Abstract
Objective
To determine whether ectopic fat depots are prospectively associated with cardiovascular disease, cancer and all-cause mortality.
Background
The morbidity associated with excess body weight varies among individuals of similar body mass index. Ectopic fat depots may underlie this risk differential. However, prospective studies of directly measured fat are limited.
Methods
Participants from the Framingham Heart Study (n=3086, 49% women, mean age 50.2 years) underwent assessment of fat depots (visceral adipose tissue, pericardial adipose tissue, and periaortic adipose tissue) using multidetector computed tomography, and were followed longitudinally for a median of 5.0 years. Cox proportional hazards regression models were used to examine the association of each fat depot (per 1 standard deviation increment) with the risk of incident cardiovascular disease, cancer, and all-cause mortality after adjustment for standard risk factors, including body mass index.
Results
Overall, there were 90 cardiovascular events, 141 cancer events, and 71 deaths. After multivariable adjustment, visceral adipose tissue was associated with cardiovascular disease (HR 1.44, 95% CI 1.08–1.92, p=0.01) and cancer (HR 1.43, 95% CI 1.12–1.84, p=0.005). Addition of visceral adipose tissue to a multivariable model that included body mass index modestly improved cardiovascular risk prediction (net reclassification improvement of 16.3%). None of the fat depots were associated with all-cause mortality.
Conclusion
Visceral adiposity is associated with incident cardiovascular disease and cancer after adjustment for clinical risk factors and generalized adiposity. These findings support the growing appreciation of a pathogenic role of ectopic fat.
Keywords: obesity, visceral fat, body fat distribution, cardiovascular disease, cancer
Introduction
Visceral adipose tissue (VAT) has been cross-sectionally associated with CVD and cancer (1,2), and is correlated with smaller ectopic fat depots, including pericardial and periaortic fat, which surround the cardiovascular system and may exert local toxic effects (3). These smaller ectopic fat depots have been associated with cardiovascular risk factors and events (4,5). Despite the interest in ectopic fat, few studies have examined prospective outcomes (6–9). In addition, little is known about CVD risk prediction, which can be useful to assess the predictive utility of new measures for incident disease. The purpose of the current study was to examine the association of directly-imaged fat measurements with incident CVD, cancer, and all-cause mortality.
Methods
Study Sample
Participants were drawn from the Framingham Heart Study Offspring and Third Generation cohorts who underwent MDCT from 2002–2005 (10). Of the 3529 participants in the MDCT sub-study, 3394 had at least one evaluable fat depot measurement, 3114 were free of CVD, 3270 were free of cancer, and 3086 had complete covariates.
The study protocol was approved by the Institutional Review Boards of Boston University Medical Center and Massachusetts General Hospital. Participants provided written informed consent.
MDCT Scan Protocol and Adipose Tissue Measurements
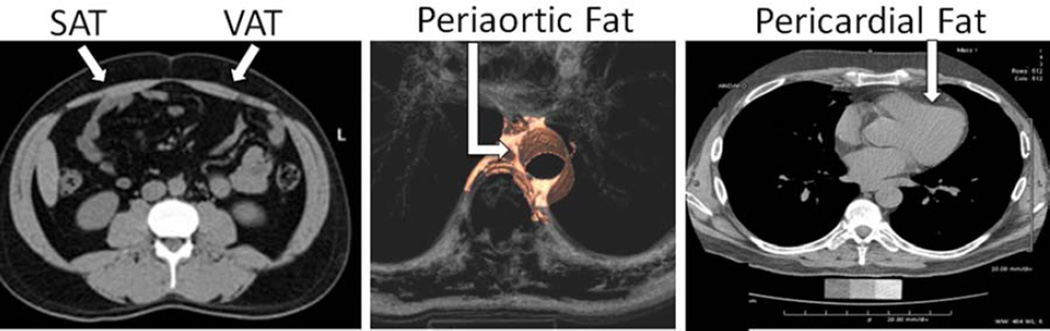
Participants underwent thoracic and abdominal MDCT using an 8-slice scanner. Details of MDCT protocols and measurement of fat volumes have been previously described (4,5,10). The estimated dose length product as a measure of radiation exposure was approximately 300 mGy-cm. Figure 1 illustrates representative images of our three fat depots.
Figure 1. Multidetector computed tomography images demonstrating SAT, VAT, periaortic fat, and pericardial fat.
Fat depots are defined by anatomic landmarks and pixels of adipose tissue within a given fat depot are identified by their characteristic Hounsfield units.
