Abstract
We previously reported encouraging results of down-staging of hepatocellular carcinoma (HCC) to meet conventional T2 criteria (one lesion 2–5 cm or two to three lesions <3 cm) for orthotopic liver transplantation (OLT) in 30 patients as a test of concept. In this ongoing prospective study, we analyzed longer-term outcome data on HCC down-staging in a larger cohort of 61 patients with tumor stage exceedingT2criteriawhowere enrolled between June 2002 and January 2007. Eligibility criteria for down-staging included: (1) one lesion >5 cm and up to 8 cm; (2) two to three lesions with at least one lesion >3 cm and not exceeding 5 cm, with total tumor diameter up to 8 cm; or (3) four to five lesions with none >3 cm, with total tumor diameter up to 8 cm. A minimum observation period of 3 months after down-staging was required before OLT. Tumor down-staging was successful in 43 patients (70.5%). Thirty-five patients (57.4%) had received OLT, including two who had undergone live-donor liver transplantation. Treatment failure was observed in 18 patients (29.5%), primarily due to tumor progression. In the explant of 35 patients who underwent OLT, 13 had complete tumor necrosis, 17 met T2 criteria, and five exceeded T2 criteria. The Kaplan-Meier intention-to-treat survival at 1 and 4 years after down-staging were 87.5% and 69.3%, respectively. The 1-year and 4-year posttransplantation survival rates were 96.2% and 92.1%, respectively. No patient had HCC recurrence after a median posttransplantation follow-up of 25 months. The only factor predicting treatment failure was pretreatment alpha-fetoprotein > 1,000 ng/mL.
Conclusion
Successful down-staging of HCC can be achieved in the majority of carefully selected patients and is associated with excellent posttransplantation outcome.
The therapeutic approach for hepatocellular carcinoma (HCC) has changed significantly in the past decade. Through the 1980s and early 1990s, surgical resection remained the treatment of choice for HCC, while orthotopic liver transplantation (OLT) was reserved mainly for patients with extensive tumor burden not amendable to surgical resection. This approach had resulted in poor posttransplantation outcome, due largely to tumor recurrence.1,2 Consequently, HCC was considered a contraindication to OLT in many transplantation centers in the early 1990s. Subsequently, the observation that small incidental HCC discovered in the liver explant had no adverse impact on posttransplantation outcome shaped the development of restrictive HCC criteria to limit the risk of tumor recurrence.3,4 The Milan criteria (one lesion ≤5 cm or two to three lesions ≤3 cm), based on the seminal publication by Mazzaferro et al. in 1996,4 have remained the paradigm for the selection of the best candidates for OLT in the past 10 years.
A critical factor determining the success of OLT for HCC is the length of wait-list time for a suitable donor. Llovet and colleagues5 were the first to use an intention-to-treat principle in analyzing the outcome of patients with HCC listed for OLT.5 Exclusion from OLT as a result of tumor progression to beyond acceptable criteria (dropout) and wait-list mortality are important endpoints in this intention-to-treat analysis. Subsequent studies from our institution and the Mayo Clinic underscored the magnitude of the problem of dropout in the United States in the absence of a prioritization scheme for HCC.6,7 In 2002, the inception of the model for end-stage liver disease (MELD) system for organ allocation adjusted for HCC has resulted in significantly reduced wait-list time and improved intention-to-treat outcome.8,9 Following the latest modification of the HCC-adjusted MELD organ allocation scheme, only patients with HCC meeting United Network for Organ Sharing T2 criteria (one lesion 2–5 cm or two to three lesions <3 cm) are eligible for priority listing for OLT.
The growing success of OLT for HCC in recent years has also fueled debates about whether the Milan criteria are too restrictive. Results from an earlier study on expanded criteria from our institution have been criticized for the small number of patients with HCC exceeding Milan criteria and that the expanded criteria were retrospectively based on explant tumor pathology.10 However, data continue to emerge suggesting that acceptable post-transplantation survival can be achieved after modest expansion of tumor size limits for OLT based on preoperative imaging in larger cohorts of patients.11,12 Down-staging of HCC to within Milan criteria is an attractive alternative to simply expanding the tumor size limits in lieu of pretransplantation loco-regional therapy. Theoretically, the down-staging process allows selection of tumors with more favorable biology that will likely respond to down-staging treatments and will also do well following OLT.13 However, concerns have been raised that expanding the indications for OLT would only inflate the waiting list with patients who are at increased risk for eventual dropout, thus compromising the intention-to-treat outcome.14 Some also believe that even if an 8-cm tumor could be successfully downsized to <5 cm (meeting Milan criteria), the risk of tumor recurrence after OLT may still be the same as that of an 8-cm HCC lesion.
Reduced waiting list time for patients with HCC as a result of the HCC-adjusted MELD system for organ allocation and the experience accumulated with loco-regional therapy—including transarterial chemoembolization (TACE) and various ablation techniques— enabled us to embark on a prospective study on tumor down-staging for OLT in 2002. We previously reported encouraging results involving the first 30 patients enrolled in our down-staging protocol as a test of concept.15 In the present study, we report longer follow-up data in a larger cohort. The primary study endpoints are intention-to-treat survival, dropout, and posttransplantation tumor recurrence after down-staging. We also examine factors that may influence response to down-staging treatments.
Patients and Methods
Down-Staging Protocol
The down-staging protocol has been described in detail.15 The criteria for inclusion based on HCC size and number are summarized in Table 1. The diagnosis of HCC for a lesion ≥2 cm was based on either quadruple-phase computed tomography (CT) or magnetic resonance imaging (MRI) with gadolinium contrast showing arterial enhancement and washout during the delayed images, or if a lesion showed interval growth by ≥2mmwith the same imaging technique. The same criteria also applied to lesions measuring 1 to 2 cm. Percutaneous biopsy for diagnosis of HCC was not routinely performed at our institution. Hepatic nodules <1 cm were not counted as HCC.
