °C solution of the cyclobutanols (0.193 g, 0.398 mmol) and triethylamine (0.25 mL, 8.0 mmol) in CH2Cl2 (2 mL) over 15 min. The reaction was allowed to slowly warm to rt and was stirred for 17 h or until no starting material remained (TLC). The reaction was diluted with CH2Cl2 (75 mL) and washed sequentially with 1N HCl (2 × 25 mL), sat aq. NaHCO3 (2 × 25 mL) and water (2 × 25 mL). The organic solution was then dried with Na2SO4 and concentrated under reduced pressure. The residue was purified by flash chromatography using 10% EtOAc/Hex to give 20 (0.206 g, 92%) as a light yellow oil consisting of a 1:1 mixture of regioisomers, each a 92:8 mixture of cis/trans stereoisomers based upon 1H NMR: Rf (10% EtOAc/Hex) = 0.31 (first trans), 0.26 (first cis/second trans), 0.21(second cis); IR: 3032, 2925, 2855, 1735, 1458, 1180, 696 cm−1; 1H NMR: δ 7.36-7.33 (m, 5H), 5.33 (d, J = 9.6 Hz, 0.92H), 5.114/5.109 (two s, total 2H), 4.84 (d, J = 7.6 Hz, 0.08H), 3.208/3.205 (two s, total 3H), 2.76-2.74 (m, 2H), 2.36/2.34 (two overlapping t, J = 7.6 Hz, total 2H), 1.66-1.60 (m, 4H), 1.46-1.26 (m, 22H), 0.88/0.87 (overlapping t, J = 6.8 Hz, total 3H). Small quantities of individual isomers could be separated for analysis by HPLC using 10% EtOAc/Hex. The stereochemical assignments were confirmed by 2D-NMR experiments and by preparation of individual methanesulfonates from individual stereoisomers of alcohol 19, prepared as described in the previous step.
First eluting trans
Rf = 0.31; IR: 3032, 2926, 2855, 1737, 1733, 1456, 1180, 697 cm−1; 1H NMR: δ 7.37-7.32 (5H), 5.12 (s, 2H), 4.84 (d, J = 7.6 Hz, 1H), 3.20 (s, 3H), 2.74-2.68 (m, 2H), 2.36 (t, J = 7.6 Hz, 2H), 1.67-1.32 (br m, 26H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR: δ 173.6, 136.1, 128.5 (two overlapping signals), 128.2 (two overlapping signals), 86.4, 85.8, 66.1, 50.5, 41.1, 39.5, 34.3, 31.8, 29.5, 29.4, 29.3, 29.2, 29.01, 28.98, 28.3, 28.1, 27.6, 27.1, 24.9, 22.6, 14.1; HRESI-MS calcd. for C28H44O5Cl2S [M+Na]+: 585.2184; found: 585.2188.
Second-eluting trans
Rf = 0.26; IR: 3040, 2927, 2855, 1734, 1456, 1179, 697 cm−1; 1H NMR: δ 7.37-7.32 (m, 5H), 5.11 (s, 2H), 4.84 (dd, J = 8.4, 0.8 Hz, 1H), 3.19 (s, 3H), 2.76-2.65 (m, 2H), 2.35 (t, J = 7.6 Hz, 2H), 1.67-1.32 (26H), 0.89 (t, J = 6.8 Hz, 3H); 13C NMR δ 173.6, 136.1, 128.5 (two overlapping signal), 128.2 (two overlapping signal), 86.4, 85.7, 66.1, 50.5, 41.1, 39.5, 34.3, 31.8, 29.6, 29.31, 29.25, 29.2, 29.0, 28.3, 28.1, 27.6, 27.0, 24.9, 22.7, 14.1: HRESI-MS; calcd. for C28H44O5Cl2S [M+Na]+: 585.2184; found: 585.2205.
First-eluting cis
Rf = 0.26; IR: 2926, 2855, 1736, 1456, 1182, 697 cm−1; 1H NMR: δ 7.37-7.32 (m, 5H), 5.33 (app d, J = 9.6 Hz, 1H), 5.11 (s, 2H), 3.19 (s, 3H), 2.80-2.72 (bm, 2H), 2.36 (app t, J = 7.6 Hz, 2H), 1.70-1.63 (m, 4H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR: δ 173.6, 136.0, 128.5 (two overlapping signal), 128.1 (two overlapping signal), 88.9, 80.7, 66.0, 49.5, 40.2, 39.2, 34.2, 31.8, 29.7, 29.3, 29.3, 29.2, 29.0, 28.8, 26.7, 25.7, 24.8, 23.7, 22.6, 14.1: HRFAB-MS (3-NBA matrix): calcd. for C28H45O5Cl2S [M+H]+: 563.2365; found: 563.2349.
Second-eluting cis
Rf = 0.21; IR: 2925, 2855, 1735, 1457, 1181, 697 cm−1; 1H NMR: δ 7.36-7.32 (m, 5H), 5.33 (app d, J = 9.6 Hz, 1H), 5.11 (s, 2H), 3.20 (s, 3H), 2.80-2.69 (bm, 2H), 2.34 (app t, J = 7.6 Hz, 2H), 1.74-1.60 (4H), 1.50-1.19 (22H); 13C NMR: δ 173.6, 136.1, 128.5 (two overlapping signal), 128.1 (two overlapping signal), 89.0, 80.7, 66.0, 49.5, 40.2, 39.3, 34.2, 31.8, 29.5, 29.5, 29.3, 29.2, 28.9, 28.9, 28.7, 26.8, 25.7, 24.8, 23.7, 22.6, 14.1; HRFAB-MS (3-NBA matrix): calcd. for C28H45O5Cl2S [M+H]+: 563.2365; found: 563.2360.