Outcome Assessment
CVD events (myocardial infarction, angina pectoris, coronary insufficiency, cerebrovascular accident, transient ischemic attack, intermittent claudication, congestive heart failure, and CVD death) and cause of death were adjudicated by 3 investigators. Cancer events were validated using medical records (pathology reports). Non-melanoma skin cancers were not included.
Risk Factor Assessment
Risk factors were measured at the 7th Offspring (1998–2001) and 1st Third Generation (2002–2005) examinations. Descriptions of the measurement of risk factors has been previously described (10).
Statistical Analysis
Cox proportional hazards regression models were used to relate each adiposity measure to A) incident CVD; B) incident cancer; and C) all-cause mortality. Individuals with prevalent disease were excluded from the respective analyses. VAT, pericardial fat, and periaortic fat were the primary exposures. Multivariable models included age, sex, systolic blood pressure, hypertension treatment, diabetes, smoking status, total and HDL cholesterol, and BMI. Hazard ratios are presented per 1 standard deviation increment of each adiposity measure.
For ectopic fat depots associated with CVD in multivariable models, we used several indices to determine the incremental predictive utility of the given fat depot for identifying individuals at CVD risk. We assessed A) increment in the c-statistic to assess discrimination, B) net reclassification index (NRI), to assess risk reclassification; C) relative integrated discrimination index, to assess risk reclassification; and D) calibration indices based on the Hosmer-Lemeshow goodness of fit test. For the NRI, we used the following categories based on the risk of CVD during our median follow-up of 5.0 years: 0–3.5%, 3.6–8.0%, 8.1–11.5%, and >11.5%. To compare risk prediction between different adiposity measures, we calculated the NRI for BMI, waist circumference and VAT in multivariable models that did not include BMI (to allow for comparison). In secondary analyses, we examined the association of pericardial fat with myocardial infarction. We assessed effect modification by age (continuous) and sex.