Table 1.
Down-Staging Protocol for Patients with HCC Initially Exceeding United Network for Organ Sharing T2 Criteria for Liver Transplantation
Inclusion criteria for down-staging based on HCC characteristics
|
Criteria for successful down-staging via imaging studies
|
Criteria for treatment failure
|
After initiation of down-staging treatments, all patients underwent CT or MRI of the abdomen about 1 month after each loco-regional treatment and at a minimum of once every 3 months during the treatment phase and after they were placed on the waiting list for OLT. The maximal diameter of residual tumors that enhanced on CT or MRI were used in tumor staging. If the original tumor showed partial necrosis with multiple areas of enhancement within the nodule, then the entire diameter, including both enhancing and nonenhancing nodule, was counted in the measurement. Chest CT was performed at baseline and every 3months to exclude metastatic disease. Bone scan was not required unless bone metastasis was suspected. The criteria for successful down-staging included a decrease in the size of a single or multiple lesions to within T2 criteria, or complete tumor necrosis without contrast enhancement based on either CT or MRI of the abdomen, equivalent to obliteration to the tumor (Table 1). For example, in a patient with four lesions, successful down-staging required complete tumor necrosis (equivalent to tumor obliteration) of at least one of these lesions so that there would be no more than three lesions with viable tumor, all within 3 cm in diameter to meet T2 criteria. Treatment was intended to achieve complete tumor necrosis of all the tumor nodules while awaiting OLT. Following successful down-staging, patients were listed with MELD priority upon approval by the regional United Network for Organ Sharing review board. A minimum follow-up of 3 months after down-staging was required before deceased donor liver transplantation or right lobe living donor liver transplantation (LDLT). Additional guidelines are summarized in Table 2.
Table 2.
Additional Guidelines in UCSF Down-Staging Protocol
|
TACE and laparoscopic radiofrequency ablation (RFA), either alone or in combination, was the mainstay of down-staging treatment. Percutaneous RFA and ethanol injection were used mainly in conjunction with TACE for residual or satellite lesions ≤4 cm. The choice of treatment was ultimately determined based on tumor size, number, location, and hypervascularity according to CT or MRI, as well as the hepatic reserve of the individual patient, upon thorough review of the clinical and radio-logic information in a multidisciplinary tumor conference. Selected patients with adequate hepatic reserve were also considered for limited hepatic resection as a down-staging procedure prior to OLT rather than as a primary treatment. Due to difficulties in obtaining approval for priority listing for OLT after resection, we have abandoned resection as a bridging procedure to OLT since 2005.
Histolopathologic Analysis
Explant tumor staging in this study was based on size and number of only the viable tumors. For example, if the explant liver showed a 5-cm, completely necrotic nodule with no viable tumor and a 3-cm nodule with HCC, the pathologic tumor stage in this case would be T2 based on only the 3-cm nodule with viable tumor. The grade of differentiation was based on the Edmondson and Steiner criteria (grade 1, well-differentiated; grade 2, moderately differentiated; grade 3, poorly differentiated).16
Statistical Analysis
The intention-to-treat survival and probabilities of treatment failure (dropout, death without OLT, or HCC recurrence after OLT) were estimated using the Kaplan-Meier method. Time zero in these analyses was defined as the date of the first down-staging treatment. A chi-squared, paired t test or Mann-Whitney U test was used to compare differences between subgroups. Cox proportional hazards models were used for the analysis of predictors of treatment failure. P < 0.05 was considered statistically significant in all analyses.
Results
Baseline Patient Characteristics
Between June 2002 and January 2007, 61 patients with tumor stage exceeding Milan criteria on CT or MRI were enrolled in our down-staging protocol. During the study period, an additional 28 patients with HCC meeting the proposed University of California, San Francisco (UCSF) criteria10 but exceeding Milan criteria underwent OLT without down-staging. After our down-staging protocol was incorporated into the United Network for Organ Sharing Region 5 policy in April 2006, all patients with HCC exceeding Milan criteria but meeting UCSF criteria were included in the down-staging protocol.
The baseline characteristics are summarized in Table 3. The most common etiology of liver disease was hepatitis C, followed by hepatitis B. Ten patients had other diagnoses, including cryptogenic cirrhosis in three, alcoholic liver disease in two, autoimmune hepatitis in two, nonalcoholic fatty liver disease in one, primary biliary cirrhosis in one, and hemochromatosis in one patient. The majority (89%) had a Child-Turcotte-Pugh (CTP)17 score <10 (Child class A and B) before down-staging.
Table 3.
Baseline Characteristics of 61 Patients Undergoing Down-Staging
| Median age, years (range) | 59 (45–73) |
| Sex (M/F) | 50/11 |
| Asian, n (%) | 19 (31%) |
| Child class cirrhosis (A/B/C)* | 29/25/7 |
| Cause of liver disease, n (%) | |
| Hepatitis C virus | 36 (59) |
| Hepatitis B virus | 15 (25) |
| Other | 10 (16) |
| Median AFP, ng/mL (range)† | 28 (3–6,539) |
| HCC characteristics before down-staging, n (median [range] of maximal tumor diameter) | |
| One lesion | 25 (6.4 cm [5.2–8 cm]) |
| Two lesions | 20 (4.2 cm [3.2–5 cm]) |
| Three lesions | 11 (4.6 cm [3.7–5 cm]) |
| Four or five lesions | 5 (2.6 cm [2.2–3 cm]) |
| Histologic confirmation of HCC at or before down-staging, n (%) | 38 (62) |
Child class A, B, and C correspond to CTP scores of 5– 6, 7–9, and 10–15, respectively.
