Maturation of Golgi cisternae into post-Golgi carriers is directly visualized using the Aspergillus nidulans Ypt31/RAB11 homologue RabE as a reporter of post-Golgi identity. A microtubule-based conveyor belt fuels carriers to actin microfilaments radiating from the apex, which carry out the membrane-proximal transport step of exocytosis.
Abstract
The mechanism(s) by which proteins traverse and exit the Golgi are incompletely understood. Using Aspergillus nidulans hyphae, we show that late Golgi cisternae undergo changes in composition to gradually lose Golgi identity while acquiring post-Golgi RabERAB11 identity. This behavior of late Golgi cisternae is consistent with the cisternal maturation model. Post-Golgi RabERAB11 carriers travel to, and accumulate at, the apex, indicating that fusion is rate limiting for exocytosis. These carriers, which are loaded with kinesin, dynein, and MyoEMYO5, move on a microtubule-based bidirectional conveyor belt relaying them to actin, which ultimately focuses exocytosis at the apex. Dynein drags RabERAB11 carriers away if engagement of MyoEMYO5 to actin cables fails. Microtubules seemingly cooperating with F-actin capture can sustain secretion if MyoEMYO5 is absent. Thus, filamentous fungal secretion involving post-Golgi carriers is remarkably similar, mechanistically, to the transport of melanosomes in melanocyte dendrites, even though melanosome biogenesis involves lysosomes rather than Golgi.
INTRODUCTION
A fascinating problem of cell biology is how lipids and proteins traverse the Golgi and are subsequently sorted into carriers bound to the plasma membrane and endosomes (Patterson et al., 2008; Glick and Nakano, 2009; Glick and Luini, 2011). One model explaining this transit is the cisternal maturation model. It implies that Golgi cisternae are transient entities that form de novo from endoplasmic reticulum (ER)–derived traffic and undergo changes in lipid and protein content until reaching a compositional stage in which trans-most cisternae enriched in cargo break up into post-Golgi carriers (Glick and Nakano, 2009). A key observation was that Golgi cisternae of Saccharomyces cerevisiae are not stacked, such that early and late cisternae labeled with appropriate fluorescent protein markers are resolvable by light microscopy (Wooding and Pelham, 1998; for fungal cisternae we use the terms “early” and “late” instead of cis and trans, as the Golgi network lacks cis-to-trans spatial organization). Intra-Golgi maturation has been directly visualized in S. cerevisiae (Losev et al., 2006; Matsuura-Tokita et al., 2006). More recently, the exit from the late Golgi of sequentially assembled adaptor-specific populations of clathrin-coated vesicles destined to endosomes has also been visualized directly (Daboussi et al., 2012). However, the transition of late Golgi cisternae to post-Golgi exocytic carriers has not.
The hyphal fungus Aspergillus nidulans is well suited to investigate exocytic traffic because early and late Golgi cisternae are also spatially resolvable by optical microscopy (Pantazopoulou and Peñalva, 2011) and secretion is polarized toward the growing tip (Taheri-Talesh et al., 2008; Peñalva et al., 2012; Pinar et al., 2013a). Another advantage of A. nidulans hyphal tip cells is that although late Golgi cisternae are strongly polarized, they are excluded from the tip region, leaving a few micrometers between them and the apex (the target of exocytic carriers; Pantazopoulou and Peñalva, 2009). The presence of this gap facilitates the filming of post-Golgi events and implies that exocytic traffic between late Golgi cisternae and the apex requires long-distance transport.