1-(Octanoic acid, 8-yl)-2-octylcyclobutene (5) was prepared from the regioisomeric mixture of dichloromethanesulfonates 20 using a reported procedure.[9,24] NH3 (~ 15 mL, liquid) was condensed into a 100 mL three-necked round bottom flask fitted with a dry ice/acetone condenser. Sodium (0.113 g, 4.90 mmol, sliced under Hex) was added in small pieces. The resulting deep blue solution was stirred for 20 min. A solution of 0.276 g (0.49 mmol) of 20 in anhydrous THF (1.5 mL) was added over 10 min. The cooling bath was removed and the reaction was allowed to stir at ~ −35 °C (refluxing NH3) 5–30 min (depending on the scale of preparation). The reaction was then recooled to −78 °C (dry ice/acetone) and stirred for 2 h or until the starting material was consumed by TLC. Saturated aq. NH4Cl was slowly added until the blue color was no longer visible. The condenser was removed and the reaction mixture was slowly allowed to warm to 0 °C with evaporation of NH3. The remaining reaction mixture was diluted with 30 mL of water and the suspension was extracted with CH2Cl2 (3 × 50 mL). The organic layers were combined and washed sequentially with 1N aq. HCl/water/saturated aq. NaHCO3/water/brine. The organic solution was then dried with NaSO4, and concentrated under reduced pressure. The residue was purified by flash column chromatography using 10% EtOAc/Hex to afford the cyclobutene carboxylic acid 5 (0.131 g, 87%) as a low-melting white solid: Rf = 0.50 (80:20:1 of Hex:EtOAc:AcOH); mp = 33–35 °C; IR: 3493, 3036, 2923, 2853, 1708,1467, 1275, 728 cm−1; 1H NMR: δ 10.94 (br s, 1H, COOH), 6.17-6.15 (br m, 2H), 2.83-2.75 (br s, 2H), 2.35 (t, J = 7.6 Hz, 2H), 1.68-1.60 (br m, 2H), 1.52-1.40 (br m, 2H), 1.35-1.20 (23H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR (found 18 C) δ 180.6, 140.0, 139.9, 46.8, 46.7, 34.1, 31.9, 29.9, 29.8, 29.8, 29.7, 29.6, 29.5, 29.3, 29.3, 29.2, 29.0, 28.3, 28.2, 24.6, 22.7, 14.1; HRFAB-MS (Gly matrix) calcd. for C20H35O2 [M-H]−: 307.2637; found: 307.2636. Purity >99 % by HPLC (retention time, 4.63 min).
cis-9,10-Ethanooctadecanoic acid (6). A solution of cyclobutene 5 (0.031 g, 0.100 mmol) and 10% Pd/C (0.020 g, 0.020 mmol) in EtOAc (1 mL) was placed in a vial under an atmosphere of H2 and stirred at rt overnight (16 h). The mixture was then filtered through a plug of Celite, which was washed with approximately 10 mL of EtOAc. The solution was then evaporated under vacuum to afford acid 6 (0.030 g, 96%) as a colorless oil which was sufficiently pure to use without chromatography: Rf = 0.50 (80:20:1 of Hex:EtOAc:AcOH); IR: 3051 (br), 2921, 2852, 1708 cm−1; 1H NMR: δ 10.93 (br s, 1H, COOH), 2.35 (t, J = 7.3, 2H), 2.24 (br s, 2H), 2.00-1.90 (br m, 1H), 1.64 (quintet J = 7.3 Hz, 2H), 1.60-1.50 (m, 2H), 1.45-1.10 (br m, 24H), 0.88 (br t, J = 7.0 Hz, 3H); 13C NMR: δ 180.2, 37.7, 37.6, 34.1, 31.9, 30.11, 30.05, 29.9, 29.7, 29.7, 29.4, 29.3, 29.1, 27.64, 27.55, 24.8, 24.7, 22.7, 14.1; HRCI-MS calcd. for C20H38O2 [M+H]+: 311.2950; found: 311.2949. Purity 100% by HPLC (retention time, 4.56 min).
2-(Octanoic acid benzyl ester, 8-yl) 3-octylcyclobutanone and 3-(Octanoic acid benzyl ester, 8-yl) 2-octylcyclobutanone (21) was prepared by a modification of a known procedure.[22–24] To a solution of the regioisomeric dichloroketones 19 (0.670 g, 1.40 mmol) in glacial acetic acid (3 mL) was added Zn(Cu) (0.450 g, 6.90 mmol). The mixture was stirred at rt under N2 until TLC showed complete disappearance of starting material (~5 h). The reaction mixture was then filtered through a plug of Celite and washed with ether. The filtrate was washed sequentially with saturated aq. NaHCO3 and water, and dried over Na2SO4. The residue obtained upon concentration was purified by flash chromatography on silica gel (5% EtOAc in Hex) to afford (0.388 g, 87%) of a mixture of cyclobutanones 21 as a colorless oil: Rf = 0.41 (10% EtOAc/Hex); IR: 3034, 2923, 2853, 1774, 1735, 1161, 696 cm−1; 1H NMR: δ 7.36-7.26 (br m, 5H), 5.115 (s, 2H), 3.28-3.17 (br m, 1H), 3.15-3.05 (br m, 1H), 2.51-2.43 (m, 1H), 2.42-2.32 (br m, 1H), 2.35/2.34 (overlapping t, J = 7.53 Hz, 2H), 1.70-1.50 (br m, 4H), 1.45-1.14 (br m, 22H), 0.88/0.87 (overlapping t, J = 7.19 Hz, 3H); 13C NMR: δ 212.0, 173.6, 136.1, 128.5, 128.1, 66.0 (br s), 61.9/61.8 (two app s), 50.1/50.1 (two app s), 34.2, 31.8, 30.1, 30.0, 29.6, 29.6, 29.5, 29.4, 29.4, 29.23, 29.17, 29.0, 28.2, 28.08, 28.05, 27.98, 27.6, 24.9, 24.3, 24.3, 22.6, 14.1; HREI-MS calcd. for C27H41O3Na [M+Na]+: 437.3032; found: 437.3029.
2-(Octanoic acid, 8-yl) 3-octylcyclobutanone and 3-(octanoic acid, 8-yl) 2-octylcyclobutanone (7). A solution of cyclobutanones 21 (0.039 g, 0.09 mmol) and 10% Pd/C (0.020 g, 0.18 mmol) in anhydrous MeOH (1 mL) in a vial was placed under an atmosphere of H2 and stirred at rt overnight (16 h). The mixture was then concentrated under vacuum. The residue was resuspended in EtOAc (~ 20 mL) and filtered through a plug of Celite. The residue obtained upon concentration was purified by flash column chromatography on silica gel (10%/25% EtOAc/Hex) to afford the regioisomeric mixture of carboxylic acids 7 (0.027 g, 87%) as a colorless oil: Rf = 0.16 (25% EtOAc/Hex); IR: 3322 (br), 2926, 2856, 1776, 1709, 913, 749 cm−1; 1H NMR: δ 3.28-3.18 (br m, 1H), 3.13/3.09 (overlapping dd, J = 9.6, 3.1 and 9.0, 3.5, 0.56H/0.44H), 2.50/2.46 (two app abnormal-shape quintets, 0.55H/0.52H), 2.42 (br dd, J = 9.6, 4.5 Hz, 1H), 2.35/2.34 (two app t, J = 7.45 Hz, 2H), 1.63-1.54 (br m, 4H), 1.43-1.18 (br m, 22H), 0.88/0.87 (overlapping br t, J = 7.0 Hz, 3H); 13C NMR: δ 212.1, 179.9, 62.0/61.9 (two app s), 50.20/50.17 (two app s), 34.0, 31.9, 30.1, 30.1, 29.7, 29.6, 29.6, 29.4, 29.3, 29.2, 29.02, 28.98, 28.2, 28.1, 28.0, 27.6, 24.6, 24.3, 24.3, 22.7, 14.1; HREI-MS calcd. for C20H36O3Na [M+Na]+: 347.2557; found: 347.2557. Purity >97% by HPLC (retention time, 6.82 min).