Baseline AFP before down-staging treatment was missed in one patient.
The baseline alpha-fetoprotein (AFP) level and tumor characteristics before down-staging are summarized in Table 3. Biopsy of the tumor was not required in this study, but many patients had biopsy-proven HCC before referral to our institution. Patients undergoing laparoscopic or open RFA also underwent intraoperative biopsy of the tumor. Overall, 38 of 61 patients (62%) had histologic confirmation of HCC at or before down-staging treatments.
Loco-Regional Therapy for Tumor Down-Staging
Loco-regional treatments used for down-staging in this cohort are summarized in Table 4. Excluding the six patients who underwent resection as the down-staging procedure, laparoscopic/open RFA and TACE were the primary treatment modalities in all 55 patients. Fourteen patients received a combination of laparoscopic RFA and TACE. Among them, TACE was used as the initial treatment to facilitate subsequent laparoscopic RFA in nine patients, and for residual or recurrent HCC after laparoscopic RFA in five patients. Fifteen patients received a combination of TACE and percutaneous RFA or percutaneous ethanol injection. Six patients underwent limited resection as a bridge to OLT.
Table 4.
Down-Staging Treatments Received by the 61 Patients
| Treatment | No. of Patients (No. of Treatments) |
|---|---|
| Laparoscopic/open RFA only* | 11 (11) |
| TACE only | 15 (34) |
| TACE + percutaneous ablation | 15 (54) |
| TACE + percutaneous ethanol ablation | 6 (27) |
| TACE + percutaneous RFA | 9 (27) |
| Laparoscopic RFA + TACE | 14 (34) |
| Resection† | 6 (6) |
Two received open RFA, nine received laparoscopic RFA.
One of these patients underwent resection despite a high preoperative CTP score of 11. This patient had a 5.3-cm lesion very close to the liver surface at risk for rupture. The other five patients had a CTP score of ≤7 before resection.
Intention-to-Treat Outcome
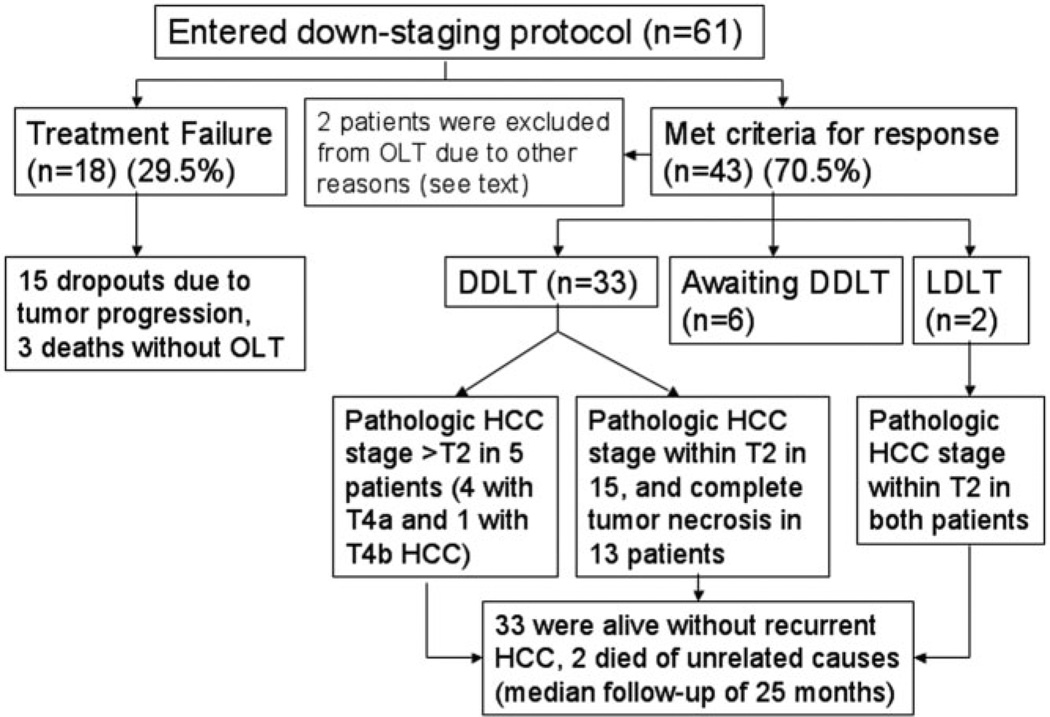
The intention-to-treat outcome for the entire cohort is summarized in Fig. 1. Down-staging was successful in 43 of 61 patients (70.5%). Among them, 35 patients had received OLT after successful down-staging, including two who underwent LDLT. Six patients were still awaiting OLT. The median time from the first down-staging procedure to OLT was 8.2 months (range, 3–25 months). Among the 35 patients who received OLT, the median posttransplantation follow-up was 25 months (range, 1.7–57 months). Two patients had died from causes unrelated to HCC recurrence. One patient died 14 months after OLT with poor graft function and biliary complications. The other patient died 12 months after OLT due to liver failure from recurrent hepatitis C infection. The other 33 patients were alive and free of HCC recurrence at last follow-up.
Fig. 1.
Summary of the intention-to-treat outcome of 61 patients undergoing down-staging of HCC. Abbreviations: DDLT, deceased donor liver transplantation; LDLT, living donor liver transplantation.
