Abstract
Background
Cadmium is a widespread toxic metal with potential cardiovascular effects, but no studies have evaluated cadmium and incident cardiovascular disease. We evaluated the association of urine cadmium concentration with cardiovascular disease incidence and mortality in a large population-based cohort.
Methods
We conducted a prospective cohort study of 3,348 American Indian adults aged 45–74 years from Arizona, Oklahoma and North and South Dakota who participated in the Strong Heart Study in 1989–1991. Urine cadmium was measured using inductively coupled plasma mass spectrometry. Follow-up extended through 31 December 2008.
Results
The geometric mean cadmium level in the study population was 0.94 μg/g (95% confidence interval= 0.92 – 0.93). We identified 1,084 cardiovascular events, including 400 deaths. After adjustment for sociodemographic and cardiovascular risk factors, the hazard ratios (comparing the 80th to the 20th percentile of urine cadmium concentrations) was 1.43 for cardiovascular mortality (95% confidence interval=1.21 – 1.70), and 1.34 for coronary heart disease mortality (1.10 – 1.63). The corresponding hazard ratios for incident cardiovascular disease, coronary heart disease, stroke, and heart failure were 1.24 (1.11 – 1.38), 1.22 (1.08 – 1.38), 1.75 (1.17 – 2.59) and 1.39 (1.01 – 1.94), respectively. The associations were similar in most study subgroups including never-smokers.
Conclusions
Urine cadmium, a biomarker of long-term exposure, was associated with increased cardiovascular mortality and with increased incidence of cardiovascular disease. These findings support that cadmium exposure is a cardiovascular risk factor.
Cadmium is a toxic metal with widespread population exposure through smoking, diet and ambient air.1 In addition to being a carcinogen, cadmium has been associated with kidney disease, bone disease and cardiovascular disease.2 In cross-sectional epidemiologic studies, low to moderate cadmium exposure has been associated with hypertension,3–5 chronic kidney disease,6–8 diabetes,9 carotid atherosclerosis,10,11 peripheral arterial disease,12,13 myocardial infarction,14,15 stroke and heart failure.16 In prospective studies, cadmium was associated with increased cardiovascular mortality in the US general population.17–18 Mechanistic animal and experimental data support a role for cadmium in atherosclerosis, including increased endothelial permeability by inhibiting cell proliferation and promoting cell death.10,19 Establishing cadmium as a cardiovascular risk factor, however, has been limited by the lack of data on its association with incident cardiovascular events and by the potential for confounding by smoking, a source of cadmium exposure.
We evaluated the prospective association of urine cadmium (an established biomarker of cumulative cadmium exposure1) with fatal and non-fatal cardiovascular disease incidence and all-cause mortality in the Strong Heart Study, a large population-based prospective cohort study of cardiovascular disease in American Indian communities in Arizona, Oklahoma and North/South Dakotas.20 This study, conducted in a population with high rates of cardiovascular disease, is one of the major cardiovascular cohorts funded by the National Heart, Lung, and Blood Institute and has served as a model for evaluation of multiple physiologic and environmental risk factors. Established risk factors for CVD in this cohort include male sex, older age, increased LDL-cholesterol, decreased HDL-cholesterol, increased dietary fat intake, smoking, hypertension, decreased kidney function, and diabetes.21–25 The cardiovascular role of environmental exposures, such as cadmium, has not been evaluated in this cohort.
METHODS
Study Population
From 1989 to 1991, men and women aged 45–75 years from 13 American Indian communities in Arizona, Oklahoma and North and South Dakota were invited to participate in this cohort study. In Arizona and Oklahoma every eligible person was invited, whereas in North and South Dakota a cluster sampling technique was used.20 The overall participation rate was 62%.26 Participants (n=4,549) were similar to non-participants in age, body mass index, and self-reported frequency of diabetes, but were more likely to be women and have hypertension.
We used data from 4,218 participants who were free of self-reported cardiovascular disease at baseline. We further excluded 517 participants who were missing urine cadmium determinations due to insufficient urine left for metal analysis, 139 participants with missing smoking information, and 214 participants missing other variables of interest, leaving 3,348 participants for this study. Included participants were similar to those excluded because of missing data (data not shown). The study protocol was approved by the Institutional and Indian Health Service Review Boards and by the participating American Indian communities. All the participants provided oral and written informed consent.
Baseline data collection
Sociodemographic data (age, sex, race/ethnicity, post-menopausal status, education), smoking history (smoking status and pack-years), alcohol drinking status, medical history and medication use were obtained from the baseline questionnaire by trained and certified staff.20 Body mass index was calculated as measured weight in kilograms divided by measured height in meters squared. Three consecutive blood pressure determinations were taken20 and the last two determinations were averaged. Hypertension was defined as a mean systolic blood pressure ≥140 mm Hg, a mean diastolic blood pressure ≥90 mm Hg, or antihypertensive medication use.
Total and HDL-cholesterol were measured in fasting serum samples on the Roche-Hitachi 717 platform (Boehringer-Mannheim/Roche Diagnostics, Indianapolis, IN). LDL-cholesterol was estimated by the Friedewald equation.27 Hyperlipidemia was defined as an estimated LDL-cholesterol ≥130 mg/dL. Plasma glucose was measured by the hexokinase method. HbA1c was measured by high-pressure liquid chromatography.28 Diabetes was defined as a fasting glucose ≥126 mg/dL, a 2-hour post-load glucose ≥200 mg/dL, an HbA1c ≥6.5%, or use of insulin or an oral hypoglycemic agent. Plasma creatinine was measured by an alkaline-picrate rate method. Estimated glomerular filtration rate was calculated from calibrated creatinine, age and sex using the Modification of Diet in Renal Disease Study formula without an ethnicity factor.25
Urine cadmium determinations
Spot (random) urine samples were collected in the morning of the baseline visit in polypropylene tubes, frozen within 1 to 2 hours, shipped buried in dry ice and stored at −80°C in the Penn Medical Laboratory, MedStar Health Research Institute (Hyattsville, MD and Washington, DC). In 2009, up to 1.0 mL of urine from each participant was transported on dry ice to the Institute of Chemistry-Analytical Chemistry, Karl Franzens University (Graz, Austria) and stored at −80°C until analyses.