2-(Octanoic acid, 8-yl) 3-octylcyclobutanol and 3-(Octanoic acid, 8-yl) 2-octylcyclobutanol (8). To a −10 °C (ice/NaCl bath) solution of the cyclobutanone 21 (0.037 g, 0.070 mmol) in anhydrous MeOH (1.5 mL) was added NaBH4 (0.005 g, 0.150 mmol) under N2. The reaction mixture was stirred at −10 °C until no starting material remained on TLC (~ 20 min). The reaction was diluted with CH2Cl2 (about 5 mL) and quenched with sat. NaHCO3 (2 mL). The mixture was stirred for 10 min. The organic layer was removed and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The organic layers were combined, dried over with Na2SO4, and evaporated to afford the alcohol as a colorless oil (0.029 g, 99%). Although the crude product appears pure by 1H NMR, it is actually a mixture of two regioisomers, each a 70:30 mixture of the 1,2,3- cis/cis and the 1,2-trans-2,3-cis isomers (identities established through 2D-NMR). The individual isomers can be detected by TLC in 20% EtOAc/Hex: Rf = 0.50 (major, all cis), 0.45 (major, all cis), 0.39 (minor, OH is trans to backbone), and 0.37 (minor, OH is trans to backbone); IR: 3396 (br), 2922, 2852, 1737, 1156, 732, 696 cm−1; 1H NMR: δ 7.39-7.28 (br m, 5H), 5.14 (s, 2H), 4.23 br q, J = 7.3, 0.7H), 3.93 (br q, J = 7.3, 0.3H), 2.42-2.36 (overlapping signal, 1H), 2.37 (t, J = 7.6, 2H), 2.15-1.95 (br m, 1H), 1.90-1.78 (br m, 1H,), 1.70-1.60 (br m, 2H), 1.60-1.48 br m, 2H), 1.45-1.20 (br m, 22H), 0.90 (br t, J = 7.6, 2H); 13C NMR: δ 173.68/173.65 (overlapping), 136.1, 128.5, 128.1, 72.2, 66.09/66.06 (two overlapping s), 66.0, 43.97/43.95 (overlapping), 37.3, 34.3, 31.9, 30.7, 30.6, 30.2, 29.94, 29.87, 29.8, 29.7, 29.62, 29.58, 29.5, 29.33, 29.29, 29.2, 29.14, 29.08, 29.0, 27.7, 27.6, 24.90, 24.88, 23.4, 23.3, 22.7, 14.1; HRESI-MS calcd. for C27H44O3Na [M+Na]+: 439.3188; found: 439.3174.
Using a procedure similar to that described for preparation of 6, the cyclobutanol benzyl esters (0.025 g, 0.060 mmol were subjected to hydrogenation in the presence of 10% Pd/C (0.013 g, 0.012 mmol) and EtOAc (0.6 mL) to afford cyclobutanol acid 8 (0.016 g, 81%) as a colorless oil. Although the product is assumed to consist of a similar mixture of regio- and stereoisomers as was present in the benzyl ester precursor, the individual isomers are not separable: Rf = 0.32 (4:6:0.05 of EtOAc:Hex:AcOH); IR: 3309 (br), 2922, 2853, 1709 cm−1; 1H NMR: δ 4.22 (q, J = 7.6, 0.7H, assumed -OH is cis to backbone from the characterization of starting material), 3.92 (q, J = 7.6, 0.3H, assumed OH is trans to backbone), 2.43-2.33 (br m, 1H), 2.34 (t, J = 7.3, 2H), 2.18-1.92 (m), 1.84 (br septet, J = 8.0, 1H), 1.68-1.58 (br quintet, J = 7.0, 2H), 1.58-1.48 (br m, 2H), 1.43-1.18 (br m, 22H), 0.88 (br t, J = 7.0 Hz, 3H); 13C NMR: δ 179.50/179.46 (two app s), 72.28, 66.16/66.12 (two app s), 48.87/48.79 (two app s), 43.97, 37.26, 35.06, 33.98, 31.89, 30.69/30.62, 30.24, 30.18, 29.90, 29.87, 29.79, 29.74, 29.63, 29.60, 29.51, 29.46, 29.34, 29.31, 29.21, 29.11, 29.06, 29.01, 28.99, 28.97, 28.67, 28.60, 28.05, 27.95, 27.92, 27.73, 27.60, 24.66/24.63, 23.38/23.34, 22.67, 14.10; HRESI-MS calcd. for C20H38O3Na [M+Na]+: 349.2719; Found 349.2715. Purity >99 % by HPLC (retention time, 11.8 and 15.9 min).
2-Chloro-4-(octanoic acid benzyl ester, 8-yl)-3-octylcyclobutanone; 2-Chloro-3-(octanoic acid benzyl ester, 8-yl) 4-octylcyclobutanone (22) was prepared based on a modification of a known procedure.[9,22,24] To a solution of dichloroketone 19 (0.992 g, 2.100 mmol) in 4 mL of glacial acetic acid was added Zn dust (0.148 g, 2.30 mmol). [Note: The amount of Zn required depended upon the purity of the dichlorocyclobutanone. and it was generally necessary to add a second equivalent after approximately 12 h of reaction.] The mixture was stirred at rt until complete disappearance of the starting material was observed (16 h, TLC). The reaction mixture was then cooled in an ice bath and diluted with water (20 mL). The resulting solution was extracted with EtOAc (3 × 50 mL) and the combined organic layers were washed sequentially with water (2 × 100 mL) and saturated NaHCO3 solution (2 × 50 mL). The resulting organic layer was dried over Na2SO4 and concentrated under reduced pressure to furnish a colorless oil (0.895 g, 97% crude yield) as a 1:1 mixture of regioisomers, each a 9:1 mixture of cis/trans stereoisomers (1H NMR); the mixture was used directly in the next step. Analysis of the 1H NMR indicated that the expected product was accompanied by recovered starting material 19 (approximately 14%) and the cyclobutanone 21 (overreduction product, approximately 10%). These byproducts could not be removed easily by flash chromatography. Rf = 0.58/0.53 (major), 0.48/0.43 (minor) (10% EtOAc in Hex); IR: 3036, 2925, 2854, 1789, 1735, 1456, 1162, 696 cm−1; 1H NMR: δ 7.36-7.25 (m, 5H), 5.12/5.11 (overlapping s, 2H), 4.97 (dd, J = 2.6, 9.5, 0.1H), 4.51 (dd, J = 2.8, 7.6 Hz, 0.9H), 3.35-3.20 (m, 1.1H), 2.75-2.65 (m, 0.1H), 2.53-2.40 (m, 0.9H), 2.36/2.35 (overlapping t, J = 7.2 Hz, 2H), 1.75-1.20 (m, 26H), 0.92-0.85 (m, 3H); 13C NMR: δ 203.48/203.45, 173.5, 136.1, 128.5, 128.1, 66.0, 65.7, 58.3, 58.2, 40.74/40.72, 34.18, 31.77/31.75, 29.5, 29.3, 29.24, 29.16, 29.14, 29.13, 29.02, 28.97, 28.90, 28.86, 28.02, 27.96, 27.92, 27.87, 25.64/25.57, 24.80/24.78, 22.59/22.58, 14.0; HRESI-MS calcd. for C27H41O3ClNa [M+Na]+: 471.2642; found 471.2635.