Treatment failure was observed in 18 patients (29.5%), including three deaths without OLT, and 15 cases of dropout due to tumor progression (Table 5). Three patients had rapid tumor progression with dropout within 3 months after down-staging. One of the 15 cases of dropout was a patient who underwent segmental resection of a 6.5-cm lesion, but was excluded for OLT due to the presence of microvascular tumor invasion in the re- section specimen. This patient was classified as treatment failure. The median time from initial down-staging to treatment failure was 6.8 months. If we excluded the patient with microvascular invasion in the resection specimen, the median time from down-staging to treatment failure was 7 months (range, 1.2–14.2 months).
Table 5.
Causes of Treatment Failure in 18 Patients
| Cause of Treatment Failure | No. of Patients |
|---|---|
| Dropout due to HCC (n = 15) | |
| Vascular invasion on imaging | 7 |
| Extrahepatic metastasis | 3 |
| Lung | 1 |
| Bone | 1 |
| Bone and lung | 1 |
| Intrahepatic tumor progression (beyond inclusion criteria) | 3 |
| Biopsy-proven lymph node invasion | 1 |
| Exclusion from OLT due to presence of vascular invasion in resection specimen | 1 |
| Death without OLT (n = 3) | |
| Liver failure after laparoscopic RFA | 1 |
| Multiorgan failure after resection | 1 |
| Sudden death at home | 1 |
Among the three deaths without OLT (Table 5), two were directly related to the down-staging procedure. One patient with a CTP score of 7 and a MELD score of 11 underwent wedge resection of a 5-cm tumor in the left lateral segment in combination with open RFA of a 2-cm lesion in the right hepatic lobe. This patient then developed respiratory failure in the early postoperative period and subsequently died of multiorgan failure 23 days after surgery. Pathology of the resection specimen in this patient showed a 6.2-cm moderately differentiated HCC with three other satellite tumor nodules, which would have precluded further consideration of OLT. The other patient, who had a CTP score of 7 and a MELD score of 14, developed severe hepatic decompensation after laparoscopic RFA and succumbed to multiorgan failure on postoperative day 29. The third patient died at home from an unidentified cause before successful tumor down-staging.
Two patients were excluded from OLT for other reasons. One had open RFA of a 5.7 cm well differentiated HCC with complete response, but was later excluded from OLT due to psychosocial contraindications. The other patient underwent resection of a 6.5 cm HCC and was listed for OLT, but subsequent petition for extension of MELD priority status for OLT was denied. This patient remained disease-free 47 months after resection. These two patients were included in the intention-to-treat outcome analysis with follow-up censored at the time of exclusion from OLT.
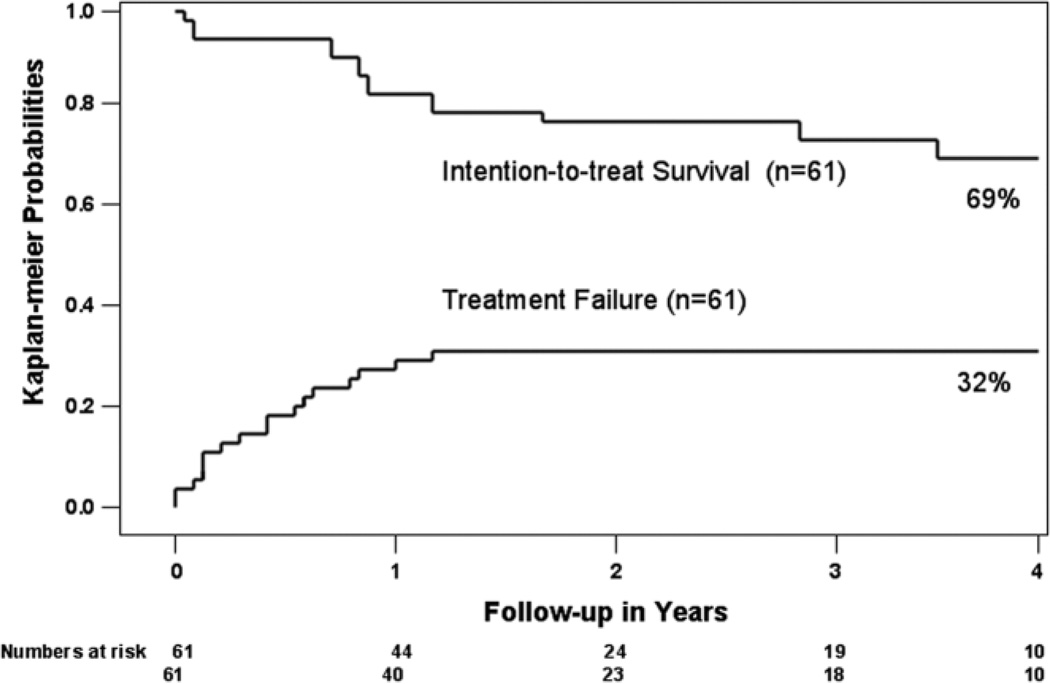
The Kaplan-Meier intention-to-treat survival rates at 1 and 4 years after initiation of down-staging were 87.5% and 69.3%, respectively (Fig. 2). The Kaplan-Meier probabilities of treatment failure, including death without OLT or dropout due to tumor progression, were 32% at 2 years and beyond (Fig. 2). Among the 35 patients who underwent OLT, the Kaplan-Meier 1-year and 4-year posttransplantation patient survival rates were 96.2% and 92.1%, respectively (Fig. 3). None had HCC recurrence after a median posttransplantation follow-up of 25 months (range, 1.7–57 months).
Fig. 2.