All analyses were conducted with SAS version 9.2 (Cary, NC). To account for three exposures, we used a p-value of <0.017 (0.05/3) for our primary analyses.
Results
The mean age was 50.2 years and 49% were women. The mean BMI was in the overweight range (Table 1).
Table 1.
Baseline characteristics of the overall sample. Data are presented as mean±SD for continuous or % for categorical characteristics
| Characteristic | N=3086 |
|---|---|
| Age (years) | 50.2 (10.0) |
| Total cholesterol (mg/dl) | 196.8 (35.1) |
| High density lipoprotein cholesterol (mg/dl) | 53.8 (16.6) |
| Systolic blood pressure (mmHg) | 121.5 (16.1) |
| Hypertension (%) | 26.7 |
| Hypertension Treatment (%) | 16.4 |
| Smoking Status, % | |
| Current | 12.5 |
| Former | 38.2 |
| Never | 49.3 |
| Diabetes, % | 5.4 |
| Adiposity Measures | |
| BMI (kg/m2) | 27.7 (5.2) |
| Waist Circumference (cm) | 96.7 (14.2) |
| Subcutaneous adipose tissue (SAT) (cm3) | 2874 (1393) |
| Visceral adipose tissue (VAT) (cm3) | 1760 (999) |
| Pericardial fat (cm3) | 111 (43) |
| Periaortic fat (cm3) | 13.2 (7.7) |
Cardiovascular Disease
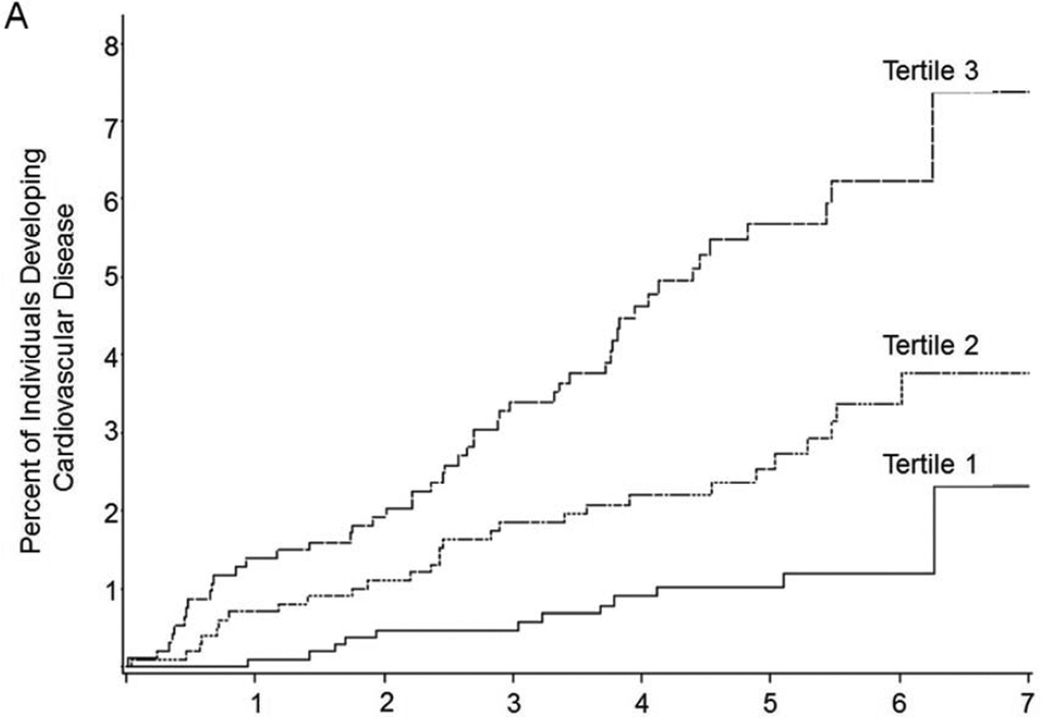
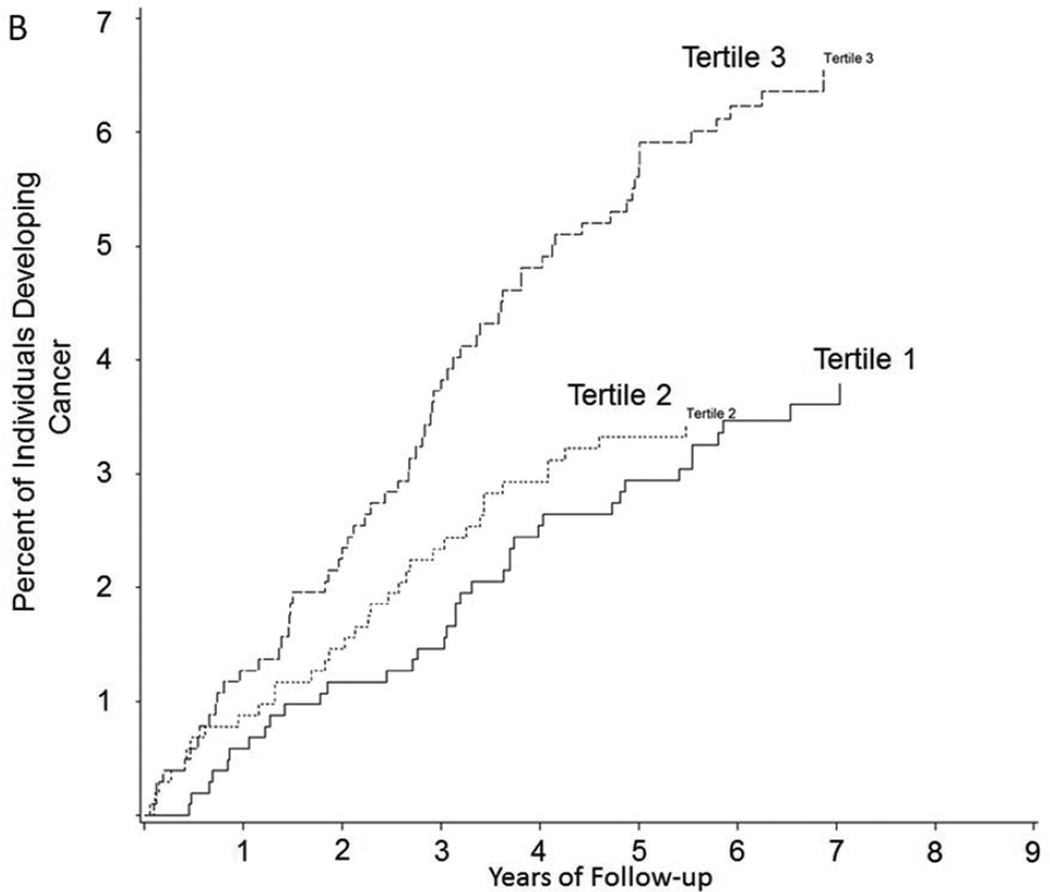
There were 90 CVD events; the median follow-up time was 5.0 years (interquartile range 3.9–6.0, maximum follow-up 7.4 years). In multivariable-adjusted models, VAT was associated with incident CVD (HR 1.44, p=0.014, Table 2), and remained associated with CVD when models were adjusted for waist circumference (HR 1.47, 95% CI 1.09–1.98, p=0.012). In contrast, there was no association of SAT and CVD. Figure 2 presents the Kaplan-Meier curves for CVD and cancer by tertile of VAT.
