Calcific aortic valve sclerosis (CAVS) cause profound morbidity in our increasingly dysmetabolic and aging citizenry1. Two to five percent of our elderly population will require aortic valve replacement (AVR) surgery to mitigate risk of death due to severe CAVS. Once considered only a passive process of dead and dying cells, arteriosclerotic calcification -- including CAVS -- has emerged as an actively regulated form of mineralized tissue metabolism1. Preclinical and epidemiological studies reveal that aging, tobacco use, renal failure, bicuspid aortic valve, and the features of metabolic syndrome – hypertension, worsening glycemic control, hypertriglyceridemia, low HDL cholesterol, and obesity2 -- are cumulative risk factors for arteriosclerotic valve calcification1. Although hypercholesterolemia certainly contributes to risk of arteriosclerotic disease, targeting cholesterol via lipid-lowering statin therapy is insufficient to fully mitigate disease progression1. Seminal histological studies by Otto and colleagues indicate that while early valve lesions do exhibit intra- and extra-cellular lipid accumulation with inflammation, features of active matrix remodeling are present as well including disruption of the elastic lamina with lamina fibrosa protein accumulation and microcalcification3. With advanced disease, remodeling woven bone can be seen in ca. 13% of specimens4 although the molecular fingerprints of active osteogenesis are uniformly present even when amorphous calcium phosphate deposits predominate 1, 4. Currently no medical therapies exist for preventing or treating CAVS – and our capacity to identify those at greatest risk for clinical progression is limited1. In the Japanese Aortic Stenosis Study (JASS), while warfarin use portended worsening disease treatment of hypertension with angiotensin receptor blockade (ARB) was associated with attenuated risk of CAVS progression 5. Interestingly, emerging data suggest that ARBs exert beneficial actions in cardiac valve biology in part via inhibition of TGF-ß signaling cascades6, 7.
In culture, TGF-ß1 clearly promotes aortic valve interstitial cell calcification8, with responses dependent upon the stiffness of the extracellular matrix9. However, the contributions of TGF-ß1 signaling to CAVS have not been fully examined in vivo – nor has the precise cellular source of TGF-ß1 important to valve pathbiology been established. In this issue of the Journal 10, Coller and colleagues begin to address these important questions. Implementing the “Reversa” LDLR−/− mouse model of vascular disease 11– first validated in CAVS by Miller, Weiss, Heistad and colleagues12 -- the authors demonstrate that diet-induced hypercholesterolemia results in valve thickening, worsening aortic valve stenosis, increased wall shear stress (WSS; determined by echocardiographic measurement of valve leaflet separation and Doppler velocimetry), and elevated circulating TGF-ß1 levels over a 12 month period. Reversing hypercholesterolemia after 6 months of dyslipidemia attenuates the severity of CAVS observed at 12 months, reflected in reductions in the rate of aortic stenosis and concomitant increases in transvalvular velocity, WSS and plasma TGF-ß110. In those animals with progressive valvular disease, a modest but significant positive relationship was observed between WSS and total circulating TGF-ß1 levels – with a strong trend for correlation exhibited at 12 months between circulating TGF-ß1 levels and the extent of aortic valve fibrosis by histological scoring (r = 0.78, p = 0.08). More importantly, surgical introduction of an ascending aortic constriction (AAC) in wild type mice – an experimental mimetic of aortic valve stenosis -- also increased WSS and circulating TGF-ß1 levels in the absence of hypercholesterolemia. The source of TGF-ß1 was firmly established to be the platelet in the AAC model; conditional deletion of TGF-ß1 in the megakaryocyte lineage (PF4-Cre;TGF-ß1(fl/fl) mice) markedly reduced time-dependent increases in total plasma TGF-ß1 following AAC10. Thus, the relationship observed between total plasma TGF-ß1 levels and WSS in wild-type mice was no longer significant in PF4-Cre;TGF-ß1(fl/fl) mice. Importantly, bioactive platelet-derived TGF-ß1 was responsible for driving Smad2/3 and ERK phosphorylation in response to AAC in both circulating leukocytes and mesenchymal cells of the ascending aorta – and these same signaling responses were phenocopied by diet-induced aortic valve disease, WSS, and plasma TGF-ß1 upregulation in the Reversa mouse model10. Ex vivo, stirring-induced shear of whole blood was shown to increase leukocyte Smad2/3 and ERK phosphorylation via mechanisms inhibited by neutralizing TGF-ß1 antibody. Thus, platelet-derived TGF-ß1 is released and signals in response to elevated aortic shear stress, activating prosclerotic signaling cascades in the arterial vasculature 10.