Genetic screens using S. cerevisiae delineated the picture of the exocytic pathway in yeast and every other eukaryotic cell (Novick et al., 1980; Bonifacino and Glick, 2004). Lipids and GTPases of the RAB family govern intracellular traffic (Behnia and Munro, 2005; Barr, 2013). Work in S. cerevisiae strongly implicated the functionally overlapping paralogues and RAB11 family members Ypt31/Ypt32 in Golgi exit and transport between the Golgi and the sites of exocytosis (Jedd et al., 1997; Morozova et al., 2006; Lipatova et al., 2008). Ypt31 is a key element of a “RAB cascade,” a regulatory switch by which one RAB, Ypt31, directs the recruitment to membranes of the downstream-acting RAB, Sec4. Sec4 would take over Ypt31 regulatory functions to mediate vesicle tethering and fusion with the plasma membrane (Ortiz et al., 2002). Similar RAB cascades would operate within the fungal Golgi (Rivera-Molina and Novick, 2009) and endosomal systems (Peplowska et al., 2007; Nordmann et al., 2010; Abenza et al., 2012).
In addition to Golgi exit, exocytic RABs regulate transport of post-Golgi carriers to the plasma membrane. In yeast this step is powered by the type V myosin Myo2. Recruitment of Myo2 to exocytic carriers involves coincidence detection of either Ypt31 or Sec4 (which bind directly to the same region in the Myo2 cargo-binding domain) and of the lipid phosphatidylinositol 4-phosphate (PtdIns4P), with involvement of the exocyst component Sec15 (Lipatova et al., 2008; Mizuno-Yamasaki et al., 2010; Jin et al., 2011; Santiago-Tirado et al., 2011). At which point of transport Sec4 replaces Ypt31 and why two, instead of one, RABs are involved, despite the fact that Ypt31 and Sec4 share a number of effectors, including the exocyst, is not fully understood.
In yeast the reason possibly reflects the fact that Ypt31 acts near Golgi exit, whereas Sec4 and its prototypic effector, the exocyst, are involved in vesicle fusion, that is, near the end of the pathway, with the “RAB cascade” ensuring order and directionality. Unlike mammalian and filamentous fungal cells S. cerevisiae exocytosis relies solely on actin-based transport, which might be reflected in the major importance of Sec4, given that Sec4 coordinates tethering and soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) regulation with the release of vesicles from Myo2 (Donovan and Bretscher, 2012).
In fungal hyphae and higher eukaryotic cells, microtubules (MTs) and F-actin cooperate in intracellular transport. In Ustilago-maydis yeasts, kinesin-1 and myosin-5 act in parallel to deliver exocytic carriers to the plasma membrane (Schuster et al., 2012). In A. nidulans, current models depict MTs as mediating long-distance exocytic transport to the tip, whereas F-actin would mediate transport to the apex within the tip-proximal region (Taheri-Talesh et al., 2008, 2012; Abenza et al., 2009; Zhang et al., 2011). Deletion of the single A. nidulans type V myosin gene, myoE, slows but does not prevent exocytosis, indicating that MT-dependent mechanisms must be operating (Taheri-Talesh et al., 2012). Indeed, cells in which MyoE is down-regulated cannot even establish polarity if MTs are depolymerized, and MyoE down-regulation becomes lethal when combined with kinA∆, removing the sole A. nidulans kinesin-1 (Requena et al., 2001; Zhang et al., 2011). However, the absence of robust exocytic markers has thus far precluded the direct confirmation of this cooperation.
Here we show how virtually every late Golgi cisterna matures into a post-Golgi structure by acquisition of the sole Ypt31 A. nidulans orthologue RabERAB11. These post-Golgi membranes engage motors and undergo movement to the apex, where they accumulate, awaiting fusion. MTs and actin cables cooperate in this transport, although MTs are sufficient if MyoE is absent. Actin depolymerization displaces exocytic carriers containing MyoE, dynein, and at least one kinesin to a MT-based conveyor belt that constantly refills the tip umbrella of actin cables with post-Golgi carriers.