Kaplan-Meier intention-to-treat survival function and probabilities of treatment failure. Time zero is the date down-staging treatment was initiated. Treatment failure was defined as death from any cause without OLT (n = 3), dropout from the waiting list due to tumor progression (n = 15), or HCC recurrence after OLT (n = 0). The number of patients at risk is shown below the horizontal axis.
Fig. 3.
Kaplan-Meier survival after liver transplantation. Time zero is the date of liver transplantation. None of the 35 patients who received OLT developed recurrent HCC.
Explant Tumor Characteristics Following Down-Staging
Explant tumor characteristics are summarized in Table 6. Down-staging treatments achieved complete tumor necrosis with no residual tumor in 13 patients (37.1%). Among these 13 patients, nine had biopsy-proven HCC prior to OLT (four percutaneously and five during open or laparoscopic RFA). The histologic grade was well-differentiated in two patients, moderately differentiated in one patient, poorly differentiated in one patient, and not specified in the other four patients.
Table 6.
Explant Histopathologic HCC Characteristics of 35 Patients
| Pathologic Tumor Stage | No. of Patients |
|---|---|
| Complete necrosis without residual tumor | 13 (37.1%) |
| Meeting T2 criteria* | 17 (48.6%) |
| Exceeding T2 criteria | 5 (14.3%) |
| T4a (≥4 lesions) | 4 |
| T4b (macrovascular invasion) | 1 |
| Histologic grade† (n = 22) | |
| Well differentiated (grade 1) | 9 (40.9%) |
| Moderately differentiated (grade 2) | 13 (59.1%) |
| Poorly differentiated (grade 3) | 0 (0%) |
| Vascular invasion | |
| None | 34 (97.1%) |
| Microvascular invasion | 0 (0%) |
| Macrovascular invasion | 1 (2.9%) |
Includes four patients with only a single satellite lesion up to 2 cm and complete necrosis of the larger tumors.
Histologic grade could not be determined in the 13 patients with complete tumor necrosis.
In the other 22 patients with residual HCC in the explant, 17 had pathologic HCC stage within T2 criteria. Four of these 17 patients had a single satellite lesion ≤2 cm and complete necrosis of the other larger tumor nodules. Five patients had explant tumor stage exceeding T2 criteria, including four patients with four or more viable tumor nodules (T4a), and one patient with a 3-cm tumor thrombus within a large branch of the portal vein (T4b) despite extensive necrosis (>90%) of the two tumor nodules in the liver. None of these five patients developed HCC recurrence after a median posttransplantation follow- up of 22 months (range, 8–46 months).
The grade of tumor differentiation could not be determined in the 13 patients with complete tumor necrosis, and was moderately differentiated in 13 patients and well-differentiated in the remaining nine patients. None had poorly differentiated tumor grade. Except for the single patient with macrovascular invasion, none of the other 34 patients had microvascular invasion (Table 6). This patient with macrovascular invasion had no sign of HCC recurrence after a follow-up of 46 months after OLT.
Correlation Between Radiologic and Pathologic Response
Among 35 patients who underwent OLT, the correlation between radiologic response and explant tumor characteristics is summarized in Table 7. Fifteen patients (42.9%) had complete radiologic response, with no residual tumor enhancement before OLT. The liver explant in these 15 patients showed complete tumor necrosis in nine patients and T2 HCC in the remaining six patients. One of these patients had wedge resection as the down-staging procedure. This patient had two residual small tumors in the liver explant measuring 0.9 cm and 0.3 cm. Of the 20 patients with partial radiologic response in which there were viable tumors meeting T2 criteria before OLT, four had complete tumor necrosis in the explant, 11 had explant tumors meeting T2 criteria, and five had HCC beyond T2 stage in the explant.
Table 7.
Correlation Between Radiologic and Explant Pathologic Tumor Characteristics
| Complete Tumor Necrosis in Explant |
Pathologic Tumor Stage Meeting T2 Criteria in Explant |
Pathologic Tumor Stage >T2 Criteria in Explant |
|
|---|---|---|---|
| Complete radiologic response with no tumor enhancement (n = 15)* | 9 | 6 | 0 |
| Radiologic stage within T2 criteria (n = 20) | 4 | 11 | 5 |
| Total (N = 35) | 13 | 17 | 5 |
Includes one patient who had wedge resection as a down-staging procedure; two small tumor nodules measuring 0.9 cm and 0.3 cm were found in the liver explant.