2-Chloro-4-(octanoic acid, 8-yl) 3-octylcyclobutanone-; 2-Chloro-3-(octanoic acid, 8-yl) 4-octylcyclobutanone (9) was prepared in a similar manner as compound 6 from reaction of benzyl ester 22 (0.085 g, 0.19 mmol), 10% Pd/C (0.040 g, 0.038 mmol) and EtOAc (1.9 mL). The product, acid 9 (0.048 g, 70%), was obtained as a colorless oil after purification by flash column chromatography (20% EtOAc/Hex). The product, which was predominantly the cis,cis-stereoisomer on the cyclobutanone, included approximately 5% of an inseparable trans-chlorocyclobutanone 11 (1H NMR signal at δ 4.37) probably arising from the epimerization of 9: Rf = 0.19 (4:1 Hex:EtOAc); IR: 3036, 2924, 2854, 1788, 1706, 1461 cm−1; 1H NMR: δ 10.74 (br s, 1H, COOH), 4.96 (dd, J = 9.4, 2.6, 0.05H), 4.50 (dd, J = 7.6, 2.8, 0.95H), 3.35-3.20 (m, 1.05H), 2.46 (quintet, J = 8.1, 0.95H), 2.74-2.62 (m, 0.05H), 2.35/2.33 (overlapping t, J = 7.3, 2H), 1.80-1.15 (m, 22H), 0.88/0.87 (overlapping t, J = 6.9, 3H); 13C NMR: δ 203.62/203.56, 180.1, 65.7, 58.30/58.26, 401.8, 34.0, 31.8, 29.5, 29.4, 29.3, 29.18/29.15, 29.04/29.00, 28.9, 28.04/27.98, 27.95/27.90, 25.67/25.59, 24.5, 22.6, 14.1; HRESI-MS calcd. for C20H35O3ClNa [M+Na]+: 381.2172; found 381.2191.
2,2-Dichloro-4-(octanoic acid benzyl ester, 8-yl) 3-octylcyclobutanone-; 2,2-Dichloro-3-(octanoic acid benzyl ester, 8-yl) 4-octylcyclobutanone (23) was prepared based on a known procedure.[22–24] To a mixture of Zn(Cu) (1.84 g, 28.0 mmol) and benzyl elaidate (2.07 g, 5.60 mmol) in 11 mL of anhydrous ether was added over 2 h (syringe pump) trichloroacetyl chloride (2.0 mL, 18 mmol). The reaction mixture was stirred at rt for 5 h (or until the starting material is consumed by TLC) and filtered through a plug of Celite. The residue was washed with ether (20 mL). The combined ether layers were transferred to a round bottom flask and cooled over ice. The black solution was then diluted with 40 mL of water and 40 mL of sat. NaHCO3. The mixture was allowed to warm to rt and stirred overnight. The aqueous layer was extracted with ether (3 × 50 mL) and the combined organic layers were dried over Na2SO4 and evaporated to dryness. The residue obtained upon concentration could be used for the next step without further purification or, alternatively, could be purified by rapid flash column chromatography through a short plug of silica (2.5%/5% EtOAc/Hex) to afford a yellow oil (1.69 g, 63%) consisting of a 1:1 mixture of regioisomers: Rf = 0.63 (20% EtOAc/Hex); IR: 2926, 2855, 1802, 1735, 1456, 1162, 696 cm−1; 1H NMR: δ 7.47-7.23 (m, 5H), 5.120/5.117 (overlapping s, 2H), 3.158/3.126 (overlapping dt, J = 7.2, 1.5 Hz, 1H), 2.56-2.46 (m, 1H), 2.37/2.34 (overlapping t, J = 7.6 Hz, 2H), 1.96-1.18 (m, 26H), 0.90/0.87 (overlapping t, J = 7.2 Hz, 3H); 13C NMR, 75 MHz : δ 196.3, 173.5, 136.0, 128.4, 128.1, 87.1, 66.0, 60.7, 60.6, 52.3, 34.2, 34.1, 31.8, 31.7, 31.37, 31.36, 29.42, 29.40, 29.3, 29.23, 29.20, 29.14, 29.12, 29.08, 28.93, 28.90, 28.85, 27.5, 27.4, 27.12, 27.05, 24.80, 24.78, 22.59, 22.57, 14.04, 14.03; HRESI-MS calcd. for C27H40O3Cl2Na [M+Na]+: 505.2252; found: 505.2248.
2,2-Dichloro-4-(octanoic acid benzyl ester, 8-yl) 3-octylcyclobutanol, methanesulfonate ester; 2,2-Dichloro-3-(octanoic acid benzyl ester, 8-yl) 4-octylcyclobutanol, methanesulfonate ester (24) was prepared by a similar procedure as 20 through reaction of dichlorocyclobutanone 23 (0.210 g, 0.400 mmol), NaBH4 (0.055 g, 1.50 mmol), and iPrOH 6 mL. The 1:1 mixture of regioisomeric dichlorocyclobutanols (0.166 g, 85%) was obtained as a colorless oil following flash chromatography (5% EtOAc/Hex): Rf = 0.66(minor)/0.61(major)/0.56(minor)/0.51(major) (10% EtOAc/Hex). IR: 3459 (br), 2924, 2853, 1736, 1456, 1161, 734, 696 cm−1; 1H NMR: δ 7.36-7.31 (m, 5H), 5.12 (s, 2H), 4.37 (br d, J = 6.4 Hz, 0.4H), 3.91 (br d, J = 8.0 Hz, 0.6H), 2.80-2.45 (br m, 2H), 2.37/2.36 (overlapping t, J = 7.6 Hz, 2H), 1.80-1.05 (br m, 26H), 0.89/0.88 (overlapping t, J = 6.7 Hz, 3H). Small quantities of individual isomers could be separated for analysis by flash column chromatography using 10% EtOAc/Hex. Stereochemical assignments are based upon 2D-NMR experiments (COSY, NOESY, and HSQC).