RESULTS
The A. nidulans exocytic Rabs, RabERAB11 and RabDRAB8
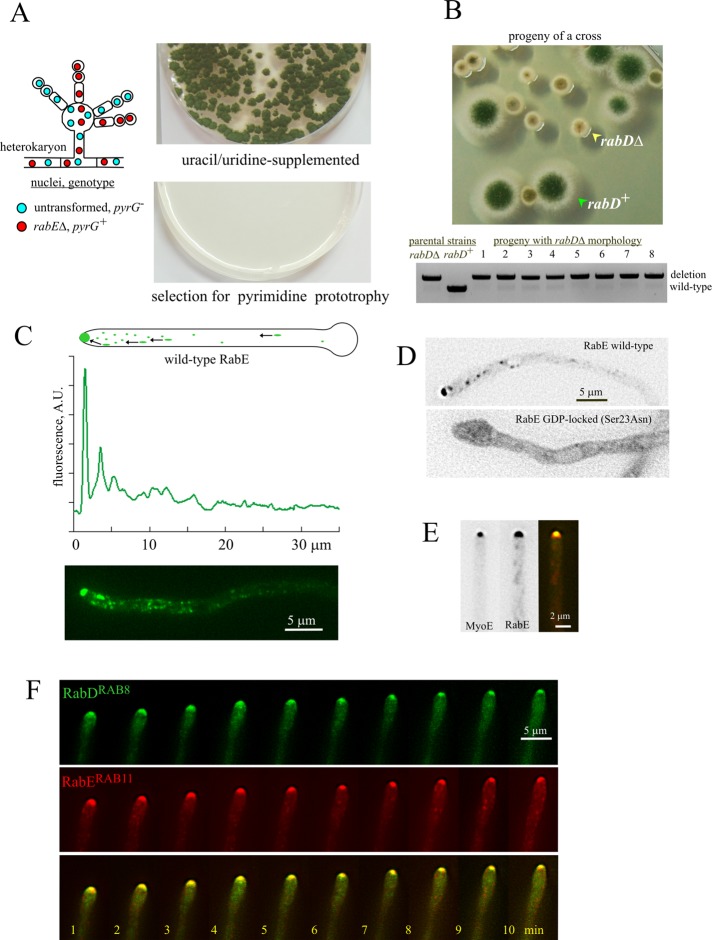
Exocytosis is required to maintain the integrity of the fungal cell wall. In S. cerevisiae, exocytosis is regulated by RAB cascade involving the pair of Ypt31/Ypt32 paralogues and Sec4 (Ortiz et al., 2002). A. nidulans has single orthologues of Ypt31/Ypt32/RAB11 and Sec4, denoted RabE and RabD, respectively. RabERAB11 is essential (Figure 1A and Supplemental Figure S1, A–C). RabDRAB8, although not essential (Supplemental Figure S1, D–F), is important for growth, as shown by the markedly reduced colony size resulting from rabD∆ (Figure 1B; the rabD∆ colony phenotype segregates as a single Mendelian character). Here we use RabERAB11 to study the biogenesis and delivery of post-Golgi exocytic carriers. The characterization of RabDRAB8 will be reported elsewhere.
FIGURE 1:
The exocytic A. nidulans RABs, RabERAB11 and RabDRAB8. (A) Heterokaryon rescue test (Osmani et al., 2006) demonstrating that rabE is essential. (B) Progeny of a cross between rabD+ and rabD∆ strains showing the rabD∆ growth phenotype, and diagnostic PCR establishing that eight random progeny with this growth phenotype contained the deletion allele. (C) Distribution of GFP-RabE. Expression was driven by the rabEp (strain MAD4120; Supplemental Table S1). The top linescan shows its marked polarization. (D) GDP-locking Sec23Asn substitution delocalizes RabERAB11 to a cytosolic haze and to a faintly labeled network of endomembranes. Wild-type and mutant GFP-RabERAB11 (strains MAD4072 and MAD4271) were expressed under the control of the alcAp, which was induced for 3 h after shifting cells to medium containing ethanol. Despite the fact that this regime results in GFP-RabERAB11 overexpression (see text and Supplemental Figure S2), the subcellular distribution of the wild-type fusion protein is indistinguishable from that attained with the natural rabE promoter. Images are middle planes of Z-stacks that have been contrasted with the “unsharp mask” filter of MetaMorph to maximize detection of endomembranes in the mutant. (E) MyoE-GFP colocalizes with mCherry-RabE at the SPK. Images of strain MAD4403, in which MyoE was endogenously tagged with GFP and mCherry-RabERAB11 expression was driven by the alcAp promoter. Cells were cultured on fructose to obtain similar levels of fluorescence, which permitted simultaneous GFP and mCherry channel recording using a Dual View. Note that the localization patterns of mCherry-RabERAB11 and GFP-RabERAB11 are indistinguishable (see Materials and Methods and Supplemental Figure S2). (F) GFP-RabD and mCherry-RabE colocalize at the SPK in a growing hypha. This time series was obtained with strain MAD4156, in which both GFP-RabDRAB8 and mCherry-RabERAB11 were expressed from single-copy alcAp-driven constructs targeted to the pyroA and argB loci, respectively.