Table 2.
The Association of Ectopic Fat Depots with Incident CVD (n=90), Cancer (n=141), and All-cause Mortality (n=71). A p-value of <0.017 was considered statistically significant
| Age-sex adjusted model | p-value | MV* Model | p-value | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| CVD | ||||
| Visceral Adipose Tissue | 1.48 (1.21–1.81) | <0.001 | 1.44 (1.08–1.92)† | 0.014 |
| Periaortic Fat | 1.41 (1.16–1.71) | <0.001 | 1.31 (1.03–1.67) | 0.03 |
| Pericardial Fat | 1.24 (1.05–1.46) | 0.01 | 1.11 (0.91–1.35) | 0.3 |
| Subcutaneous Adipose Tissue | 1.23 (0.99–1.53) | 0.06 | 0.99 (0.66–1.49) | 1.0 |
| Cancer | ||||
| Visceral Adipose Tissue | 1.22 (1.03–1.46) | 0.03 | 1.43 (1.12–1.84) | 0.005 |
| Periaortic Fat | 1.21 (1.02–1.44) | 0.03 | 1.22 (0.99–1.50) | 0.06 |
| Pericardial Fat | 1.20 (1.05–1.39) | 0.02 | 1.17 (0.98–1.38) | 0.08 |
| Subcutaneous Adipose Tissue | 0.98 (0.82–1.18) | 0.9 | 0.90 (0.65–1.26) | 0.5 |
| All-Cause Mortality | ||||
| Visceral Adipose Tissue | 1.26 (1.01–1.57) | 0.04 | 1.05 (0.77–1.43) | 0.7 |
| Periaortic Fat | 1.28 (1.05–1.55) | 0.01 | 1.08 (0.85–1.38) | 0.5 |
| Pericardial Fat | 1.31 (1.10–1.55) | 0.002 | 1.17 (0.95–1.44) | 0.1 |
| Subcutaneous Adipose Tissue | 1.07 (0.81–1.40) | 0.7 | 0.72 (0.45–1.15) | 0.2 |
Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; VAT, visceral adipose tissue
MV (multivariable) models - adjusted for age, sex, systolic blood pressure, diabetes, total cholesterol, HDL cholesterol, current smoking, hypertension treatment, and BMI
For the CVD outcome, the integrated discrimination index was 0.082 (95% confidence interval −0.003, 0.176) after the addition of VAT to the multivariable model. The chi square statistic (p-value) for model calibration was 12.9 (p=0.2), suggesting good calibration.
Figure 2. Kaplan Meier Curve of Time to A) CVD and B) Cancer by Tertile of VAT (cm3).
Vertical axes show the percent of subjects developing A) CVD and B) cancer during follow-up; horizontal axes reflect years of follow-up. Tertile cutpoints for VAT are 1242.2 and 2168.4 cm3
CVD Risk Prediction
The c-statistic of the multivariable model was similar before (c-statistic 0.775) and after (c-statistic 0.781) the addition of VAT (p value for difference between models=0.18) suggesting similar discrimination of the models. However, the c-statistic has been recognized as relatively insensitive at comparing models after the inclusion of standard risk factors (11). Thus, we calculated the NRI, and observed modest improvement in risk prediction in models containing VAT (NRI 16.3%, 95% CI 2.8%–39.3%), with 14.2% reflecting appropriate upward reclassification and 2.1% reflecting appropriate downward reclassification of individuals. When the NRI of BMI, waist circumference and VAT were compared (in multivariable models that did not contain BMI to allow comparison), the NRI for VAT [10.1% (95% CI 2.5%, 17.6%)] was higher than that of BMI [−2.7% (95% CI −9.0%, 3.5%)] or waist circumference [0.3% (95% CI −5.7%, 6.3%)].
Cancer Events
Supplemental Table 1 displays the cancer types (141 events). VAT, but not SAT, was associated with incident cancer in multivariable models (HR 1.43, 95% CI 1.12–1.84, p=0.005, Table 2). Findings were similar after additional adjustment for waist circumference instead of BMI (HR 1.41, 95% CI 1.09–1.83, p=0.008).