In Graz, the sample was thawed and a portion of the supernatant was diluted ten-fold with 10% v/v nitric acid (containing the internal standards Ge, In, Lu at a concentration of 44 μg/L) and centrifuged for ten minutes at 1.3 ×104 g. The cadmium concentration in this solution was determined using inductively coupled plasma mass spectrometry (Agilent 7700x ICPMS; Agilent Technologies, Waldbronn Germany). The “Seronorm° trace elements urine blank” (SERO AS, Billingstad, Norway), with a labeled cadmium concentration of 0.35 μg/L, was used for quality control. We obtained a cadmium mean value of 0.38 (standard deviation= 0.07) μg/L (n=66). Additionally, the cadmium concentration in the Certified Reference Material NIST 1643e “Trace elements in water” (NIST, Gaithersburg, USA), with a mean certified concentration of 6.57 μg/L (standard deviation 0.07), was determined with each run. We obtained a mean value of 6.42 (standard deviation 0.38) μg/L (n=79).
The limit of detection for urine cadmium was 0.015 μg/L. In one participant below the limit of detection, the cadmium concentration was imputed as the limit of detection divided by the square root of two. Urine cadmium concentrations were corrected for molybdenum oxide interference using the formula [Cd]corr = [Cd]-0.0016*[Mo].29 To account for urine dilution, urine cadmium concentrations were expressed in μg per g of urine creatinine. Urine creatinine was measured at the Laboratory of the National Institute of Diabetes and Digestive and Kidney Disease, Epidemiology and Clinical Research Branch (Phoenix, AZ) by an alkaline picrate rate method.20
Incidence and mortality follow-up
Cardiovascular incidence, including both fatal and non-fatal events, was the primary outcome of this study. Incident cardiovascular end-points during follow-up were assessed by annual mortality and morbidity surveillance reviews of hospitalization and death records and at two research clinic visits conducted in 1993–1995 and 1998–1999. Follow-up was 99.8% complete for mortality and 99.2% complete for morbid events.26 When possible cardiovascular events were identified, medical records were reviewed by mortality and morbidity review committees composed of physician reviewers who assigned cardiovascular events. Detailed definitions of fatal and nonfatal events have been described.20,30 Incident coronary heart disease was defined as the first occurrence of definite fatal myocardial infarction, sudden death due to coronary heart disease, non-fatal myocardial infarction or definite non-fatal coronary heart disease. Incident cases of stroke were defined as the first occurrence of definite or possible fatal or definite non-fatal stroke. Incident cases of heart failure were defined as the first occurrence of definite or possible fatal or definite non-fatal heart failure. The composite end-point of all cardiovascular disease was defined as the occurrence of a cardiovascular death or of definite non-fatal coronary heart disease, stroke or heart failure. All-cause mortality included all causes of death and was a secondary outcome in the study.
Time to event was calculated as the difference between age at the date of the baseline examination and the age at the date of the cardiovascular event, age at the date of death, age 90, or age at 31 December 2008, whichever occurred first. The mean follow-up time among participants who did not develop a cardiovascular event over follow-up was 15 years.
Statistical methods
Creatinine-corrected urine cadmium levels were markedly right-skewed, and we log-transformed them for statistical analyses. The prospective association of urine cadmium concentrations with cardiovascular disease incidence was evaluated using Cox-proportional hazards models with age as time scale and individual starting follow-up times (age at baseline examination) treated as staggered entries. Creatinine-corrected urine cadmium concentrations were introduced in the models as cadmium quartiles or as log-transformed cadmium concentrations to compare 80th vs. 20th percentile (interquintile range). The non-parametric underlying baseline hazards were allowed to differ by study region (Arizona, Oklahoma, and North and South Dakota), using the function strata() in the Cox regression model. The assumption of hazards proportionality was visually assessed based on the smoothed association between age and scaled Schoenfeld residuals,31 with no major departures from proportionality.
We used three statistical models with progressive adjustment for the following variables at baseline. Model 1 adjusted for sex. Model 2 additionally adjusted for post-menopausal status for women (no, yes), education (<12, ≥12 years of education completed), body mass index (continuous), diabetes (no, yes), total cholesterol (continuous), estimated LDL cholesterol (continuous), hypertension (no, yes) and estimated glomerular filtration rate (continuous). Model 3 additionally adjusted for smoking status (never, former, current) and cumulative smoking dose (pack-years modeled as restricted cubic splines with knots at 10, 20 and 30 pack-years). Adjustment for age was not needed because the time scale in our Cox model was age (i.e. intrinsically conditioning on age). Alternative Cox model specification using time since examination as time scale with adjustment for age resulted in similar results. P-values for linear trend were obtained from Wald tests for log-transformed urine cadmium in regression models. We modeled non-linear relationships between cadmium levels and incident cardiovascular endpoints by using restricted quadratic splines with knots at the 10th, 50th and 90th percentiles of the creatinine-corrected urine cadmium distribution (0.40, 0.92 and 2.14 μg/g, respectively).
Exploratory subgroup analyses were conducted by including interaction terms for log-transformed urine cadmium concentrations with indicator variables for subgroups defined by age (<65, ≥65 years), sex (men, women), education (<12, ≥12 years of education completed), body mass index (<30, ≥30 Kg/m2), smoking status (never, former, current), hypertension (no, yes), diabetes (no, yes), reduced estimated glomerular filtration rate (<60, ≥60 ml/min/1.72m2) and hyperlipidemia (no, yes) in separate models. P-values for interaction were obtained by using Wald tests for multiple coefficients. We conducted sensitivity analysis by modeling urine cadmium concentrations in μg/L and adjusting for urine creatinine concentrations instead of dividing urine cadmium by urine creatinine concentrations, with consistent findings (data not shown). Since cadmium is a well-known nephrotoxicant and could potentially mediate part of the cardiovascular effects induced by cadmium, adjustment for estimated glomerular filtration rate in our final model could have resulted in an attenuation of the association. We conducted a sensitivity analysis removing estimated glomerular filtration rate from the final model with no changes to the association (data not shown). Finally, to eliminate the possibility of a birth cohort effect, we ran additional models allowing the non-parametric underlying baseline hazards to differ by the interaction of study region and age groups (<54, 54–64 and ≥64 years), with consistent findings. All statistical analysis were conducted using R-software.32
RESULTS
The overall median of urine cadmium concentrations at baseline was 0.92 (IQR= 0.61 – 1.45) and the geometric mean was 0.94 μg/g creatinine. Increasing urine cadmium concentrations were associated with less education, higher age, body mass index and cigarette pack-years, increasing prevalence of ever-smoking status, and decreasing prevalence of current drinking status, diabetes and hypertension (Table 1).