First-eluting H1-H3 trans
Rf =0.66; IR: 3463 (br), 2927, 2855, 1738, 1453, 1158 cm−1; 1H NMR: δ 7.37-7.31 (m, 5H), 5.12 (s, 2H), 4.38 (ddd, 3JH1,H4 = 6.8 Hz, 3JH1,OH = 5.1 Hz, 4JH1,H3(trans) = 1.3 Hz, 1H, CH-OH), 2.62-2.55 (m, 1H, CH-CCl2), 2.44 (d, 3JH1,OH = 5.1 Hz, -OH), 2.36 (t, J = 7.6 Hz, 2H), 2.18-2.08 (m, 1H, CH-CHOH), 1.80-1.10 (br m, 26H), 0.89 (t, J = 6.7 Hz, 3H); 13C NMR (NOESY): δ 173.6, 136.1, 128.5, 128.2, 90.2, 78.5, 66.1, 57.0, 42.0, 34.3, 31.9, 31.3, 29.7, 29.5, 29.4, 29.3, 29.0, 27.7, 27.5, 26.6, 24.9, 22.7, 14.1; HRESI-MS calcd. for C27H40O3Cl2 [M+Na]+: 507.2409; found: 507.2409.
First-eluting H1-H3 cis
Rf =0.61; IR: 3448 (br), 2925, 2854, 1737, 1456, 1163, 734, 697 cm−1; 1H NMR: δ 7.37-7.31 (m, 5H), 5.12 (s, 2H), 3.91 (dd, 3JH1,OH = 10.8 Hz, 3JH1,H4(trans) = 8.0 Hz, 4JH1,H3(cis) = 0 Hz, 1H, CH-OH), 2.46 (d, J = 10.8 Hz, 2H, OH), 2.36 (t, J = 7.6 Hz, 2H), 2.11-2.04 (m, 1H, CH-CCl2), 1.75-1.18 (m, 27H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR, 75 MHz (NOESY): δ 173.6, 136.1, 128.5, 128.2, 91.0, 81.6, 66.1, 51.9, 48.1, 34.3, 33.0, 31.8, 30.0, 29.6, 29.41, 29.38, 29.2, 29.0, 27.3, 26.8, 24.9, 22.6, 14.1; HRESI-MS calcd. for C27H40O3Cl2 [M+Na]+: 507.2409; found: 507.2415.
Second-eluting H1-H3 trans
Rf = 0.56; IR: 3463 (br), 2925, 2854, 1736, 1456, 1163, 696 cm−1; 1H NMR: δ 7.36-7.31 (m, 5H), 5.11 (s, 2H), 4.40-4.34 (m, 1H), 2.64-2.55 (m, 1H, CH-OH), 2.64-2.55 (m, 1H, CH-CCl2), 2.46 (d, J = 4.8 Hz, 1H, OH), 2.35 (t, J = 7.6 Hz, 2H), 2.20-2.03 (m, 1H, CH-CHOH), 1.80-1.10 (br m, 26H), 0.88 (t, J = 6.4 Hz, 3H); 13C NMR, 75 MHz: δ 173.7, 136.1, 128.5, 128.2, 90.2, 78.4, 66.1, 57.0, 42.0, 34.3, 31.9, 31.3, 29.6, 29.5, 29.41, 29.39, 29.2, 29.1, 29.0, 27.6, 27.5, 26.7, 24.9, 22.7, 14.1; HRESI-MS calcd. for C27H40O3Cl2 [M+Na]+: 507.2409; found: 507.2398.
Second-eluting H1-H3 cis
Rf = 0.51; IR: 3444 (br), 2924, 2854, 1734, 1162, 734, 696 cm−1; 1H NMR: δ 7.37-7.31 (m, 5H), 5.11 (s, 2H), 3.90 (dd, 3JH1,OH = 10.8 Hz, 3JH1,H4(trans) = 8.0 Hz, 4JH1,H3(cis) = 0 Hz, 1H, CH-OH), 2.52 (OH, d, J = 10.8 Hz, 2H), 2.35 (t, J = 7.6 Hz, 2H), 2.13-2.02 (m, 1H, CH-CCl2), 1.75-1.18 (m, 27H), 0.89 (t, J = 6.8 Hz, 3H); 13C NMR: (75 MHz): δ 173.7, 136.1, 128.5, 128.1, 91.0, 81.6, 66.1, 51.9, 48.0, 34.2, 33.0, 31.8, 30.0, 29.6, 29.4, 29.3, 29.2, 29.02, 28.97, 28.9, 27.2, 26.8, 24.9, 24.8, 22.6, 14.1; HRESI-MS calcd. for C27H40O3Cl2: [M+Na]+ 507.2409; found:507.2409.
The mixture of dichlorocyclobutanols was converted to the methanesulfonates (mesylate) in a similar manner as for 20. Reaction of methanesulfonyl chloride (0.05 mL, 0.70 mmol), the dichlorocyclobutanols (0.166 g, 0.300 mmol), and triethylamine (0.20 mL, 1.7 mmol) in CH2Cl2 (2 mL), followed by purification using flash chromatography (5% EtOAc/Hex) provided dichloromesylate 24 as a light yellow oil (0.146 g, 86 %) as a mixture of inseparable region- and stereoisomers: Rf = 0.40/0.38 (20% EtOAc/Hex); IR: 2925, 2851, 1734, 1368, 1180, 964, 697 cm−1; 1H NMR: δ 7.38-7.30 (m, 5H), 5.20 (dd, J = 7.1, 1.2 Hz, 0.4H), 5.115/5.112 (two overlapping s, total 2H), 4.84 (d, J = 8.8 Hz, 0.6H), 3.202/3.199 (two overlapping s, 1.8H), 3.166/3.163 (two overlapping s, total 1.2H), 2.66-2.58 (m, 0.4H), 2.40-2.30 (m, 2H), 2.21-2.12 (m, 0.6H), 2.10-1.98 (m 0.6H), 1.80-1.15 (m, 26.4H), 0.91-0.86 (m, 3H); 13C NMR: 173.6, 136.10/136.08, 128.5/128.1, 86.45/86.38, 84.09, 83.86/83.82, 66.03/66.01, 57.18, 51.81/51.80, 44.51/44.50, 41.71, 39.5, 39.0, 34.2, 32.21/32.18, 31.78/31.77, 31.41/31.36, 30.1, 29.49 29.46, 29.42, 29.34, 29.32, 29.27, 29.22, 29.16, 28.95, 28.90, 28.2, 27.0, 26.9, 26.8, 26.7, 26.6, 26.5, 26.4, 26.3, 24.8, 22.6, 14.1; HRESI-MS C28H44O5Cl2SNa [M+Na]+: 585.2184; found: 585.2186.