To investigate the subcellular localization of RabERAB11, for a majority of experiments we used a construct driving expression of green fluorescent protein (GFP)–RabE under the control of its own promoter, integrated at the rabE locus through a single crossover, which resulted in a tandem duplication of rabE in which only one of the copies is tagged with GFP (strain MAD4120 in Supplemental Table S1; complete description in Supplemental Figure S2 and Materials and Methods). We chose this genetic arrangement involving a “backup copy” of untagged RabERAB11 to avoid hypomorphic effects due to impaired function and/or reduced steady-state levels potentially resulting from endogenous GFP tagging (rabE is stringently essential); indeed, Western blot analyses with anti-RabERAB11 polyclonal antiserum showed that the steady-state levels of GFP-RabE in MAD4120 were 3.5 times lower than those of untagged RabERAB11, showing that the GFP-tagging procedure impairs protein expression/stability (Supplemental Figure S2). In parallel experiments, we showed by gene replacement that endogenous tagging of RabERAB11 with GFP (without a backup untagged copy) resulted in a similar 3.5-times reduction in steady-state levels (Supplemental Figure S2). However, even though this gene-replaced allele impairs growth, it rescues lethality and supports vegetative growth very substantially (Supplemental Figure S2). We also confirmed that the RabERAB11 subcellular distribution visualized by endogenous tagging was comparable to that seen with the tandem duplication allele (Supplemental Figure S2). Data detailed later showed that GFP-RabERAB11 recruitment to membranes depends on the GTP switch and on normal Golgi function. For some localization experiments, including all of those requiring mCherry-RabERAB11, we used the alcohol dehydrogenase promoter (alcAp), which allows manipulation of fusion protein levels by the carbon source of the medium (these alcAp strains also contain the resident untagged rabE copy). Supplemental Figure S2 includes comparative Western blot analyses showing the different expression levels of RabERAB11 attainable with the alcAp by manipulating the carbon source.
RabERAB11 is present in three types of structures (Figure 1C and Supplemental Movie S1): 1) punctate cytosolic structures, polarized toward the tip; 2) rapidly moving cytosolic structures; and 3) a conspicuous accumulation of membranes at the apex, subjacent to the plasma membrane. RabERAB11 localization to all these structures is nucleotide switch dependent because Ser23Asn substitution in RabERAB11 resulted in marked relocalization to the cytosol, with faint, diffuse fluorescence in endomembranes possibly representing the ER (Figure 1D). (The ER seems to be the “default” localization of the proportion of Rab-GDP that constantly sounds membranes; Cabrera and Ungermann, 2013.) Later we demonstrate that RabERAB11 cytosolic structures correspond to post-Golgi membrane domains forming by maturation of late Golgi cisternae (LGC), moving structures represent exocytic RabERAB11 carriers deriving from these domains and captured during their journey toward the site of delivery, and the apical dome accumulation reflects a dense concentration of these carriers once they have been transported to the apex but have not yet fused with the plasma membrane. This accumulation corresponds to the Spitzenkörper (SPK), a fungal-specific structure that in electron microscope images consists of vesicles intermingled with a mesh of actin microfilaments (Harris et al., 2005; Hohmann-Marriott et al., 2006). RabERAB11 colocalizes at this structure with both the myosin-5 MyoE and RabDRAB8 (Figure 1, E and F; for MyoE, see Supplemental Movie S2), which is consistent with the role attributed to Sec4/RAB8 proteins in the fusion of exocytic carriers with the plasma membrane, acting downstream of Ypt31/RAB11.