Table 1.
Baseline characteristics of study participants overall and by urine cadmium quartiles (n=3,348)
| Urine Cadmium Quartiles (μg/g creatinine) |
||||||
|---|---|---|---|---|---|---|
| Overall | ≤0.61 | 0.62–0.92 | 0.93–1.45 | > 1.45 | Test for trend | |
| Age (years); mean (SE), | 56.0 (0.1) | 55.0 (0.3) | 55.9 (0.3) | 56.0 (0.3) | 57.1 (0.3) | P<0.001 |
| Men; % (SE) | 40 (0.8) | 64 (1.6) | 40 (1.7) | 35 (1.6) | 18 (1.3) | P<0.001 |
| Post-menopausal women; % (SE)a | 76 (0.9) | 73 (2.5) | 75 (2.0) | 74 (1.9) | 80 (1.5) | P= 0.005 |
| < High school; % (SE) | 47 (0.9) | 39 (1.7) | 46 (1.7) | 49 (1.7) | 53 (1.7) | P <0.001 |
| BMI (Kg/m2); mean (SE) | 30.8 (0.1) | 31.9 (0.2) | 31.5 (0.2) | 30.4 (0.2) | 29.4 (0.2) | P <0.001 |
| Smoking | ||||||
| Former smoking; % (SE) | 31 (0.8) | 42 (1.7) | 36 (1.7) | 25 (1.5) | 23 (1.5) | P <0.001 |
| Current smoking; % (SE) | 34 (0.8) | 21 (1.4) | 29 (1.6) | 42 (1.7) | 46 (1.7) | P <0.001 |
| Cumulative smoking (pack-years); mean (SE) | 10 (0.3) | 7 (0.6) | 9 (0.6) | 12 (0.6) | 14 (0.6) | P <0.001 |
| Current drinking; % (SE) | 42 (0.9) | 46 (1.7) | 43 (1.7) | 43 (1.7) | 38 (1.7) | P= 0.003 |
| LDL-cholesterol (mg/dL); mean (SE) | 116.8 (0.6) | 114.8 (1.1) | 115.9 (1.2) | 116.2 (1.2) | 120.1 (1.2) | P= 0.002 |
| Hypertension; % (SE) | 37 (0.8) | 40 (1.7) | 38 (1.7) | 36 (1.7) | 33 (1.6) | P= 0.001 |
| Diabetes; % (SE) | 48 (0.9) | 52 (1.7) | 51 (1.7) | 45 (1.7) | 43 (1.7) | P<0.001 |
| Estimated glomerular filtration rate < 60 ml/min/1.73m2; % (SE) | 9.2 (0.5) | 9.3 (1.0) | 10.6 (1.1) | 7.9 (0.9) | 9.1 (1.0) | P= 0.48 |
Subsample of 2,027 women
During follow-up, a total of 1,084 participants developed cardiovascular disease, 766 coronary heart disease, 244 with stroke, and 328 with heart failure. The numbers of all-cause, cardiovascular disease and coronary heart disease deaths were 1,382, 400 and 307, respectively. Creatinine-corrected urine cadmium concentrations were associated with mortality endpoints in all 3 models (Table 2). Fully-adjusted hazard ratios (HRs) comparing the highest to the lowest quartile of urine cadmium concentrations for all-cause, cardiovascular and coronary heart disease mortality were 1.58 (95% confidence interval [CI]= 1.32 – 1.89), 1.87 (1.34 – 2.60) and 1.51 (1.04 – 2.20), respectively (Table 2, model 3). Creatinine-corrected urine cadmium concentrations were also associated with incident events. The fully adjusted hazard ratio for all cardiovascular disease incidence, comparing the highest to the lowest quartiles of urine cadmium concentrations, was 1.48 (1.21 – 1.80) (Table 3, model 3). The corresponding hazard ratios for incident coronary heart disease, stroke and heart failure were 1.33 (1.05 – 1.68), 1.87 (1.22 – 2.86) and 1.61 (1.10 – 2.36), respectively.
Table 2.
Hazard ratios (95% confidence interval) for mortality endpoints by urine cadmium concentrations (n=3,348)
| Cadmium Quartiles (μg/g) | 80th vs 20th | |||||
|---|---|---|---|---|---|---|
| ≤ 0.61a HR (95% CI) | 0.62–0.92 HR (95% CI) | 0.93–1.45 HR (95% CI) | > 1.45 HR (95% CI) | percentiles HR (95% CI) | Test for trendb | |
| Total Mortality | ||||||
| No. Cases/Non Cases | (317 / 545) | (337 / 480) | (355 / 482) | (373 / 459) | (1,382 / 1,966) | |
| Model 1c | 1.00 | 1.25 (1.07–1.46) | 1.36 (1.16–1.59) | 1.56 (1.32–1.84) | 1.34 (1.23–1.47) | P< 0.001 |
| Model 2d | 1.00 | 1.29 (1.10–1.51) | 1.44 (1.22–1.69) | 1.68 (1.41–1.99) | 1.38 (1.26–1.52) | P< 0.001 |
| Model 3e | 1.00 | 1.26 (1.07–1.48) | 1.36 (1.16–1.61) | 1.58 (1.32–1.89) | 1.34 (1.22–1.48) | P< 0.001 |
| Cardiovascular Disease Mortality | ||||||
| No. Cases / Non Cases | (90 / 772) | (101 / 716) | (94 / 743) | (115 / 717) | (400 / 2,948) | |
| Model 1c | 1.00 | 1.30 (0.97–1.73) | 1.24 (0.93–1.67) | 1.64 (1.21–2.11) | 1.35 (1.15–1.58) | P< 0.001 |
| Model 2d | 1.00 | 1.41 (1.05–1.89) | 1.48 (1.09–2.01) | 2.01 (1.46–2.76) | 1.47 (1.25–1.73) | P< 0.001 |
| Model 3e | 1.00 | 1.33 (0.99–1.79) | 1.37 (1.00–1.88) | 1.87 (1.34–2.60) | 1.43 (1.21–1.70) | P< 0.001 |
| Coronary Heart Disease Mortality | ||||||
| No. Cases / Non Cases | (79 / 783) | (71 /746) | (78 / 759) | (79 / 753) | (307 / 3,041) | |
| Model 1c | 1.00 | 1.07 (0.78–1.48) | 1.21 (0.88–1.67) | 1.35 (0.96–1.90) | 1.27 (1.06–1.52) | P= 0.01 |
| Model 2d | 1.00 | 1.15 (0.83–1.60) | 1.40 (1.00–1.96) | 1.59 (1.11–2.28) | 1.36 (1.13–1.63) | P =0.001 |
| Model 3e | 1.00 | 1.09 (0.78–1.53) | 1.32 (0.93–1.87) | 1.51 (1.04–2.20) | 1.34 (1.10–1.63) | P =0.005 |
Reference category.
