Abstract
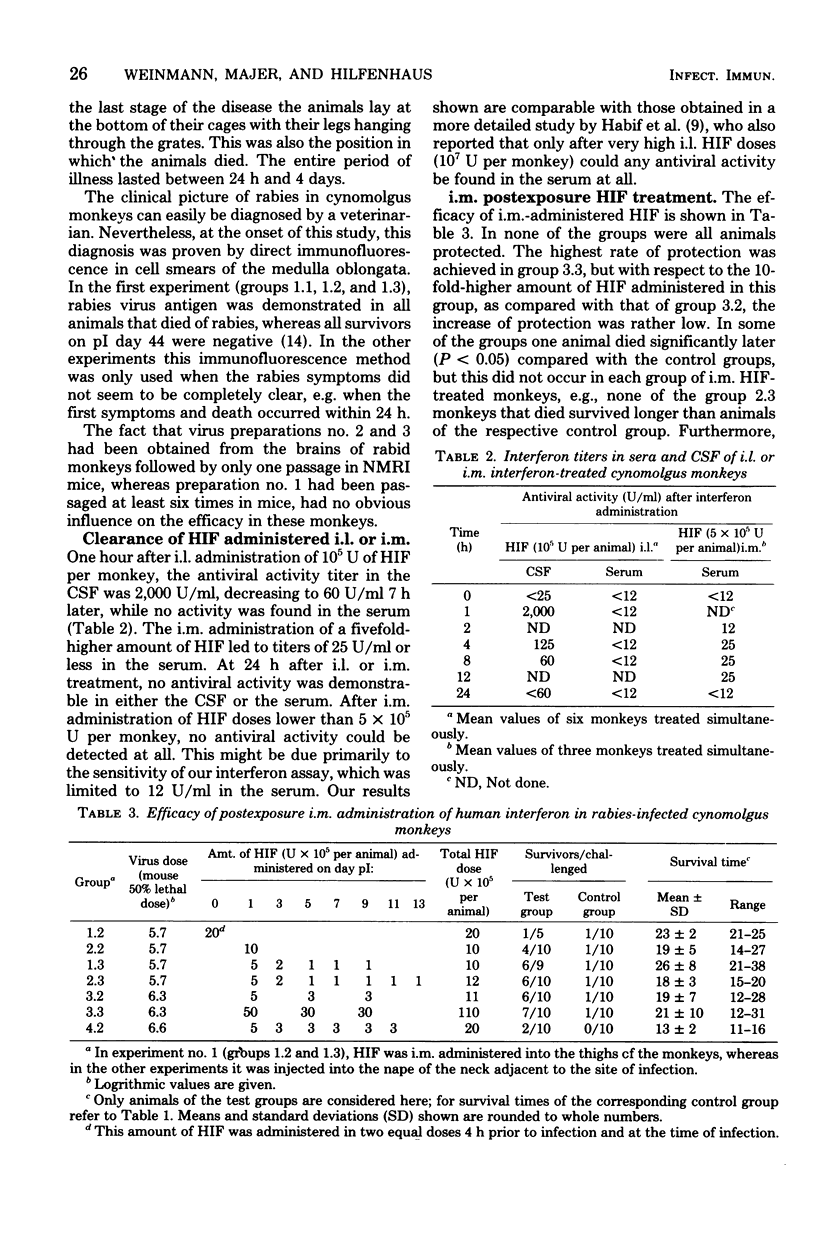
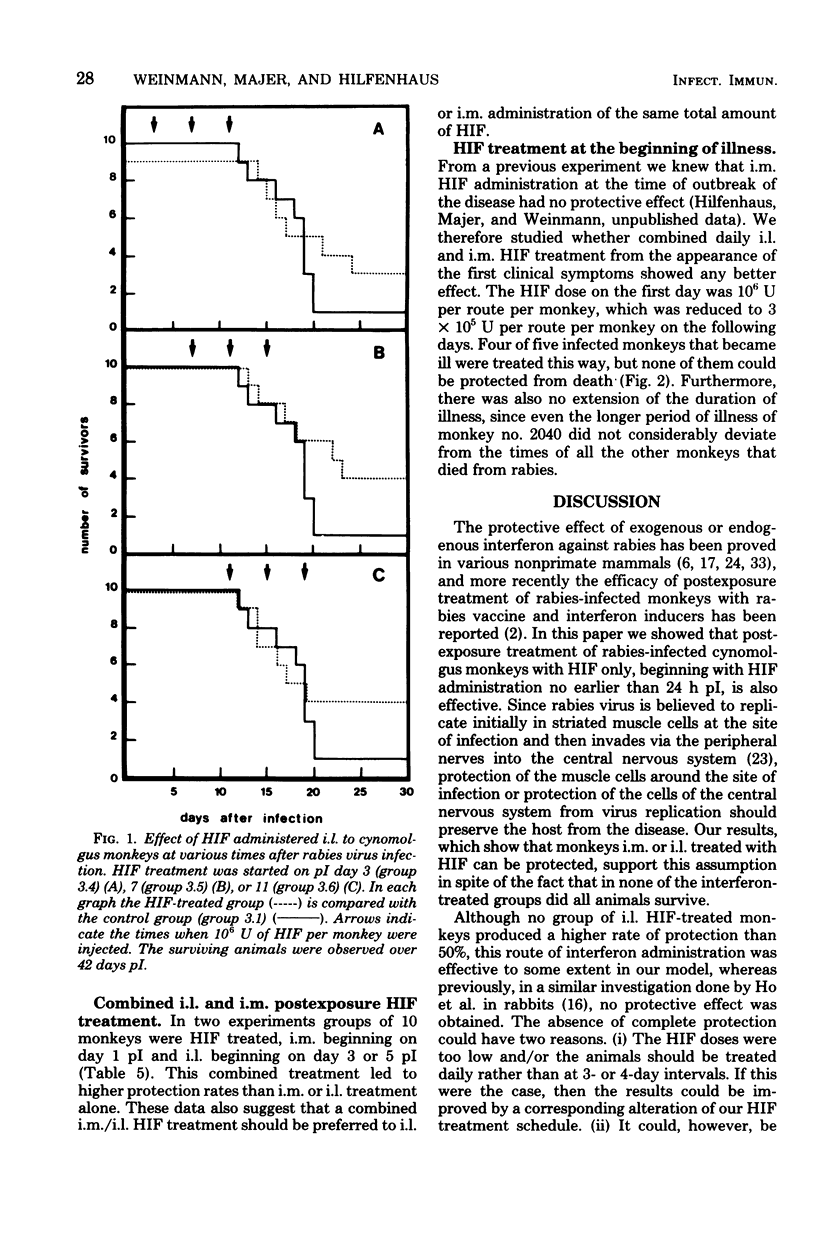
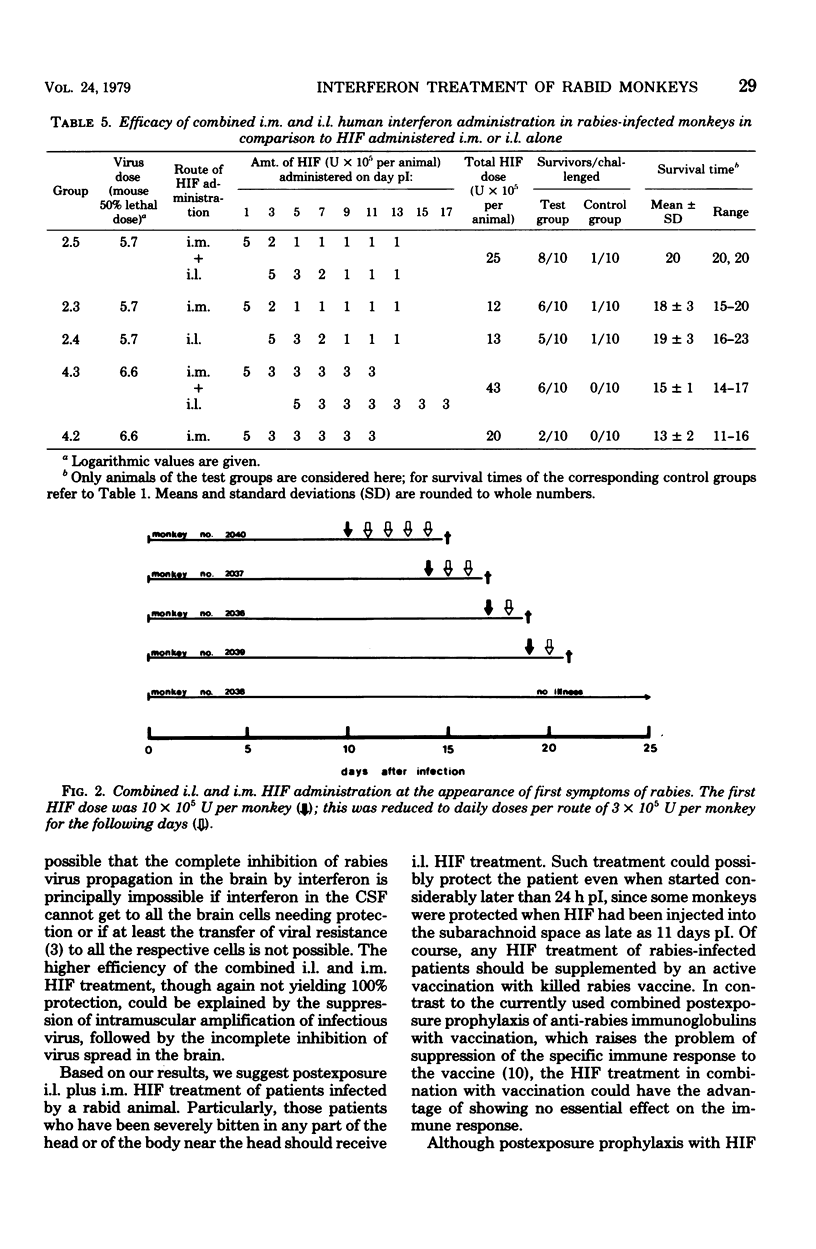
From 9 to 10 of 10 cynomolgus monkeys infected with rabies street virus died of rabies about 20 days postinfection (pI). Symptoms of illness appeared 1 to 4 days before death. In an attempt to protect infected animals from the disease, human leukocyte interferon (HIF) was administered intramuscularly (i.m.) near the site of infection or into the cerebrospinal fluid between the first and second lumbar vertebrae (i.e., intralumbarly [i.l.]). Multiple HIF doses given over a period of several days proved more effective than a single HIF dose. In every experiment, i.m. HIF treatment was started 1 day pI. The best result obtained was a survival rate of 7 of 10 monkeys. The i.l. HIF administration schedules, consisting of multiple doses given over a period of at least 8 days, were started on day 3, 7, or 11 pI. Here the best result noted was the protection of 5 of 10 treated monkeys. The latest successful postexposure i.l. HIF treatment began on day 11 pI. The highest protection rate, 8 survivors of 10 treated monkeys, was achieved by a combined i.m. and i.l. HIF treatment. From these results we conclude that human patients severely bitten by rabid animals should in addition to an active immunization be i.m. and i.l. treated with HIF. Particularly, i.l. HIF administration could be effective, even when given several days pI. Whether an HIF administration starting after the appearance of clinical symptoms of rabies can help cannot be decided upon from the studies made in this monkey model. The most obvious difference between rabies in humans and cynomolgus monkeys is the duration of illness between the outbreak of the disease and death (1 to 4 days only in this animal model). It might have been due to this short period of illness that i.l. and i.m. HIF treatment at the appearance of clinical symptoms failed to help any of the monkeys treated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atanasiu P. Quantitative assay and potency test of antirabies serum and immunoglobulin. Monogr Ser World Health Organ. 1973;(23):314–318. [PubMed] [Google Scholar]