All-Cause Mortality
There were 71 all-cause deaths. We observed no association between ectopic fat and all-cause mortality in multivariable models (HR for VAT 1.05, 95% CI 0.77–1.43, p=0.8) (Table 2).
Secondary Analyses
We observed no association between pericardial fat and myocardial infarction (n=47 events, HR 0.97, 95% CI 0.73–1.28, p-value=0.8). There was no effect modification by age or sex. Given the reported difference in fat distribution by sex (12), sex-stratified analyses were performed (Table 3). Despite the non-significant p-values for sex interaction, the hazard ratios for CVD and cancer tended to be higher for men.
Table 3.
Association of multiple fat depots with incident CVD (40 events in women, 50 events in men) and incident cancer (68 events in women, 73 events in men) stratified by sex.
| Adiposity Measure | Women (N=1519) | Men (N=1567) | p-interaction† | ||
|---|---|---|---|---|---|
| Age-adjusted | MV Model | Age-adjusted | MV Model | ||
| Incident CVD | |||||
| HR (95% CI) | HR (95% CI) | ||||
| Visceral Adipose Tissue | 1.20 (0.90–1.59) | 1.04 (0.65–1.65) | 1.64 (1.27–2.11) | 1.66 (1.16–2.39) | 0.1 |
| Periaortic Fat | 1.19 (0.91–1.56) | 1.08 (0.75–1.55) | 1.47 (1.16–1.85) | 1.37 (1.02–1.84) | 0.5 |
| Pericardial Fat | 1.13 (0.85–1.49) | 0.97 (0.69–1.36) | 1.28 (1.05–1.55) | 1.16 (0.91–1.47) | 0.4 |
| Subcutaneous Adipose Tissue | 1.24 (0.90–1.71) | 1.33 (0.72–2.45) | 1.22 (0.93–1.61) | 0.79 (0.47–1.33) | 1.0 |
| Incident Cancer | |||||
| Visceral Adipose Tissue | 1.19 (0.94–1.49) | 1.27 (0.88–1.82) | 1.20 (0.96–1.49) | 1.43 (1.06–1.94) | 0.4 |
| Periaortic Fat | 1.28 (1.02–1.62) | 1.31 (0.97–1.77) | 1.11 (0.90–1.38) | 1.13 (0.86–1.49) | 1.0 |
| Pericardial Fat | 1.06 (0.83–1.35) | 0.96 (0.72–1.29) | 1.25 (1.05–1.48) | 1.24 (1.02–1.51) | 0.06 |
| Subcutaneous Adipose Tissue | 1.00 (0.78–1.28) | 0.80 (0.49–1.30) | 0.98 (0.76–1.27) | 0.99 (0.64–1.54) | 0.9 |
| All–cause Death | |||||
| Visceral Adipose Tissue | 1.12 (0.77–1.62) | 1.18 (0.66–2.14) | 1.27 (0.99–1.62) | 0.92 (0.65–1.28) | 0.3 |
| Periaortic Fat | 1.06 (0.72–1.55) | 1.05 (0.64–1.71) | 1.28 (1.04–1.58) | 0.95 (0.72–1.24) | 0.2 |
| Pericardial Fat | 1.11 (0.77–1.60) | 1.09 (0.73–1.64) | 1.36 (1.12–1.65) | 1.16 (0.91–1.46) | 0.2 |
| Subcutaneous Adipose Tissue | 0.84 (0.54–1.32) | 0.73 (0.32–1.63) | 1.26 (0.91–1.74) | 0.74 (0.41–1.33) | 0.2 |
Abbreviations: HR, hazard ratio; CI, confidence interval; BMI, body mass index; VAT, visceral adipose tissue
MV (multivariable) models - adjusted for age, systolic blood pressure, diabetes, total cholesterol, HDL cholesterol, current smoking, hypertension treatment, and BMI
p-interaction is age-adjusted
Comment
Visceral adiposity was associated with incident CVD and cancer above and beyond BMI or waist circumference. In contrast to prior studies of BMI and waist circumference, visceral adiposity modestly improved CVD risk prediction. These results support the hypothesis that visceral fat may partially underlie the association of clinical adiposity measures with CVD and cancer.
Cross-sectional studies have demonstrated associations between ectopic fat and CVD and cancer (1,2), but few prospective studies are available (6–9,13). Our findings expand the literature with the use of a prospective cohort that avoided selection or sampling bias and a sample size of over 3000 individuals with a wide age range. Unlike prior studies, we assessed risk prediction. Our finding of a modest improvement in risk prediction with VAT stands in contrast to the lack of improvement with waist circumference (14). One potential explanation is that waist circumference is a surrogate marker of visceral adiposity and reflects both SAT and VAT.