Models comparing the 80th (1.62 μg/g) to the 20th (0.55 μg/g) percentiles of urine cadmium distributions and associated test for trend were obtained from Cox proportional hazards models with log-transformed cadmium as a continuous variable.
Model 1 adjusted for sex
Model 2 further adjusted for post-menopausal status for women (yes, no), education (< high school, ≥ high school), body mass index (kg/m2), total cholesterol (mg/dL), estimated
LDL- cholesterol (mg/dL), hypertension (yes, no), diabetes (yes, no), and estimated glomerular filtration rate (ml/min/1.73m2).
Model 3 further adjusted for smoking status (never, former, current) and cumulative dose (pack-years modeled as restricted cubic splines with knots at 10, 20 and 30 pack-years).
Table 3.
Hazard ratios (95% confidence interval) for incident cardiovascular endpoints by urine cadmium concentrations (n=3,348)
| Cadmium Quartiles (μg/g) | 80th vs 20th | |||||
|---|---|---|---|---|---|---|
| ≤ 0.61a HR (95% CI) | 0.62 – 0.92 HR (95% CI) | 0.93 – 1.45 HR (95% CI) | > 1.45 HR (95% CI) | percentile HR (95% CI) | Test for trendb | |
| Cardiovascular Disease | ||||||
| No. Cases / Non Cases: | (249 / 613) | (263 / 554) | (269 / 568) | (303 / 529) | (1084 / 2264) | |
| Model 1c | 1.00 | 1.17 (0.98–1.40) | 1.21 (1.01–1.46) | 1.34 (1.11–1.62) | 1.19 (1.07–1.31) | P= 0.001 |
| Model 2d | 1.00 | 1.26 (1.05–1.50) | 1.38 (1.14–1.66) | 1.58 (1.30–1.92) | 1.28 (1.16–1.42) | P <0.001 |
| Model 3e | 1.00 | 1.20 (1.00–1.44) | 1.30 (1.07–1.58) | 1.48 (1.21–1.80) | 1.24 (1.11–1.38) | P< 0.001 |
| Coronary Heart Disease | ||||||
| No. Cases / Non Cases | (193 / 669) | (182 / 635) | (198 / 639) | (193 / 639) | (766 / 2582) | |
| Model 1c | 1.00 | 1.09 (0.89–1.34) | 1.19 (0.96–1.47) | 1.18 (0.95–1.47) | 1.15 (1.02–1.30) | P =0.02 |
| Model 2d | 1.00 | 1.18 (0.96–1.45) | 1.38 (1.11–1.71) | 1.41 (1.12–1.78) | 1.26 (1.12–1.42) | P <0.001 |
| Model 3e | 1.00 | 1.13 (0.92–1.40) | 1.31 (1.05–1.63) | 1.33 (1.05–1.68) | 1.22 (1.08–1.38) | P =0.002 |
| Stroke | ||||||
| No. Cases / Non Cases | (51 / 811) | (51 / 766) | (53 / 784) | (89 / 743) | (244 / 3104) | |
| Model 1c | 1.00 | 1.14 (0.78–1.68) | 1.14 (0.77–1.68) | 1.91 (1.30–2.80) | 1.73 (1.22–2.45) | P =0.006 |
| Model 2d | 1.00 | 1.24 (0.84–1.84) | 1.22 (0.81–1.82) | 2.03 (1.35–3.06) | 1.82 (1.25–2.65) | P =0.006 |
| Model 3e | 1.00 | 1.21 (0.81–1.80) | 1.11 (0.73–1.69) | 1.87 (1.22–2.86) | 1.75 (1.17–2.59) | P =0.03 |
| Heart Failure | ||||||
| No. Cases / Non Cases | (60 / 802) | (81 / 736) | (87 / 750) | (100 / 732) | (328 / 3020) | |
| Model 1c | 1.00 | 1.35 (0.96–1.89) | 1.38 (0.98–1.95) | 1.41 (0.98–2.03) | 1.20 (0.88–1.63) | P =0.03 |
| Model 2d | 1.00 | 1.49 (1.06–2.11) | 1.65 (1.15–2.35) | 1.78 (1.22–2.59) | 1.46 (1.06–2.02) | P =0.002 |
| Model 3e | 1.00 | 1.40 (0.99–1.99) | 1.52 (1.06–2.18) | 1.61 (1.10–2.36) | 1.39 (1.01–1.94) | P =0.01 |
Reference category.
Models comparing the 80th (1.62 μg/g) to the 20th (0.55 μg/g) percentiles of urine cadmium distributions and associated test for trend were obtained from Cox proportional hazards models with log-transformed cadmium as a continuous variable, except for stroke and heart failure where cadmium was introduced as restricted quadratic splines with knots at percentiles 10th, 50th and 90th because of the non-linear relationships.
Model 1 adjusted for sex
Model 2 further adjusted for post-menopausal status for women (yes, no), education (< high school, ≥ high school), body mass index (kg/m2), total cholesterol (mg/dL), estimated LDL- cholesterol (mg/dL), hypertension (yes, no), diabetes (yes, no), and estimated glomerular filtration rate (ml/min/1.73m2).