- Baer G. M., Shaddock J. H., Moore S. A., Yager P. A., Baron S. S., Levy H. B. Successful prophylaxis against rabies in mice and Rhesus monkeys: the interferon system and vaccine. J Infect Dis. 1977 Aug;136(2):286–291. doi: 10.1093/infdis/136.2.286. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Baron S. Interferon-induced transfer of viral resistance between animal cells. Nature. 1977 Sep 29;269(5627):422–425. doi: 10.1038/269422a0. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Mechanism of the suppressive effect of interferon on antibody synthesis in vivo. J Immunol. 1975 Apr;114(4):1323–1328. [PubMed] [Google Scholar]
- Desmyter J., De Groote J., Desmet V. J., Billiau A., Ray M. B., Bradburne A. F., Edy V. G., De Somer P. Administration of human fibroblast interferon in chronic hepatitis-B infection. Lancet. 1976 Sep 25;2(7987):645–647. doi: 10.1016/s0140-6736(76)92460-0. [DOI] [PubMed] [Google Scholar]
- Fenje P., Postic B. Protection of rabbits against experimental rabies of poly I-poly C. Nature. 1970 Apr 11;226(5241):171–172. doi: 10.1038/226171a0. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Pollard R. B., Lutwick L. I., Gregory P. B., Robinson W. S., Merigan T. C. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med. 1976 Sep 2;295(10):517–522. doi: 10.1056/NEJM197609022951001. [DOI] [PubMed] [Google Scholar]
- Habif D. V., Lipton R., Cantell K. Interferon crosses blood-cerebrospinal fluid barrier in monkeys. Proc Soc Exp Biol Med. 1975 May;149(1):287–289. doi: 10.3181/00379727-149-38790. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus F. The impossibility of defining unequivocally the antiviral activity of human fibroblast interferon in terms of a human leukocyte interferon standard. J Biol Stand. 1978;6(2):159–164. doi: 10.1016/s0092-1157(78)80046-8. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus J., Damm H., Karges H. E., Manthey K. F. Growth inhibition of human lymphoblastoid Daudi cells in vitro by interferon preparations. Arch Virol. 1976;51(1-2):87–97. doi: 10.1007/BF01317837. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus J., Karges H. E. Growth inhibition of human lymphoblastoid cells by interferon preparations, obtained from human leukocytes. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):618–622. doi: 10.1515/znc-1974-9-1027. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus J., Karges H. E., Weinmann E., Barth R. Effect of administered human interferon on experimental rabies in monkeys. Infect Immun. 1975 May;11(5):1156–1158. doi: 10.1128/iai.11.5.1156-1158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfenhaus J., Weinmann E., Majer M., Barth R., Jaeger O. Administration of human interferon to rabies virus-infected monkeys after exposure. J Infect Dis. 1977 May;135(5):846–849. [PubMed] [Google Scholar]
- Ho M., Nash C., Morgan C. W., Armstrong J. A., Carroll R. G., Postic B. Interferon administered in the cerebrospinal space and its effect on rabies in rabbits. Infect Immun. 1974 Feb;9(2):286–293. doi: 10.1128/iai.9.2.286-293.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis B., Habel K. Rabies in rabbits and mice: protective effect of polyriboinosinic-polyribocytidylic acid. J Infect Dis. 1972 Apr;125(4):345–352. doi: 10.1093/infdis/125.4.345. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Baron S. The nature of the suppressive effect of interferon and interferon inducers on the in vitro immune response. Cell Immunol. 1976 Jul;25(1):106–115. doi: 10.1016/0008-8749(76)90100-3. [DOI] [PubMed] [Google Scholar]
- Jones B. R., Coster D. J., Falcon M. G., Cantell K. Topical therapy of ulcerative herpetic keratitis with human interferon. Lancet. 1976 Jul 17;2(7977):128–128. doi: 10.1016/s0140-6736(76)92850-6. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr Antiviral and cell growth inhibitory activities reside in the same glycoprotein of human fibroblast interferon. Nature. 1976 Jul 22;262(5566):302–303. doi: 10.1038/262302a0. [DOI] [PubMed] [Google Scholar]
- Koprowski H. Laboratory techniques in rabies: vaccine for man prepared in human diploid cells. Monogr Ser World Health Organ. 1973;(23):256–260. [PubMed] [Google Scholar]