Predictors of Treatment Failure
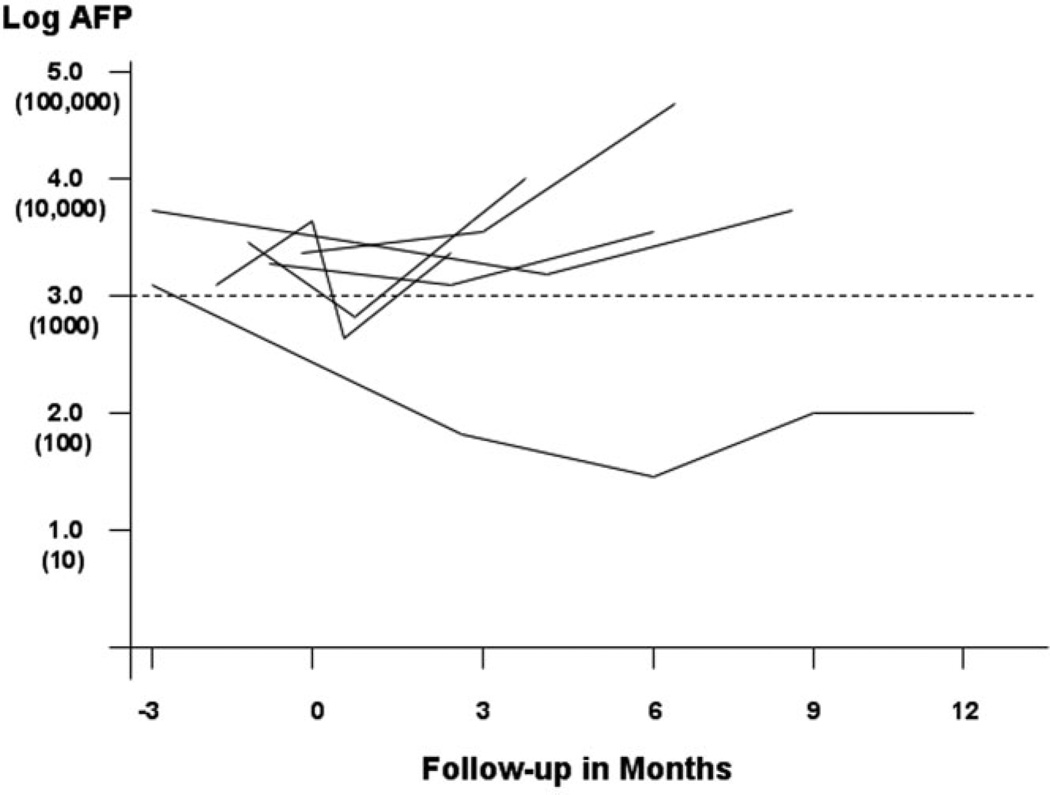
When the clinical characteristics of the 35 patients who received OLT were compared with the 18 patients with treatment failure, only the median AFP level within 3months prior to OLT or treatment failure was significantly different between the two groups (Table 8). The Cox proportional hazard model was also used to identify predictors of treatment failure (Table 9). Only AFP > 1,000 ng/mL was a significant predictor of treatment failure, with a hazard ratio of 7.75 (P < 0.001). Treatment failure was the eventual outcome in seven of the eight patients with pretreatment AFP > 1,000 ng/mL. The AFP level was only slightly improved or became further elevated over time in five patients despite treatment (Fig. 4) and was not repeated in the patient who died from accelerated liver failure shortly after down-staging treatment. Another patient had microvascular invasion in the resection specimen and were excluded from OLT. The remaining patient had a significant decrease in AFP and response to down-staging, but was subsequently excluded from OLT for psychosocial reasons.
Table 8.
Comparison of Clinical Characteristics of 35 Liver Transplantation Patients and 18 Patients with Treatment Failure
| Characteristics | Transplantation (n = 35) |
Treatment Failure (n = 18) |
|---|---|---|
| Median age, years | 58.2 | 59.5 |
| Sex (M/F) | 29/6 | 14/4 |
| Asian, n (%) | 13 (37.1) | 4 (22.2) |
| Child class cirrhosis (A/B/C) | 15/16/4 | 8/8/2 |
| Cause of liver disease, n (%) | ||
| Hepatitis C virus | 24 (68.6) | 9 (50.0) |
| Hepatitis B virus | 15 (25.0) | 6 (33.3) |
| Other | 4 (11.4) | 3 (16.7) |
| Median AFP, ng/mL (range)* | 12.9 (2.1–760) | 271.9 (5.3–58,096) |
| HCC characteristics before down-staging, n (%) | ||
| One lesion | 11 (31.4) | 9 (25.7) |
| Two lesions | 14 (40.0) | 5 (27.8) |
| Three lesions | 7 (20.0) | 3 (16.7) |
| Four or five lesions | 3 (8.6) | 1 (5.6) |
The difference in the median AFP level within 3 months prior to OLT and the median AFP within 3 months before treatment failure was statistically significant (P = 0.001; Mann-Whitney U test). None of the other characteristics are statistically different between the two groups.
Table 9.
Cox Proportional Hazard Model for Predictors of Treatment Failure
| Variable | Hazard Ratio (95% CI) |
P Value |
|---|---|---|
| AFP ≥1,000 ng/mL | 7.75 (2.9–20.7) | <0.0001 |
| Multifocal HCC (versus single lesion) | 0.76 (0.31–1.88) | 0.56 |
| Three or more treatments (versus one treatment) | 0.51 (0.18–1.41) | 0.19 |
| Combination treatment (versus same treatment modality) | 0.67 (0.27–1.66) | 0.39 |
| Child class B or C cirrhosis (versus class A) | 0.97 (0.4–2.40) | 0.95 |
| Cause of liver disease | ||
| Hepatitis C versus hepatitis B | 0.50 (0.14–1.8) | 0.29 |
| Hepatitis C versus other | 0.50 (0.11–2.2) | 0.36 |
Fig. 4.
Changes in AFP level in eight patients with pretreatment AFP >1000 ng/mL. Time zero is the date of first down-staging treatment. Data were not shown for the patient excluded from OLT when microvascular invasion was found in the resection specimen. AFP was not repeated in another patient who died from accelerated liver failure shortly after down-staging treatment. Treatment failure was the eventual outcome in seven of eight patients. Only one patient had significant decrease in the AFP and initial response to down-staging, but was excluded from OLT due to psychosocial reasons.