Model 3 further adjusted for smoking status (never, former, current) and cumulative smoking dose (pack-years modeled as restricted cubic splines with knots at 10, 20 and 30 pack-years).
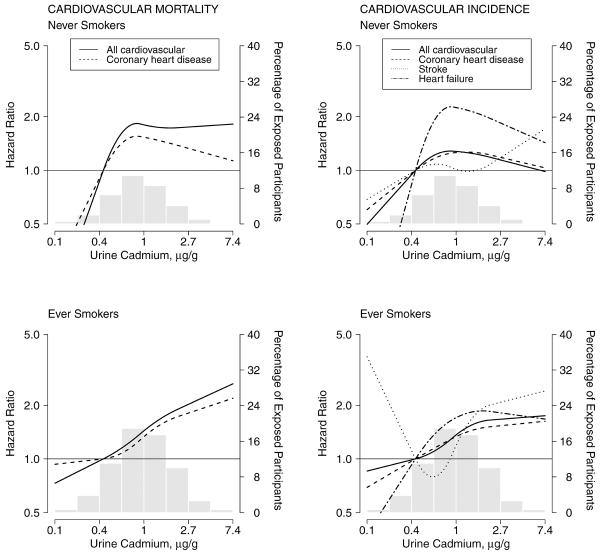
In dose-response analyses, the association between cadmium concentrations and cardiovascular endpoints showed positive dose-response relationships (Tables 2 and 3, and Figure 1), with no statistically significant departures from linearity except for incident stroke and heart failure (p-value for non-linear terms = 0.001 and 0.02, respectively).
Figure 1.
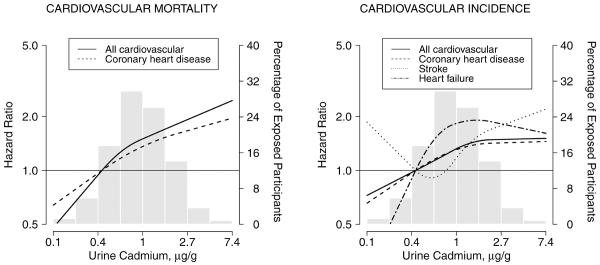
Hazard ratios for cardiovascular mortality and incidence by urine cadmium concentrations (n=3,348). Lines represent adjusted hazard ratios based on restricted quadratic splines for log-transformed cadmium concentrations with knots at the 10th, 50th and 90th percentiles, which correspond to 0.40, 0.92 and 2.14 μg/g creatinine, respectively. The reference was set at the 10th percentile of cadmium distribution. Vertical bars represent the histogram of urine cadmium distribution in the study population. The models were adjusted for sex, post-menopausal status for women (yes, no), education (< high school, ≥ high school), body mass index (kg/m2), total cholesterol (mg/dL), estimated LDL- cholesterol (mg/dL), hypertension (yes, no), diabetes (yes, no), estimated glomerular filtration rate (ml/min/1.73m2), smoking status (never, former, current) and cumulative smoking dose (pack-years modeled as restricted cubic splines with knots at 10, 20 and 30 pack-years). Cardiovascular incidence included fatal and non-fatal events.
The associations between cadmium and cause-specific cardiovascular endpoints were similar in most subgroups including never-smokers (Figure 2 and eFigure 1 and eFigure 2, eAppendix available online), except for the association between cadmium and incident stroke by smoking status (eFigure 2). Participants with diabetes and without chronic kidney disease showed stronger associations with most cardiovascular endpoints.
Figure 2.
Hazard ratios for cardiovascular disease mortality and incidence by urine cadmium concentrations in smoking status subgroups (never-smokers, n=1,145; ever-smokers, n=2,203) Methodological details are the same as for Figure 1.
DISCUSSION
This is the first study to evaluate the prospective association between cadmium and cardiovascular disease, including both fatal and non-fatal events. Cadmium exposure, measured by urine cadmium concentrations, was associated with increased risk of all-cause and cardiovascular mortality and with increased incidence of cardiovascular events in middle-aged to elderly men and women followed for up to 19 years. The associations persisted after adjusting for traditional cardiovascular risk factors, and were similar in most subgroups evaluated, including never-smokers. Our findings, together with evidence on the association between cadmium and cardiovascular mortality17,18,33,34 and risk factors,3–11 and with mechanistic evidence,10,19 support the role of cadmium as a cardiovascular risk factor.
Cadmium was used extensively in consumer products (e.g., pigments, batteries, coatings and plastic stabilizers) throughout the 20th century.1 Presently, cadmium is still used in batteries and solar panels. Moreover, cadmium remains an important soil contaminant from industrial releases and the use of cadmium-containing phosphate fertilizers.1,35 Exposure to cadmium in the population occurs through tobacco smoke, certain foods (shellfish, offal and some vegetables and grains that concentrate cadmium from soil) and ambient air, particularly in the vicinity of industrial and hazardous waste sites and in occupational settings.1,36,37 No information is available on specific sources of cadmium exposure in American Indian populations, but urine cadmium concentrations in the Strong Heart Study participants were higher than in the general US population for the same age group and time period.38 While the prevalence of smoking is higher in some American Indian communities compared with other US ethnic groups,39,40 their intensity of smoking is markedly lower,40 suggesting that sources of exposure other than smoking may be responsible for the increased urine cadmium concentrations. Studies of other Native American populations from the US, Canada and Mexico have suggested that sources of cadmium include nearby contaminating factories and mining,41,42 and surface dust in jewelry-making homes.43 Another activity that could be relevant for cadmium exposure in our population is small-scale-motor vehicle repair.37
Several studies have found no association between occupational cadmium exposure and cardiovascular disease.44–46 Occupational studies, however, have been limited in the quantification of cadmium exposure, assessment of cardiovascular outcomes and measurement of relevant confounders. Workers could also be affected by the healthy-worker effect. Of the few studies that have evaluated the prospective association between cadmium exposure and cardiovascular mortality, most have found positive associations.17,18,33,34 In the Japanese Kakeshi river basin, markedly high urine cadmium concentrations were associated with heart failure mortality but not with cerebrovascular mortality.33 In that study, however, the number of deaths was small and the associations were not adjusted for smoking. In the general US population, urine cadmium concentrations were positively associated with cardiovascular mortality in men who participated in the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994)17 and (consistent with our findings) in both men and women who participated in NHANES 1999–2004.18 In NHANES 1999–2004, the hazard ratio for cardiovascular mortality, comparing the 80th (0.57 μg/g creatinine) vs. 20th (0.14 μg/g creatinine) percentile of urine cadmium concentrations, was 1.74 (95% CI= 1.07 – 2.83).18 In a Belgian population, urine cadmium concentrations at baseline were associated with all-cause mortality (HR= 1.22 [95% CI= 1.06 – 1.40]) and to a lesser extent with cardiovascular death (1.11 [95% CI=0.89 – 1.38]) after 20 years of follow-up.34 Blood cadmium was also associated with increased cardiovascular death (1.29 [95% CI= 0.99 – 1.67]).