Why is this manuscript so intriguing? Firstly, the storage and release of platelet derived TGF-ß1 has significant implications vis-á-vis the emerging bone-vascular axis and the endocrine regulation of arteriosclerosis13. By mobilizing and recruiting mesenchymal progenitors capable of directing robust collagen deposition and matrix mineralization, TGF-ß1 signaling coordinates normal bone turnover and fracture repair14. Cao and colleagues recently identified that with tissue injury, increases in circulating TGF-ß1 mobilizes and recruit bone marrow-derived mesenchymal stem cells (MSCs; Sca1+CD29+CD11B-CD45-) to sites vascular remodeling – including mechanically initiated neointima formation15. Therefore, by analogy, increases in WSS that promote platelet-derived TGF-ß release are posited to increase circulating levels of MSCs capable of contributing to pro-sclerotic vascular responses including CAVS. Moving forward, it will be important to examine whether circulating MSCs are increased in response to ascending aortic constriction and are recruited only to that site of vascular injury -- or potentially populate other vascular venues with disturbed flow and dependent upon platelet-derived TGF-ß1. Secondly, this study once again points to the limitations of targeting only cholesterol in CAVS – even though lipoprotein metabolism is one critically important component of disease initiation1. Once valve hemodynamics are perturbed in CAVS, a vicious “feed-forward” cycle arising from increased WSS may continue to condition the dysmetabolic milieu with circulating TGF-ß1. It should be noted that (Smad) and noncanonical (ERK) pathway activation persisted in Reversa mice; pharmacokinetic-pharmacodynamic relationships between platelet-derived TGF-ß1 release and arteriosclerotic disease remain to be established. Independent of whether TGF-ß1 functions by paracrine vascular actions or via MSC mobilization, the administration of TGF-ß1 neutralizing antibody is predicted to help mitigate CAVS disease progression, although this remains to be directly tested. Finally, this study suggests that other platelet-derived molecules such as PDGF16 and Dkk117 – the latter participating in arteriosclerotic responses via the Smad-dependent endothelial-mesenchymal transition 18– will be relevant to CAVS biology. Moreover, in addition to affording useful plasma biomarkers predictive of CAVS progression, targeted inhibition of TGF-ß1 and other pro-sclerotic platelet products may represent effective strategy for mitigating disease in those individuals at greatest clinical risk. Indeed, fresolimumab -- a monoclonal antibody neutralizing all forms of TGF-ß and under evaluation for treatment of focal segmental glomerulosclerosis19 and advanced melanoma 20 -- might impact CAVS progression in those individuals with high plasma TGF-ß1 or elevated leukocyte Smad activation. Of note, circulating levels of Dkk1 are increased in patients with severe CAVS and decrease following surgical aortic valve replacement21. Intriguingly, Dkk1 levels apparently do not change following transcatheter aortic implantation21; whether other platelet-derived signals including TGF-ß1 are differentially impacted by surgical intervention remains to be assessed – and the clinical impact if any upon arteriosclerotic disease progression in other vascular venues remains to be determined. All in all, the seminal work of Coller and colleagues10 provides us with an enlightened view of CAVS biology, offering new hope for diagnostic and therapeutic intervention in patients afflicted with arteriosclerotic valve and vascular disease.
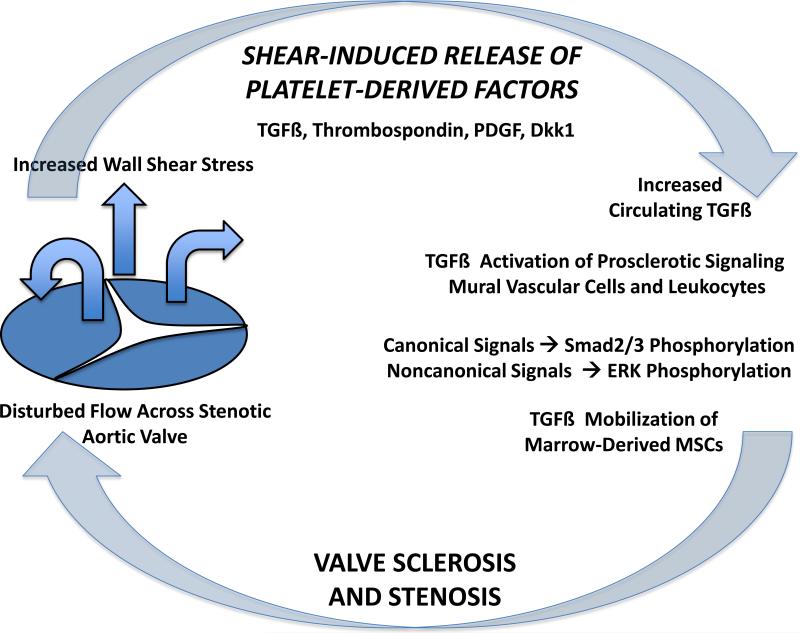
FIGURE. Shear – dependent TGF-ß release and the pathobiology of CAVS.