1-(Octanoic acid, 8-yl)-2-octylcyclobutene (10) was prepared from reductive fragmentation and deprotection of dichloromethansulfonate 24 (0.143 g, 0.250 mmol) in THF (1 mL), NH3 (~ 10 mL) and Na (~ 0.090 g, 4.10 mmol) by a procedure similar to that employed for 5. The crude product was purified by flash chromatography (10% EtOAc/Hex) to afford the acid 10 (0.035 g, 46%) as a colorless oil: Rf = 0.35 (60:40 of Hex:EtOAc); IR: 3124, 2923, 2852, 1708, 910, 736 cm−1; 1H NMR: δ 6.12 (br s, 2H), 2.35 (t, J = 7.2 Hz, 2H), 2.26 (t, J = 7.2 Hz, 2H), 1.64 (quintet, J = 7.2 Hz, 2H), 1.44 (br m, 4H), 1.35-1.20 (23H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR: δ 179.7, 139.2, 139.1, 50.4, 50.3, 34.02, 33.97, 31.9, 29.9, 29.6, 29.3, 29.2, 29.0, 28.4, 28.3, 24.7, 22.7, 14.1; HRFAB-MS (3-NBA matrix): calcd. for C20H35O2Li2 [M-H+2Li]+: 321.2957; found: 321.2969. Purity 100% by HPLC (retention time, 4.73 min).
2-Chloro-4-(octanoic acid, 8-yl) 3-octylcyclobutanone-; 2-Chloro-3-(octanoic acid, 8-yl) 4-octylcyclobutanone (11) was prepared by a similar procedure as described for compound 9. Reaction of benzyl ester 23 (0.500 g, 1.000 mmol), Zn(Cu) (0.074 g, 1.10 mmol) and glacial acetic acid (2 mL) furnished, after flash chromatography (5% EtOAc/Hex), trans-monochlorocyclobutanone (0.313 g, 70%) as a colorless oil which included an equal mix of the 2-chloro-4-octyl and 2-chloro-3-octyl regioisomers, each of which included both epimers at the chloride-bearing carbon: Rf = 0.42/0.39 (minor) and 0.33 (major) (10% EtOAc/Hex). The amount of Zn dust varied from 1.1–2.2 equivalents; generally, a second equivalent of zinc dust was added to the incomplete reaction mixture after stirring for approximately 12 h. The residual dichlorocyclobutanone was easily removed by flash column chromatography. However, the cyclobutanone product of overreduction was formed in significant amounts (28% based upon 1H NMR and was nearly inseparable by flash column chromatography: IR: 2924, 2854, 1788, 1735, 1457, 1161, 733, 696 cm−1; 1H NMR: δ 7.40-7.32 (m, 5H), 5.113/5.110 (overlapping s, 2H), 4.95 (dd, J = 3.1, 9.4, 0.3H), 4.37 (dt, J = 2.7, 7.8 Hz, 0.7H), 2.97-2.90 (m, 0.3H), 2.84-2.74 (m, 0.7H), 2.40-2.30 (overlapping m, 2.3H), 2.08-1.97 (m, 0.7H), 1.85-1.10 (m, 26H), 0.92-0.84 (m, 3H); 13C NMR: δ 204.6, 202.0, 173.5, 136.1, 128.5, 128.1, 66.0, 65.52/65.46, 63.98, 63.78/63.75, 63.57/63.52, 60.74/60.69, 49.8, 43.7, 37.4, 36.63/36.59, 35.12/35.10, 34.2, 31.8, 31.3, 30.3, 30.1, 29.5, 29.42, 29.38,29.32, 29.25, 29.19, 29.03, 29.00, 28.96, 28.93, 28.88, 28.2, 27.67, 27.60, 27.55, 27.48, 27.38, 27.3, 27.2, 27.1, 24.83/24.80, 22.6, 14.0; HRESI-MS calcd. for C27H41O3ClNa [M+Na]+: 471.2642; found: 471.2630.
The resulting mixture of monochloroketone mixtures was subjected to debenzylation described in the preparation of compound 6. Reaction of the benzyl ester mixture (0.034 g, 0.11 mmol), with 10% Pd/C (0.024 g, 0.022 mmol) and EtOAc (1 mL) afforded the unprotected acid (0.013 g, 54%) as a white solid: Rf = 0.24 (6:4:0.05 of Hex:EtOAc:AcOH); mp = 74–75 °C; IR: 3062, 2924, 2854, 1789, 1708, 1464 cm−1; 1H NMR: δ 8.70 (br s, 1H), 4.95 (dd, J = 2.9, 9.2, 0.3H), 4.37 (ddd, J = 7.8, 2.5, 1.3, 0.7H), 2.97-2.91 (m, 0.3H), 2.85-2.73 (m, 0.7H), 2.40-2.22 (m, 22H), 2.11-2.98 (m 0.7H), 1.85-1.10 (m 26H), 0.92-0.82 (m, 3H); 13C NMR δ 211.7, 204.7, 202.2, 179.9, 65.6/65.5, 64.0, 63.85/63.82, 63.64/63.58, 60.82/60.77, 49.81, 43.8, 37.5, 36.69/36.65, 35.2, 34.0, 31.8, 31.3, 30.4, 30.2, 29.55, 29.48, 29.44, 29.38, 29.31, 29.25, 29.2, 29.06, 29.06, 28.9, 28.3, 28.2, 27.73, 27.67, 27.62, 27.44, 27.37, 27.26, 27.20, 27.17, 24.63/24.60, 22.7, 14.1; HRESI-MS calcd. for C20H35O3ClNa [M+Na]+: 381.2172; found: 381.2170.
Determination of inhibitor aqueous stability and solubility
One-dimensional (1D) 1H-NMR spectroscopy was used to verify the chemical purity, aqueous stability, and concentration dependent micelle formation of eleven fatty acid analogs. Each compound was dissolved in deuterated dimethyl sulfoxide (DMSO-d6) to obtain a stock concentration of 20 mM. Six different concentrations were prepared from the stock solutions for NMR analysis: 1.00 mM, 750 μM, 500 μM, 100 μM, and 0 μM. All NMR samples consisted of 600 μL of a deuterated 50 mM potassium phosphate buffer at pH 7.2 with 50 μM of 3 (trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TMSP). Each 600 μL NMR sample contains 30 μL (5%) of DMSO-d6 and was transferred to a 5 mm NMR tube for analysis.