Our results are supported by experimental evidence showing roles of cadmium in oxidative stress,47 endothelial dysfunction,10,48,49 atherosclerosis formation,10,50 hypertension51,52 and kidney disease.53 Cadmium-related vascular damage could be partly promoted by dysfunctional metallothionein production in endothelial cells.54 In addition, cadmium could induce cardiovascular disease through epigenetic and endocrine disruption mechanisms, involving aberrant DNA-methylation, histone modifications and changes in miRNA expression,55 and activation of the estrogen receptor.56–58 The associations between cadmium and cardiovascular endpoints observed in the Strong Heart Study are also consistent with reported cross-sectional associations of cadmium exposure with peripheral arterial disease,12,13 myocardial infarction diagnosed by the Cardiac Infarction Injury Score (an electrocardiographic classification system to assess the extent of cardiac injury in post-myocardial infarction59)14 carotid atherosclerosis,10,11 and self-reported coronary heart disease, stroke and heart failure15,16 – further supporting a role for cadmium in cardiovascular disease development.
Smoking is an important determinant of cadmium exposure1 and a major cardiovascular risk factor.60 It has been suggested that cadmium could be a mediator of tobacco-related cardiovascular disease.12,61 Our findings support the hypothesis that cadmium is an independent cardiovascular risk factor, as the association remained after we adjusted for smoking status and cumulative smoking dose. Residual confounding by smoking is possible, however, because smoking status and pack-years were defined by self-report and we do not have information on secondhand smoke exposure. Nevertheless, the association between urine cadmium and cardiovascular mortality remained in previous studies17,18 after adjustment for serum cotinine, an objective biomarker of cigarette smoking. Moreover, the similar association between cadmium exposure and cardiovascular endpoints in never-smokers compared with ever-smokers makes residual confounding less likely.
Strengths of this study include the prospective design and the long follow-up, the low rate of losses to follow-up, the high number of events, the low limit of detection for urine cadmium, and the standardization and quality control of data collection, laboratory analyses, and identification of incident cardiovascular events.20 These data from American Indians provide information for an understudied ethnic group.
Our study has several limitations. First, we used a single baseline urine cadmium determination as a biomarker of exposure. While urine cadmium has a half-life of decades,1 regression-dilution bias due to non-differential measurement error may have resulted in an underestimation of the associations. Second, American Indian communities have a higher burden of cardiovascular disease and its risk factors, as well as higher mortality due to non-cardiovascular causes, compared with some other populations.62 It is uncertain whether our results can be completely generalized to populations with a different cardiovascular-risk-factor profile, although cadmium toxicity pathways are likely to be common to many populations. Our population, for instance, is characterized by a high burden of diabetes. In our exploratory subgroup analysis, we found that the association was stronger among participants with diabetes. although those findings were not statistically significant and must be taken with caution. Finally, there is a concern that end-stage renal disease associated with diabetic nephropathy and consequent renal replacement therapies could change chronic cadmium accumulation in the body. In our study, we had only 2 participants at baseline with kidney transplantation and 14 participants with end-stage renal disease (defined as a glomerular filtration rate <15 mL/min/1.73m2) – none of them under dialysis. The exclusion of those participants did not change the observed associations (data not shown).
In conclusion, cadmium exposure was prospectively associated with increased cardiovascular disease and mortality in both men and women. The findings of this study, together with accumulated evidence on cadmium cardiovascular toxicity and previous epidemiologic findings can contribute to evaluate causality of the association between cadmium and cardiovascular disease based on established criteria,63 including consistency, temporality, dose-response relationship and biological plausibility. Cardiovascular disease, including coronary heart disease, heart failure and stroke, are major causes of death, long-term functional disability and medical costs around the world.63,64 If cadmium is confirmed as a cause of cardiovascular disease, population-based preventive strategies to decrease cadmium exposure, including tobacco control measures and reduction of cadmium in air, soils and food could potentially contribute to reduce the burden of cardiovascular disease.
Supplementary Material
Acknowledgments
sources of funding: This work was supported by the National Heart Lung and Blood Institute (grant HL090863 and by SHS grants HL41642, HL41652, HL41654 and HL65521). Dr. Maria Tellez-Plaza was supported by a Rio Hortega training grant (Funds for Research in Health Sciences, Ministry of Science and Innovation, Spain).
Footnotes
Conflict of interest The authors have no other conflict of interest to declare.