In this issue of the Journal10, Coller and colleagues demonstrate that shear-induced release of TGF-ß from platelet alpha granules activates pro-sclerotic canonical and noncanonical signals that can drive systemic arteriosclerotic disease. This portends a feed-forward vicious cycle in CAVS. Thus, in addition to providing potential biomarkers for those individuals at greatest risk for clinical progression, platelet-derived factors such as TGF-ß afford novel therapeutic targets for medical treatment of CAVS. See text for details. TGFß, transforming growth factor ß; PDGF, platelet-derived growth factor; Dkk1, Dickkopf-1; MSCs, mesenchymal stem cells.
Acknowledgements
D.A.T. is supported by grants HL69229, HL81138, HL114806 from the National Institutes of Health.
REFERENCES
- 1.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 4.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto K, Yamamoto H, Yoshida K, Kisanuki A, Hirano Y, Ohte N, Akasaka T, Takeuchi M, Nakatani S, Ohtani T, Sozu T, Masuyama T. Prognostic factors for progression of early- and late-stage calcific aortic valve disease in japanese: The japanese aortic stenosis study (jass) retrospective analysis. Hypertens Res. 2010;33:269–274. doi: 10.1038/hr.2009.225. [DOI] [PubMed] [Google Scholar]
- 6.Wylie-Sears J, Levine RA, Bischoff J. Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-ß-induced phosphorylation of erk. Biochemical and biophysical research communications. 2014;446:870–875. doi: 10.1016/j.bbrc.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, Hashim S, Tellides G. Modulation of transforming growth factor-ß signaling and extracellular matrix production in myxomatous mitral valves by angiotensin ii receptor blockers. Circulation. 2012;126:S189–197. doi: 10.1161/CIRCULATIONAHA.111.082610. [DOI] [PubMed] [Google Scholar]
- 8.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: Tgf-ß1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. The Annals of thoracic surgery. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. discussion 465-456. [DOI] [PubMed] [Google Scholar]
- 9.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Vootukuri S, Meyer A, Ahamed J, Coller BS. Association between shear stress and platelet-derived tgf-β1 release and activation in animal models of aortic valve stenosis. Arteriosclerosis, thrombosis, and vascular biology. 2014 doi: 10.1161/ATVBAHA.114.303852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieu HD, Withycombe SK, Walker Q, Rong JX, Walzem RL, Wong JS, Hamilton RL, Fisher EA, Young SG. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107:1315–1321. doi: 10.1161/01.cir.0000054781.50889.0c. [DOI] [PubMed] [Google Scholar]
- 12.Miller JD, Weiss RM, Serrano KM, Brooks RM, 2nd, Berry CJ, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation. 2009;119:2693–2701. doi: 10.1161/CIRCULATIONAHA.108.834614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadini GP, Rattazzi M, Matsumoto T, Asahara T, Khosla S. Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation. 2012;125:2772–2781. doi: 10.1161/CIRCULATIONAHA.112.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane JL, Cao X. Bone marrow mesenchymal stem cells and tgf-ß signaling in bone remodeling. The Journal of clinical investigation. 2014;124:466–472. doi: 10.1172/JCI70050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan M, Li C, Zhen G, Jiao K, He W, Jia X, Wang W, Shi C, Xing Q, Chen YF, Jan De Beur S, Yu B, Cao X. Injury-activated transforming growth factor ß controls mobilization of mesenchymal stem cells for tissue remodeling. Stem cells. 2012;30:2498–2511. doi: 10.1002/stem.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowen-Pope DF, Raines EW. History of discovery: Platelet-derived growth factor. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2397–2401. doi: 10.1161/ATVBAHA.108.179556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 18.Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and msx2-wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1679–1689. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JP, Streisand JB, Hard ML, Ledbetter SR, Vincenti F. A phase 1, single-dose study of fresolimumab, an anti-tgf-ß antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney international. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, Hsu FJ, Berzofsky JA, Lawrence DP. Phase i study of gc1008 (fresolimumab): A human anti-transforming growth factor-ß (tgfß) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PloS one. 2014;9:e90353. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motovska Z, Vichova T, Tousek P, Dusek L, Widimsky P. Circulating osteoprotegerin and dickkopf-1 changed significantly after surgical aortic valve replacement but remained without any significant differences after transcatheter aortic valve implantation. International journal of cardiology. 2012;158:300–301. doi: 10.1016/j.ijcard.2012.04.115. [DOI] [PubMed] [Google Scholar]