- Merigan T. C., Rand K. H., Pollard R. B., Abdallah P. S., Jordan G. W., Fried R. P. Human leukocyte interferon for the treatment of herpes zoster in patients with cancer. N Engl J Med. 1978 May 4;298(18):981–987. doi: 10.1056/NEJM197805042981801. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Bauer S. P. Early street rabies virus infection in striated muscle and later progression to the central nervous system. Intervirology. 1974;3(4):256–268. doi: 10.1159/000149762. [DOI] [PubMed] [Google Scholar]
- Postic B., Fenje P. Effect of administered interferon on rabies in rabbits. Appl Microbiol. 1971 Sep;22(3):428–431. doi: 10.1128/am.22.3.428-431.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes R. K., Cleary W. F., Koprowski H., Wiktor T. J., Kaplan M. M. Effective protection of monkeys against death from street virus by post-exposure administration of tissue-culture rabies vaccine. Bull World Health Organ. 1971;45(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Skurkovich S. V., Klinova E. G., Eremkina E. I., Levina N. V. Immunosuppressive effect of an anti-interferon serum. Nature. 1974 Feb 22;247(5442):551–552. doi: 10.1038/247551a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gresser I., Tovey M. G., Bandu M., Le Goff S. Identification of the cell multiplication inhibitory factors in interferon preparations as interferons. Nature. 1976 Jul 22;262(5566):300–302. doi: 10.1038/262300a0. [DOI] [PubMed] [Google Scholar]
- Strander H., Cantell K. Production of interferon by human leukocytes in vitro. Ann Med Exp Biol Fenn. 1966;44(2):265–273. [PubMed] [Google Scholar]
- Strander H. Interferons: anti-neoplastic drugs? Blut. 1977 Sep 18;35(4):277–288. doi: 10.1007/BF00996140. [DOI] [PubMed] [Google Scholar]
- Sundmacher R., Neumann-Haefelin D., Cantell K. Letter: Interferon treatment of dendritic keratitis. Lancet. 1976 Jun 26;1(7974):1406–1407. doi: 10.1016/s0140-6736(76)93052-x. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A., Yamazaki S. Antigenic, physicochemical, and biologic characterization of human interferons. Ann N Y Acad Sci. 1977 Mar 4;284:703–710. doi: 10.1111/j.1749-6632.1977.tb22006.x. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Postic B., Ho M., Koprowski H. Role of interferon induction in the protective activity of rabies vaccines. J Infect Dis. 1972 Oct;126(4):408–418. doi: 10.1093/infdis/126.4.408. [DOI] [PubMed] [Google Scholar]