A Bruker Avance DRX 500-MHz spectrometer equipped with a 5 mm triple-resonance cryoprobe (1H, 13C, 15N) with a z-axis gradient was utilized for all 1D 1H NMR experiments. Acquisition of NMR spectra was automated using a BACS-120 sample changer and Icon NMR software. All spectra were acquired at 298.15 K with 16 dummy scans, 64 scans, 32K data points, a spectral width of 5482.46 Hz, and a relaxation delay of 1.5 s. The NMR spectra were processed and analyzed using ACD/1D NMR Manager (Advanced Chemistry Development). The resulting 1D 1H NMR spectra were visually inspected for evidence of micelle formation (peak broadening), or chemical instability/impurities (additional peaks).
Measurement of nonspecific cytotoxicity
RAW 264.7 macrophages were incubated with 0–100 mM of oleic acid or the C18 cyclobutene fatty acid 2, each delivered as bovine serum albumin complexes. After 24 h, cell viability was relative to untreated controls was assessed using an IN CTYOTOX-CVDE Crystal violet Dye Elution Kit. The values shown in Figure 2 are means ± SEM based upon measurement in triplicate.
Bacterial strains and culture conditions
Bacterial strains used in this study are the E. coli wild type strain G58-1, the genome sequencing Msm strain mc2155, and the Mtb strains CDC 1551 and H37Rv. Cells were grown shaking at 37 °C in LB broth for E. coli or complete Middlebrook 7H9 broth supplemented with 0.05% v/v Tween 80 to an O.D.600 of 0.6–1.2 for mycobacteria. For MIC determinations, cells were inoculated into LB for E. coli or a modified previously reported minimal medium for both E. coli and mycobacteria.[26] The minimal media components and final concentrations are as follows: 22 mM dibasic potassium phosphate, 16 mM monobasic potassium phosphate, 2.8 × 10−5 mM ferric chloride, 8.7 × 10−3 mM zinc sulfate, 8.4 × 10−4 mM cobalt(II) chloride, 1.0 × 10−2 mM manganese chloride, 6.8 × 10−2 mM calcium chloride, 2.4 mM magnesium sulfate, 5.0 mM ammonium chloride, 25 mM glycerol, and 0.02% v/v Tyloxapol.
Drug susceptibility assays
MICs were determined by a 96-well microplate method, as described by Chacon.[26] Bacteria were harvested, washed 2× with minimal media, and inoculated to an initial concentration of approximately 1.0 × 105 colony forming units (CFU) per well. The initial inoculum was plated to verify retrospectively the desired CFU/ml for each strain. Stocks of fatty acid analog compounds were prepared in 100% DMSO-d6, as this solvent was chosen to provide consistency with the NMR studies. Appropriate doubling dilution gradients (μg/mL of compound dissolved in the corresponding % v/v DMSO- d6) were prepared in the following ranges for each compound tested: E. coli, 8 to 512; Msm, 16 to 1024; and Mtb, 0.25 to 256 (e.g., 64 μg/mL of compound corresponded to 0.064% v/v of DMSO-d6). Alternatively, the concentration of DMSO-d6 was adjusted in all wells to its maximum concentration in the corresponding gradient (e.g., 64 μg/ml of compound corresponded to 0.256% v/v of DMSO-d6). We tested cells in the absence of compound and DMSO-d6 to verify cell viability. The effect of DMSO-d6 was also tested by growing cells in minimal media with DMSO-d6 and no compound. We observed no significant effect of DMSO-d6 on cell viability for E. coli and Msm. However for Mtb, we observed some variability with anomalous inhibitory results in a few technical replicate wells containing DMSO-d6 alone at higher concentrations. Nonetheless, most of the replicate wells displayed no inhibition allowing us to eliminate these anomalous results. Moreover, the effect of the compound was observed at DMSO-d6 concentrations having no inhibitory action. Plates were incubated at 37 °C for 2 days (E. coli), 4 days (Msm) or 7 weeks (Mtb). MIC values were determined by the consistent results of three biological and three technical replicates. The MIC was defined by taking the mode of three independent cultures where the MIC did not differ by more than one doubling dilution, discarding any results that are two doubling dilutions away from the mode.
Supplementary Material
Acknowledgments
The research was funded from the Life Sciences Interdisciplinary Research Program (University of Nebraska-Lincoln). Portions of this research were performed in facilities renovated with support from the NIH (RR016544). We wish to acknowledge useful discussions with Prof. Ken Nickerson and Prof. Martha Morton (University of Nebraska-Lincoln) and Dr. Chris Schwartz. We also acknowledge technical assistance from Ms. Wendy Austin, Ms. Sara Basaiga, and Mr. Shingo Ishihara (Univ. of Nebraska-Lincoln).
Abbreviations
- ATR
Attentuated total reflection
- COSY
Correlation spectroscopy
- DCC
dicyclohexyl carbodiimide
- DCS
D-cycloserine
- DMF
N,N′-dimethyl formamide
- DMSO-d6
Hexadeutero dimethyl sulfoxide
- DSC/TGA
Differential scanning calorimetry/thermal gravimetric analysis
- EtOAc
Ethyl acetate
- Hex
Hexane
- HPLC
High-performance liquid chromatography
- HRESI
High resolution mass spectrometry via electrospray ionization
- HRFAB
High resolution mass spectrometry via fast atom bombardment
- HSQC
Heteronuclear single quantum coherence spectroscopy
- DMAP
4-dimethylaminopyridine
- iPrOH
isopropa nol
- MDR
Multidrug resistant
- MIC
Minimum inhibitory concentration
- Msm
Mycobacterium smegmatis
- Mtb
Mycobac terium tuberculosis
- NOESY
Nuclear Overhauser effect spectroscopy
- NMR
Nuclear magnetic resonance
- THF
tetrahydrofuran
- TLC
thin layer chromatography
- UV
ultraviolet
Footnotes
Supporting information for this article is available on the WWW under http://www.chemmedchem.org or from the author
References
- 1.Raviglione M, Marais B, Floyd K, Lönroth K, Getahun H, Migliori GB, Harries AD, Nunn P, Lienhardt C, Graham S, Chakaya J, Weyer K, Cole S, Kaufmann SHE, Zumla A. Lancet. 2012;379:1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 2.a) Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. Nature. 2011;469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]; b) Zhang Y. Annu Rev Pharmacol Toxicol. 2005;45:529–564. doi: 10.1146/annurev.pharmtox.45.120403.100120. [DOI] [PubMed] [Google Scholar]
- 3.Ballell L, Field RA, Duncan K, Young RJ. Antimicrob Agents Chemother. 2005;49:2153–2163. doi: 10.1128/AAC.49.6.2153-2163.2005. (erratum, 2007, 51, 1888) [DOI] [PMC free article] [PubMed] [Google Scholar]; van den Boogard J, Kibiki GS, Kisanga ER, Boerjee MJ, Aarnoutse RE. Antimicrob Agents Chemother. 2009;53:849–862. doi: 10.1128/AAC.00749-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayama K, Wang C, Besra GS. Clin Microbiol Rev. 2005;18:81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer L, Dover LG, Morbidoni HR, Vilchèze C, Maughan WN, Baulard A, Tu SC, Honoré N, Deretic V, Sacchettini JC, Locht C, Jacobs WR, Jr, Besra GS. J Biol Chem. 2003;278:20547–20554. doi: 10.1074/jbc.M302435200. [DOI] [PubMed] [Google Scholar]
- 6.Several reports have described incorporation of labeled or modified fatty acids into mycolic acid biosynthesis: Morbidoni HR, Vilcheze C, Kremer L, Bittman R, Sacchettini JC, Jacobs WR. Chem Biol. 2006;13:297–307. doi: 10.1016/j.chembiol.2006.01.005.Wheeler PR, Besra GS, Minnikin DE, Ratledge C. Biochim Biophys Acta. 1993;1167:182–188. doi: 10.1016/0005-2760(93)90160-b.Dreher RK, Poralla K, Kong WA. J Bacteriol. 1976;127:1136–1140. doi: 10.1128/jb.127.3.1136-1140.1976.Kaneda T. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991.