Numerous experimental studies support a potential link between VAT and biological pathways important in the pathogenesis of multiple disease outcomes. Adipokines, biologically active molecules secreted from adipose tissue, are key components of these pathways and include inflammatory cytokines, angiogenic factors, lipid metabolites, and extracellular matrix components (15). Adipokine secretion appears to differ between specific fat depots (16) with VAT demonstrating greater expression of pro-inflammatory and proangiogenic genes compared with SAT (17). Furthermore, arterioles within VAT compared with SAT were more likely to exhibit endothelial dysfunction (17), suggesting a potential toxic effect of VAT on the vasculature.
Given the worldwide obesity epidemic, identification of high risk individuals is important as it allows targeting of preventive and therapeutic measures. Furthermore, markers of risk may provide insight into the biology linking body fat distribution and outcomes. Our findings that the association of VAT with events was not completely explained by standard risk factors is consistent with evidence suggesting that the link between VAT and obesity-related complications may involve novel biologic pathways.
Some limitations deserve comment. Our sample is predominantly white. Our study is observational, preventing inferences of causality and treatment recommendations. Weight change data was not available on our participants during the follow-up period. Finally, these findings do not imply that MDCT fat quantification should be used clinically.
Visceral adiposity is associated with incident CVD and cancer after adjustment for clinical risk factors and generalized or central adiposity. These findings support the growing appreciation of a pathogenic role of ectopic fat depots.
Acknowledgments
Drs. Britton and Fox had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract N01-HC-25195). Dr. Britton was supported by a Research Career Development Award (K12 HL083786) from the NHLBI.
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- HDL
high density lipoprotein
- HR
hazard ratio
- IDI
integrated discrimination index
- NRI
net reclassification index
- SAT
subcutaneous adipose tissue
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no conflicts of interest.
References
- 1.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaji T, Iwasaki M, Sasazuki S, et al. Visceral Fat Volume and the Prevalence of Colorectal Adenoma. American Journal of Epidemiology. 2009;170:1502–1511. doi: 10.1093/aje/kwp311. [DOI] [PubMed] [Google Scholar]
- 3.Greenstein AS, Khavandi K, Withers SB, et al. Local Inflammation and Hypoxia Abolish the Protective Anticontractile Properties of Perivascular Fat in Obese Patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Massaro JM, Schlett CL, et al. Peri-Aortic Fat Deposition Is Associated with Peripheral Arterial Disease: The Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3:515–519. doi: 10.1161/CIRCIMAGING.110.958884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 6.Cheng VY, Dey D, Tamarappoo B, et al. Pericardial Fat Burden on ECG-Gated Noncontrast CT in Asymptomatic Patients Who Subsequently Experience Adverse Cardiovascular Events. JACC: Cardiovascular Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Hsu F-C, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral Fat Is an Independent Predictor of All-cause Mortality in Men[ast] Obesity. 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 9.Nicklas BJ, Penninx BWJH, Cesari M, et al. Association of Visceral Adipose Tissue with Incident Myocardial Infarction in Older Men and Women. American Journal of Epidemiology. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 10.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association With Metabolic Risk Factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 11.Cook NR, Ridker PM. Advances in Measuring the Effect of Individual Predictors of Cardiovascular Risk: The Role of Reclassification Measures. Annals of Internal Medicine. 2009;150:795-W.143. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovejoy JC, Sainsbury A the Stock Conference Working G. Sex differences in obesity and the regulation of energy homeostasis. Obesity Reviews. 2009;10:154–167. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 13.McNeely MJ, Shofer JB, Leonetti DL, Fujimoto WY, Boyko EJ. Associations Among Visceral Fat, All-Cause Mortality, and Obesity-Related Mortality in Japanese Americans. Diabetes Care. 2012;35:296–298. doi: 10.2337/dc11-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Emerging Risk Factors Collaboration. Separate and combined associations of bodymass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. The Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hocking SL, Wu LE, Guilhaus M, Chisholm DJ, James DE. Intrinsic Depot-Specific Differences in the Secretome of Adipose Tissue, Preadipocytes, and Adipose Tissue–Derived Microvascular Endothelial Cells. Diabetes. 2010;59:3008–3016. doi: 10.2337/db10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farb MG, Ganley-Leal L, Mott M, et al. Arteriolar Function in Visceral Adipose Tissue Is Impaired in Human Obesity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:467–473. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]