Maturation of late Golgi cisternae into exocytic post-Golgi carriers
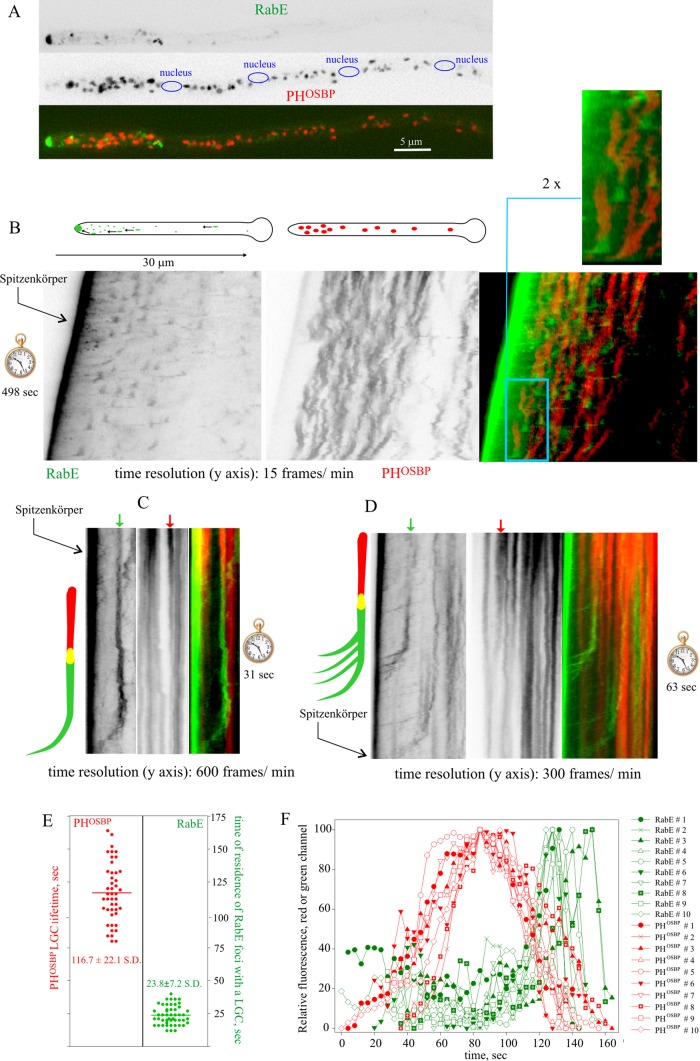
According to the cisternal maturation model, newly formed Golgi cisternae undergo changes in protein/lipid composition until reaching a stage at which they become competent for tearing off into post-Golgi carriers. Maturation of early into late Golgi cisternae has been observed in vivo in S. cerevisiae (Losev et al., 2006; Matsuura-Tokita et al., 2006). More recently, progression of LGC into Gga- and AP-1–containing clathrin-coated carriers destined to the endosomes has been thoroughly documented (Daboussi et al., 2012). In contrast, the transition of LGC into post-Golgi carriers has not been visualized in vivo. To this end, we used monomeric red fluorescent protein (mRFP)–PHOSBP as specific reporter of the late Golgi (Pantazopoulou and Peñalva, 2009, 2011; Pinar et al., 2013a). PHOSBP is a peripheral protein recruited to membranes through coincidence detection of PtdIns4P and Arf1 (Levine and Munro, 2002). We previously showed that PHOSBP colocalizes with the A. nidulans Sec7 orthologue, HypBSec7 (Pantazopoulou and Peñalva, 2009). Cytosolic RabERAB11 structures showed little colocalization with PHOSBP puncta corresponding to LGC (Figure 2A), which is consistent with the former representing post-Golgi. Next we cofilmed RabERAB11 and PHOSBP, acquiring Z-stacks of the green and red channels simultaneously every 3–4 s for several minutes. Z-stack projections were used to build up movies (Supplemental Movie S3), which were analyzed with kymographs. In these plots, LGC appeared as tilted vertical lines that gradually lose intensity until they become undetectable (Figure 2B), indicating that LGC are transient structures that eventually dissipate, an observation consistent with the cisternal maturation model (Matsuura-Tokita et al., 2006). The RabERAB11-channel kymographs (Figure 2B), “capped” at the apexes by a prominent line corresponding to the SPK, suggested that cytosolic RabERAB11 structures arise by recruitment of RabERAB11 to dissipating LGC. As a result, PHOSBP and RabERAB11 overlapped, but only momentarily, because the RabERAB11 signal associated with any given LGC increased with time as the PHOSBP signal declined until becoming barely, if at all, detectable (89% of n = 141 mRFP-PHOSBP LGC from six different hyphae were observed to mature to GFP-RabERAB11; Figure 2, B–D and F), and because coinciding with the peak of RabERAB11 fluorescence, RabERAB11 membranes underwent rapid movement toward the SPK, where they accumulated (Supplemental Movie S3; see later discussion). These departure events were seen as RabERAB11 diagonal tracks “capping’” vertical LGC signals at their bottom ends (Figure 2, B–D). In summary, cytosolic RabERAB11 structures originate from LGC that, we presume, undergo compositional changes to facilitate RabERAB11 recruitment.