REFERENCES
- 1.Nordberg GF, Nogawa K, Nordberg M, Friberg L. Cadmium. In: Nordberg GF, Fowler BF, Nordberg M, Friberg L, editors. Handbook on the toxicology of metals. Elsevier; Amsterdam: 2007. pp. 445–486. [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry [Accessed November 11, 2012];Toxicological Profile for Cadmium. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15. [PubMed]
- 3.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) Environ Health Perspect. 2008;116(1):51–56. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ Res. 2010;110(6):612–6. doi: 10.1016/j.envres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Lee BK, Kim Y. Association of blood cadmium with hypertension in the Korean general population: Analysis of the 2008–2010 Korean national health and nutrition examination survey data. Am J Ind Med. 2012;55(11):1060–7. doi: 10.1002/ajim.22078. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom L, Elinder CG, Dahlberg B, Lundberg M, Jarup L, Persson B, Axelson O. Cadmium exposure and end-stage renal disease. Am J Kidney Dis. 2001;38(5):1001–8. doi: 10.1053/ajkd.2001.28589. [DOI] [PubMed] [Google Scholar]
- 7.Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170(9):1156–64. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Punta B, Funkhiew T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: a five-year follow-up. Environ Res. 2012;112:194–8. doi: 10.1016/j.envres.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, Il'yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26(2):468–70. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- 10.Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, Shen YH, Zeller I, Willeit J, Laufer G, Wick G, Kiechl S, Bernhard D. Cadmium Is a Novel and Independent Risk Factor for Early Atherosclerosis Mechanisms and In Vivo Relevance. Arterioscler Thromb Vasc Biol. 2009;29(9):1392–8. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- 11.Fagerberg B, Bergstrom G, Boren J, Barregard L. Cadmium exposure is accompanied by increased prevalence and future growth of atherosclerotic plaques in 64-year-old women. J Intern Med. 2012 Jul 20; doi: 10.1111/j.1365-2796.2012.02578.x. doi: 10.1111/j.1365-2796.2012.02578.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109(25):3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 13.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, Guallar E. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;172(6):671–81. doi: 10.1093/aje/kwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environ Res. 2008;106(2):284–6. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Lee MS, Park SK, Hu H, Lee S. Cadmium exposure and cardiovascular disease in the 2005 Korea National Health and Nutrition Examination Survey. Environ Res. 2011;111(1):171–6. doi: 10.1016/j.envres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res. 2010;110(2):199–206. doi: 10.1016/j.envres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117(2):190–6. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect. 2012;120(7):1017–22. doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messner B, Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals. 2010;23(5):811–22. doi: 10.1007/s10534-010-9314-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 21.Lee ET, Howard BV, Wang W, Welty TK, Galloway JM, Best LG, Fabsitz RR, Zhang Y, Yeh J, Devereux RB. Prediction of coronary heart disease in a population with high prevalence of diabetes and albuminuria: the Strong Heart Study. Circulation. 2006;113(25):2897–905. doi: 10.1161/CIRCULATIONAHA.105.593178. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Eilat-Adar S, Loria C, Goldbourt U, Howard BV, Fabsitz RR, Zephier EM, Mattil C, Lee ET. Dietary fat intake and risk of coronary heart disease: the Strong Heart Study. Am J Clin Nutr. 2006;84(4):894–902. doi: 10.1093/ajcn/84.4.894. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, Kizer JR, Howard BV, Cowan LD, Yeh J, Howard WJ, Wang W, Best L, Lee ET. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118(15):1577–84. doi: 10.1161/CIRCULATIONAHA.108.772285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnick HE, Carter EA, et al. Incidence of lower-extremity amputation in American Indians: the Strong Heart Study. Diabetes Care. 2004;27(8):1885–91. doi: 10.2337/diacare.27.8.1885. [DOI] [PubMed] [Google Scholar]
- 25.Shara NM, Resnick HE, Lu L, Xu J, Vupputuri S, Howard BV, Umans JG. Decreased GFR estimated by MDRD or Cockcroft-Gault equation predicts incident CVD: the strong heart study. J Nephrol. 2009;22(3):373–80. [PMC free article] [PubMed] [Google Scholar]
- 26.Stoddart ML, Jarvis B, Blake B, Fabsitz RR, Howard BV, Lee ET, Welty TK. Recruitment of American Indians in epidemiologic research: the Strong Heart Study. Am Indian Alsk Native Ment Health Res. 2000;9(3):20–37. doi: 10.5820/aian.0903.2000.20. [DOI] [PubMed] [Google Scholar]
- 27.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23(1):97–104. [PubMed] [Google Scholar]
- 28.Little RR, England JD, Wiedmeyer HM, McKenzie EM, Mitra R, Erhart PM, Durham JB, Goldstein DE. Interlaboratory standardization of glycated hemoglobin determinations. Clin Chem. 1986;32(2):358–60. [PubMed] [Google Scholar]
- 29.Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, Pollak J, Tellez-Plaza M, Silbergeld EK, Guallar E, Navas-Acien A. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods. 2012;4(2):406–413. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108(1):150–8. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 31.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 32.R Development Core Team [Accessed November 11, 2012];R: A language and environment for statistical computing. Available at: http://www.R-project.org.
- 33.Nakagawa H, Nishijo M, Morikawa Y, Miura K, Tawara K, Kuriwaki J, Kido T, Ikawa A, Kobayashi E, Nogawa K. Urinary cadmium and mortality among inhabitants of a cadmium-polluted area in Japan. Environ Res. 2006;100(3):323–9. doi: 10.1016/j.envres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Nawrot TS, Van Hecke E, Thijs L, Richart T, Kuznetsova T, Jin Y, Vangronsveld J, Roels HA, Staessen JA. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ Health Perspect. 2008;116(12):1620–8. doi: 10.1289/ehp.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staessen JA, Vyncke G, Lauwerys RR, Roels HA, Celis HG, Claeys F, Dondeyne F, Fagard RH, Ide G, Lijnen PJ, et al. Transfer of cadmium from a sandy acidic soil to man: a population study. Environ Res. 1992;58(1):25–34. doi: 10.1016/s0013-9351(05)80202-6. [DOI] [PubMed] [Google Scholar]