Discussion
Amid the controversy surrounding expanded criteria for OLT in patients with HCC, it is generally agreed that some patients with tumors exceeding Milan criteria would clearly benefit from OLT with an acceptably low risk of posttransplantation tumor recurrence.11,12,18,19 The concept of using loco-regional therapy such as TACE to reduce the size of HCC, thereby facilitating resection or OLT, was first tested by Majno et al.20 for patients with one or more lesions >3 cm. Subsequently, Roayaie et al.18 from Mount Sinai Medical Center, New York, applied adjuvant chemotherapy in combination with TACE in 80 patients with HCC exceeding Milan criteria based on one or more tumors >5 cm. Thirty-seven patients (46%) were excluded from OLT mainly due to tumor progression, and the other 43 patients ultimately received OLT in the pre-MELD era. The subgroup with the largest tumor diameter between 5 and 7 cm had significantly better 5-year posttransplantation survival than those with tumors >7 cm in diameter (55% versus 34%; P = 0.024). Patients treated under their adjuvant protocol were not required to demonstrate objective response to preoperative therapy prior to OLT. Consequently, the impact of successful down-staging on outcome after OLT was unknown.
The fundamental principle behind down-staging is to select a subset of tumors with more favorable biology that are more likely to respond to treatment and do well after OLT.13 This concept was highlighted in a study by Otto et al.21 in which TACE was used exclusively in controlling tumor progression prior to OLT in 62 patients with HCC initially exceeding Milan criteria and in 34 patients meeting Milan criteria. Using the response evaluation criteria in solid tumors (RECIST) criteria, response to TACE was defined as a 30% decrease in the sum of the largest tumor diameter. Patients were excluded from OLT if they had significant tumor progression, defined as a 20% increase in the sum of the largest tumor diameter or the development of new lesions. Patients with minimal disease progression but not meeting the RECIST criteria also underwent transplantation. Fifty patients eventually underwent OLT, including 27 of 62 patients (44%) with tumors initially exceeding Milan criteria, and 22 of 34 patients (65%) with tumors initially meeting Milan criteria. The 39 patients demonstrating response to treatment or no tumor progression had a significantly better 5-year recurrence-free probability than the 11 patients with minimal tumor progression before OLT (94.5% versus 35.4%, respectively; P = 0.0017). Patients with tumor stage initially exceeding Milan criteria had a 5-year recurrence- free survival not significantly different from those meeting Milan criteria (74.5% versus 93.8%, respectively).
In a subsequent study, Millonig et al.22 applied TACE before OLT in 68 patients within Milan criteria, 33 patients exceeding Milan but within the UCSF criteria,10 and 15 patients exceeding both criteria. The dropout rates for the subgroups within Milan and UCSF criteria were 2.9% and 12.1%, respectively (P = 0.08). For the group exceeding both the Milan and UCSF criteria, the dropout rate was significantly higher at 26.7% (P = 0.009). The 5-year intention-to-treat survival for the group within Milan criteria was 70.3% versus 65.9% for the group within UCSF criteria but exceeding Milan criteria (P = 0.08) and 24.5% for the group exceeding both criteria (P < 0.001). Among patients within the UCSF but exceeding Milan criteria who responded to TACE by RECIST criteria (effectively down-staged), the intention-to-treat 5-year survival was 66.6% for the subgroup with complete response, 63.7% for partial response (30% reduction in tumor size), and only 25% for no response.
The present study was designed to evaluate the intention-to-treat outcome of tumor down-staging to meet Milan criteria, with well-defined eligibility criteria for down-staging. Tumor understaging to beyond Milan criteria was observed in five of 22 patients (23%) with viable tumors in the explant, similar to the 20% rate of under-staging for patients meeting Milan criteria prior to OLT in a previous report from our center.12 We observed no posttransplantation HCC recurrence after down-staging, but the median follow-up of 25 months after OLT may be too short to fully ascertain the recurrence risk. The number of patients with ≥4 tumor nodules is very small, and more data are needed. The extremely low rate of poorly differentiated tumor grade and vascular invasion in the explant is surprising, but this observation may reflect selection of tumors with more favorable biology before OLT, in accord with the fundamental principle behind the down-staging process. There are technical and anatomical factors, in addition to tumor biology, that influence initial response to loco-regional therapy, but the likelihood of tumor progression despite treatment is expected to be higher in those with microvascular invasion and other unfavorable tumor characteristics, The lower dropout rate in our study compared with that reported by Otto (30% versus 55%, respectively)21 may be due to differences in the definition of treatment response and the lack of upper limits in tumor size and number for inclusion in the study by Otto. We believe that well-defined upper limits in tumor size and number in determining eligibility for down-staging is crucial in achieving favorable outcome.
A minimal observation period of 3 months between down-staging treatment and OLT was arbitrarily determined in our protocol. Only three patients had rapid tumor progression leading to dropout within 3 months after down-staging. There is a complex interplay of unfavorable tumor biology and waiting list time on the risk of dropout due to tumor progression. It has been argued that short waiting list time or “fast-tracking” under MELD or with LDLT allows transplantation of aggressive tumors that would have been selected out with traditionally longer waiting list time. This would in turn lead to a higher risk for posttransplantation tumor recurrence.23 This hypothesis was disputed in a recent study from our institution in which waiting list time did not correlate with the risk of tumor recurrence when tumor stage was within Milan criteria prior to OLT.24 In patients with larger tumors undergoing down-staging, however, the proportion of tumors with unfavorable histologic tumor characteristics such as microvascular invasion is expected to be higher,25 which may also be associated with poor treatment response and eventual dropout. While there is still uncertainty regarding what the optimal observation period for down-staging should be, we feel that the 3-month minimal interval between down-staging and OLT is reasonable as long as the criteria for successful down-staging are met before OLT.
The heterogeneity of loco-regional therapies may be considered a weakness of this study.26 We designed the down-staging protocol with the understanding that response to specific loco-regional therapy might vary according to individual tumor characteristics and for technical reasons. Consequently, applying exclusively one type of treatment to all patients might not produce the most optimal response. Instead, the best treatment was determined on a case-by-case basis. Neither the number nor the type of treatment was predictive of treatment failure. The limited sample size precluded more in-depth analysis of the best treatment strategies in achieving successful down-staging.