FIGURE 2:
Maturation of LGC into post-Golgi carriers. Strain MAD4440. (A) GFP-RabE structures and mRFP-PHOSBP LGC do not colocalize. (B) Kymographs of GFP-RabE and mRFP-PHOSBP channels derived from time series constructed with projections of Z-stacks acquired every 4 s. The blue inset in the merge image is shown at double magnification. (C, D) Kymographs of GFP-RabE and mRFP-PHOSBP derived from middle-plane time series with the indicated time resolution. Schemes illustrating the two modes of movement of post-Golgi carriers to the apex also depict an interpretation of the changes undergone by the arrowed cisternae. (E) Left, average lifetimes of mRFP-PHOSBP LGC. Right, time of residence of GFP-RabE foci associated with a maturing LGC before it undergoes movement toward the SPK. (F) Quantitation of mRFP-PHOSBP and GFP-RabE fluorescence data for n = 10 cisternae over ∼160 s. The sharp decay of GFP-RabE fluorescence corresponds to the time at which the corresponding focus undergoes movement toward the SPK.
To increase time resolution, we acquired middle sections of growing hyphae every 100–250 ms (250–300 frames in total; Supplemental Movie S4), focusing kymographs on the transition between LGC and RabERAB11 structures. These plots confirmed that the latter derive from the former (Figure 2, C and D). In addition, kymographs revealed that nascent RabERAB11 structures could move toward the SPK following two different patterns: in some cases, the structure moved as whole entity (Figure 2C), whereas in other cases, once the RabERAB11 structure was formed, it broke up into smaller “particles” (example in Figure 2D). Taken together, these experiments strongly suggest that Golgi exit involves a process by which mRFP-PHOSBP membranes undergo changes in composition to mature into RabERAB11 ones. Once RabERAB11 reaches a certain level, these membranes undergo movement toward the site of exocytosis. These observations bear resemblance to the progressive PtdIns4P-dependent assembly of adaptor-specific clathrin coats during Golgi exit in the Golgi-to-endosome pathway (Daboussi et al., 2012). By analyzing individual examples (Figure 2E, left) we determined that the average lifetime of LGCs was 120 s. Given that individual RabERAB11 structures cannot be reliably tracked after they move toward the SPK, we determined their time of residence at the position of the “parental” LGC structure, before undergoing movement, which was 20 s (Figure 2E, right). Figure 2F provides a quantitative depiction of the transition between LGC and RabERAB11 carriers for 10 different events.