- 36.Nawrot TS, Kuenzli N, Sunyer J, Shi T, Moreno T, Viana M, Heinrich J, Forsberg B, Kelly FJ, Sughis M, Nemery B, Borm P. Oxidative properties of ambient PM2.5 and elemental composition: heterogeneous associations in 19 European cities. Atmos Environ. 2009;43:4595–4602. [Google Scholar]
- 37.Yassin AS, Martonik JF. Urinary cadmium levels in the U S working population, 1988–1994. J Occup Environ Hyg. 2004;1(5):324–33. doi: 10.1080/15459620490445499. [DOI] [PubMed] [Google Scholar]
- 38.Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environ Health Perspect. 2012;120(2):204–9. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabsitz RR, Sidawy AN, Go O, Lee ET, Welty TK, Devereux RB, Howard BV. Prevalence of peripheral arterial disease and associated risk factors in American Indians: the Strong Heart Study. Am J Epidemiol. 1999;149(4):330–8. doi: 10.1093/oxfordjournals.aje.a009817. [DOI] [PubMed] [Google Scholar]
- 40.From the Centers for Disease Control and Prevention Cigarette smoking among American Indians, Alaskan Natives--behavioral risk factor surveillance system, 1987–1991. JAMA. 1992;268(21):3052, 3055. [PubMed] [Google Scholar]
- 41.Schmitt CJ, Brumbaugh WG, Linder GL, Hinck JE. A screening-level assessment of lead, cadmium, and zinc in fish and crayfish from Northeastern Oklahoma, USA. Environ Geochem Health. 2006;28(5):445–71. doi: 10.1007/s10653-006-9050-4. [DOI] [PubMed] [Google Scholar]
- 42.Moon J, Smith TJ, Tamaro S, Enarson D, Fadl S, Davison AJ, Weldon L. Trace metals in scalp hair of children and adults in three Alberta Indian villages. Sci Total Environ. 1986;54:107–25. doi: 10.1016/0048-9697(86)90259-7. [DOI] [PubMed] [Google Scholar]
- 43.Gonzales M, Shah V, Bobelu A, Qualls C, Natachu K, Bobelu J, Jamon E, Neha D, Paine S, Zager P. Concentrations of surface-dust metals in Native American jewelry-making homes in Zuni Pueblo, New Mexico. Arch Environ Health. 2004;59(5):245–9. doi: 10.3200/AEOH.59.5.245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elinder CG, Kjellstrom T, Hogstedt C, Andersson K, Spang G. Cancer mortality of cadmium workers. Br J Ind Med. 1985;42(10):651–5. doi: 10.1136/oem.42.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorahan T, Waterhouse JA. Mortality study of nickel-cadmium battery workers by the method of regression models in life tables. Br J Ind Med. 1983;40(3):293–300. doi: 10.1136/oem.40.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kazantzis G, Lam TH, Sullivan KR. Mortality of cadmium-exposed workers. A five-year update. Scand J Work Environ Health. 1988;14(4):220–3. doi: 10.5271/sjweh.1929. [DOI] [PubMed] [Google Scholar]
- 47.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 48.Bilgen I, Oner G, Edremitlioglu M, Alkan Z, Cirrik S. Involvement of cholinoceptors in cadmium-induced endothelial dysfunction. J Basic Clin Physiol Pharmacol. 2003;14(1):55–76. doi: 10.1515/jbcpp.2003.14.1.55. [DOI] [PubMed] [Google Scholar]
- 49.Majumder S, Gupta R, Reddy H, Sinha S, Muley A, Kolluru GK, Chatterjee S. Cadmium attenuates bradykinin-driven nitric oxide production by interplaying with the localization pattern of endothelial nitric oxide synthase. Biochem Cell Biol. 2009;87(4):605–20. doi: 10.1139/o09-018. [DOI] [PubMed] [Google Scholar]
- 50.Knoflach M, Messner B, Shen YH, Frotschnig S, Liu G, Pfaller K, Wang X, Matosevic B, Willeit J, Kiechl S, Laufer G, Bernhard D. Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circ J. 2011;75(10):2491–5. doi: 10.1253/circj.cj-11-0196. [DOI] [PubMed] [Google Scholar]
- 51.Varoni MV, Palomba D, Satta M, Anania V. Low urinary kallikrein rats: different sensitivity of verapamil on hypertensive response to central acute cadmium administration. Vet Hum.Toxicol. 2003;45(4):202–206. [PubMed] [Google Scholar]
- 52.Gokalp O, Ozdem S, Donmez S, Dogan M, Demirin H, Kara HY, Sutcu R, Cicek E, Ozer MK, Delibas N. Impairment of endothelium-dependent vasorelaxation in cadmium-hypertensive rats. Toxicol Ind Health. 2009;25(7):447–53. doi: 10.1177/0748233709106822. [DOI] [PubMed] [Google Scholar]
- 53.Sabolic I. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol. 2006;104(3):107–14. doi: 10.1159/000095539. [DOI] [PubMed] [Google Scholar]
- 54.Fujiwara Y, Banno H, Shinkai Y, Yamamoto C, Kaji T, Satoh M. Protective effect of pretreatment with cilostazol on cytotoxicity of cadmium and arsenite in cultured vascular endothelial cells. J Toxicol Sci. 2011;36(2):155–61. doi: 10.2131/jts.36.155. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Li Y, Shao C, Tan Y, Cai L. Cadmium and its epigenetic effects. Curr Med Chem. 2012;19(16):2611–20. doi: 10.2174/092986712800492913. [DOI] [PubMed] [Google Scholar]
- 56.Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol. 2000;14(4):545–53. doi: 10.1210/mend.14.4.0441. [DOI] [PubMed] [Google Scholar]
- 57.Fechner P, Damdimopoulou P, Gauglitz G. Biosensors paving the way to understanding the interaction between cadmium and the estrogen receptor alpha. PLoS One. 2011;6(8):e23048. doi: 10.1371/journal.pone.0023048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kluxen FM, Hofer N, Kretzschmar G, Degen GH, Diel P. Cadmium modulates expression of aryl hydrocarbon receptor-associated genes in rat uterus by interaction with the estrogen receptor. Arch Toxicol. 2012;86(4):591–601. doi: 10.1007/s00204-011-0787-x. [DOI] [PubMed] [Google Scholar]
- 59.van Domburg RT, Klootwijk P, Deckers JW, van Bergen PF, Jonker JJ, Simoons ML. The Cardiac Infarction Injury Score as a predictor for long-term mortality in survivors of a myocardial infarction. Eur Heart J. 1998;19(7):1034–41. doi: 10.1053/euhj.1998.1011. [DOI] [PubMed] [Google Scholar]
- 60.US Department of Health and Human Services [Accessed November 11, 2012];A Report of the Surgeon General: How Tobacco Smoke Causes Disease. The Biology and Behavioral Basis for Smoking-Attributable Diseases. Available at: http://www.surgeongeneral.gov/library/tobaccosmoke/report/index.html.
- 61.Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology. 2011;62(5):422–9. doi: 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- 62.US Department of Health and Human Services [Accessed November 11, 2012];The federal health program for American Indians and Alaska Natives. Indian Health Disparities. Available at: http://info.ihs.gov/Disparities.asp.
- 63.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 64.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124(3):314–23. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.