High AFP of > 1000 ng/mL was a significant predictor of treatment failure. There is growing evidence that this degree of elevation in the AFP level predicts a greater risk of tumor recurrence after OLT.27,28 High AFP may be a marker for vascular invasion or extrahepatic disease that escapes detection by conventional imaging techniques. CTP score as a marker for severity of liver disease was not shown to be a significant predictor of treatment failure. Intuitively, patients with severely decompensated cirrhosis and large tumors are not likely to tolerate aggressive down-staging treatments. Although we did not exclude any patient for down-staging on the basis of CTP score or other indices of liver disease severity, a referral bias probably existed in that only patients with adequate functional reserve were referred for consideration of tumor down-staging. The two deaths in our series precipitated by hepatic decompensation shortly after down-staging treatments underscore the importance of counseling patients about the potential risks for tumor down-staging.
In conclusion, our results suggest that tumor down-staging to meet conventional criteria for OLT among carefully selected patients is associated with excellent posttransplantation outcome. Down-staging put selection pressure against aggressive tumors that are likely to progress despite treatment, whereas tumors with more favorable histology are more likely to respond to treatment and do well after OLT. Balancing the risk of dropout due to long waiting list time and the selection of candidates with more favorable tumor biology remains a challenge. There is still a need for refinement of down-staging treatment strategies to further improve intention-to-treat outcome.
Acknowledgment
We thank Chengshi Jin and Peter Bacchetti for statistical assistance.
Supported in part by a grant from the National Institutes of Health to the University of California, San Francisco Liver Center (P30DK26743).
Abbreviations
- AFP
alpha-fetoprotein
- CT
computed tomography
- CTP
Child-Turcotte-Pugh
- DDLT
deceased donor liver transplantation
- HCC
hepatocellular carcinoma
- LDLT
living donor liver transplantation
- MELD
model for end-stage liver disease
- MRI
magnetic resonance imaging
- OLT
orthotopic liver transplantation
- RECIST
response evaluation criteria in solid tumors
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
- UCSF
University of California, San Francisco
Footnotes
Presented at the Presidential plenary session of the 58th Annual American Association for the Study of Liver Diseases meeting, Boston, MA, November 2007 (Hepatology 2007;46(Suppl 1):309A).
Potential conflict of interest: Nothing to report.
References
- 1.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726–734. [PubMed] [Google Scholar]
- 2.Pichlmayr R, Weimann A, Ringe B. Indications for liver transplantation in hepatobiliary malignancy. Hepatology. 1994;20:33S–40S. doi: 10.1016/0270-9139(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 3.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinoma in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Fuster J, Bruix J for the Barcelona Clinic Liver Cancer (BCLC) group. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 6.Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle, and dropout from the waiting list. Liver Transpl. 2002;8:873–883. doi: 10.1053/jlts.2002.34923. [DOI] [PubMed] [Google Scholar]
- 7.Maddala YK, Stadheim L, Andrews JC, Burgart LJ, Rosen CB, Kremers WK, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: outcome with chemoembolization. Liver Transpl. 2004;10:449–455. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 8.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(Suppl 1):S261–S267. doi: 10.1053/j.gastro.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 10.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 11.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded. A 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–511. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF expanded criteria based on pre-operative imaging. Am J Transpl. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 13.Yao FY. Expanding criteria for hepatocellular carcinoma: down-staging with a view to liver transplantation—yes. Semin Liv Dis. 2006;26:239–247. doi: 10.1055/s-2006-947295. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Fuster J, Llovet J. Liver transplantation for hepatocellular carcinoma. Foucault pendulum versus evidence-based decision. Liver Transpl. 2003;9:700–702. doi: 10.1053/jlts.2003.50124. [DOI] [PubMed] [Google Scholar]
- 15.Yao FY, Hirose R, LaBerge J, Davern T, II, Bass NM, Kerlan R, et al. A prospective study on down-staging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 16.Edmondson H, Steiner P. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;1:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 18.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinoma larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128:1752–1764. doi: 10.1053/j.gastro.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 22.Millonig G, Graziadei I, Freund MC, Jaschke W, Stadimann S, Ladurner R, et al. Response to chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–279. doi: 10.1002/lt.21033. [DOI] [PubMed] [Google Scholar]
- 23.Kulik L, Abeccassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127(Suppl 1):S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Chao SD, Roberts JP, Farr M, Yao FY. Short waitlist time does not adversely impact outcome after liver transplantation for hepatocellular carcinoma. Am J Transpl. 2007;7:1594–1600. doi: 10.1111/j.1600-6143.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- 25.Pawlik TM, Delman KA, Vauthey JN, Nagomey DM, Ng ID, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 26.Gamblin TC, Geller DA. Down-staging hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1466–1468. doi: 10.1002/lt.20528. [DOI] [PubMed] [Google Scholar]
- 27.Figueras J, Ibanez L, Ramos E, Jaurrieta E, Ortiz-de-Urbina J, Pardo F, et al. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multi-center study. Liver Transpl. 2001;7:877–883. doi: 10.1053/jlts.2001.27856. [DOI] [PubMed] [Google Scholar]
- 28.Yao FY, Kinkhabwala M, LaBerge J, Bass NM, Brown R, Kerlan R, et al. The impact of pre-operative loco-regional treatments on survival following liver transplantation for hepatocellular carcinoma. Am J Transpl. 2005;5:795–804. doi: 10.1111/j.1600-6143.2005.00750.x. [DOI] [PubMed] [Google Scholar]