- 7.For an overview of application of fatty acids as antimycobacterial activity. see: Carballeira NM. Prog Lipid Res. 2008;47:50–61. doi: 10.1016/j.plipres.2007.10.002.
- 8.Mahindroo VK, Singh V, Dev S. Ind J Chem, Sect B. 1988;27B:1080–1089. [Google Scholar]
- 9.Powers DC, Leber PA, Gallagher SS, Higgs AT, McCullough LA, Baldwin JE. J Org Chem. 2007;72:187–194. doi: 10.1021/jo061964x. [DOI] [PubMed] [Google Scholar]
- 10.a) Hyatt JA, Raynolds PW. Organic Reactions. 1994;45:159–646. [Google Scholar]; b) Darses B, Greene AE, Coote SC, Poisson JF. Org Lett. 2008;10:821–824. doi: 10.1021/ol702977x. [DOI] [PubMed] [Google Scholar]; c) Darses B, Greene AE, Poisson J-F. Org Lett. 2010;12:3994–3997. doi: 10.1021/ol101559b. In our hands, the use of Zn and not the more active Zn/Cu was important in obtaining the cis-cyclobutene and not a cis/trans mixture. [DOI] [PubMed] [Google Scholar]
- 11.Blaszczyk K, Koenig H, Mel K, Paryzek Z. Tetrahedron. 2006;62:1069–1078. [Google Scholar]
- 12.Hassner A, Krepski LR. J Org Chem. 1978;43:2879–2882. [Google Scholar]
- 13.Frey HM, Marshall DC, Skinner RF. Trans Faraday Soc. 1965;61:861–865. [Google Scholar]
- 14.Davis BD, Dubos RJ. J Exp Med. 1947;86:215–228. doi: 10.1084/jem.86.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Even simple fatty acids can display toxicity towards a macrophage line at concentrations beyond 50 – 100 μM: Martins de Lima T, Cury-Boaventura TMF, Giannocco G, Nunes MT, Curi R. Clin Sci(Lond) 2006;111:307–317. doi: 10.1042/CS20060064.
- 16.a) Rishton GM. Drug Discov Today. 1997;2:382–384. doi: 10.1016/s1359644602025722. [DOI] [PubMed] [Google Scholar]; b) McGovern SL, Caselli E, Grigorieff N, Shoichet BK. J Med Chem. 2002;45:1712–1722. doi: 10.1021/jm010533y. [DOI] [PubMed] [Google Scholar]; c) McGovern SL, Helfand BT, Feng B, Shoichet BK. J Med Chem. 2003;46:4265–4272. doi: 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]; d) Seidler J, McGovern SL, Doman TN, Shoichet BK. J Med Chem. 2003;46:4477–4486. doi: 10.1021/jm030191r. [DOI] [PubMed] [Google Scholar]
- 17.Camargo EE, Kertcher JA, Larson SM, Tepper BS, Wagner HN., Jr Int J Lepr Other Mycobact Dis. 1982;50:200–204. [PubMed] [Google Scholar]
- 18.Barkan D, Liu Z, Sacchettini JC, Glickman MS. Chem Biol. 2009;16:499–509. doi: 10.1016/j.chembiol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Sinninghe Damsté JS, Strous M, Rijpstra WI, Hopnams EC, Geenevasen JA, van Duin AC, van Niftrik LA, Jetten MS. Nature. 2002;419:708–712. doi: 10.1038/nature01128. [DOI] [PubMed] [Google Scholar]; b) Sinninghe Damsté JS, Rijpstra WIC, Geenevasen JAJ, Strous M, Jetten MS. FEBS J. 2005;272:4270–4283. doi: 10.1111/j.1742-4658.2005.04842.x. [DOI] [PubMed] [Google Scholar]
- 20.Moore BS, Floss HG. In: Comprehensive Natural Products Chemistry. Sankawa U, Barton DHR, Nakanishi K, Meth-Cohn O, editors. Vol. 1. Elsevier; New York: 1999. 61 pp.81 pp. [Google Scholar]
- 21.Zhang Y, Heym B, Allen B, Young D, Cole S. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- 22.a) Deprés JP, Coelho F, Greene AE. J Org Chem. 1985;50:1972–1973. [Google Scholar]; b) Bak DA, Brady WT. J Org Chem. 1979;44:107–110. [Google Scholar]; c) Greene AE, Deprés JP. J Am Chem Soc. 1979;101:4003–4005. [Google Scholar]; d) Brady WT, Waters OH. J Org Chem. 1967;32:3703–3705. [Google Scholar]
- 23.Zn dust was used instead of Zn(Cu) and 2.5 equiv of Cl3CCOCl was employed
- 24.Baldwin JD, Belfield KD. J Org Chem. 1987;52:4772–4776. [Google Scholar]
- 25.a) Seidl PR, Dias JF. In: The Chemistry of Cyclobutanes. Rappoport Z, Liebman JF, editors. John Wiley & Sons, Ltd; Chichester: 2005. pp. 213–256. [Google Scholar]; b) Pretsch E, Buhlmann P, Affolter C. Structure Determination of Organic Compounds: Tables of Spectral Data. Springer-Verlag; Berlin: 2000. p. 203. [Google Scholar]
- 26.Chacon O, Feng Z, Harris NB, Cáceres NE, Adams LG, Barletta RG. Antimicrob Agents Chemother. 2002;46:47–54. doi: 10.1128/AAC.46.1.47-54.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.