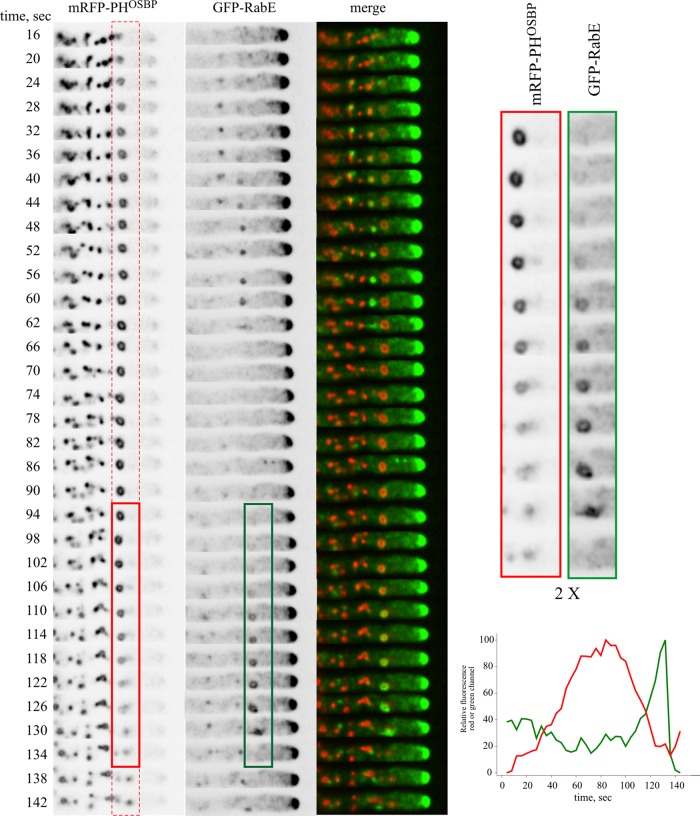
Figure 3 (also see Supplemental Movie S3) shows a typical example in its cellular context, focusing on a LGC that can be easily tracked due to its characteristic ring shape (see magnified inset on the right). The biogenesis of post-Golgi carriers is preceded by the formation of an mRFP-PHOSBP cisterna (boxed in red). After reaching a plateau, the mRFP-PHOSBP content of the cisterna decreases, whereas RabERAB11, initially undetectable, becomes visible and progressively increases, such that at late steps of the “cycle” the structure is detectable mostly for its RabERAB11 signal (boxed in green). Soon after RabERAB11 becomes the “dominant” signal, the cisterna, initially spherical/ellipsoid, becomes stretched, as if submitted to tension, and moves toward the apex, accumulating under the apical dome plasma membrane.
FIGURE 3:
Maturation of LGC into post-Golgi carriers: an example in its cellular context. Boxed in red and green is an mRFP-PHOSBP cisterna that matures into a GFP-RabE post-Golgi structure that undergoes movement at the 130-s time point. The relative fluorescence in the red and green channels is plotted against time. Relevant time points of the two channels corresponding to this structure are magnified on the right to illustrate the match in shape between mRFP-PHOSBP and GFP-RabE. Frames are from Supplemental Movie S3 (strain MAD4440).
To further confirm the maturation pathway of LGC into post-Golgi RabERAB11 carriers, we used HypBSec7 endogenously tagged with GFP (Pantazopoulou and Peñalva, 2009) to monitor LGC in combination with mCherry-RabERAB11. Owing to the weaker signal of HypBSec7, we expressed mCherry-RabERAB11 under the control of the alcAp promoter on fructose (which results in relatively low levels of expression of the protein; Supplemental Figure S2) in a strain containing an untagged copy of rabE. Even though the weaker signals limited the time extension of four-dimensional image acquisition, movies derived from maximal-intensity projections of Z-stacks showed remarkably similar results to those described earlier (Supplemental Figure S3 and Supplemental Movie S5). Thus kymographs revealed that at the end of their lifespan, HypBSec7 LGC are “capped” by mCherry-RabERAB11 signals subsequently moving toward the apex. Analysis of the movies revealed that individual cisternae maturing into mCherry-RabERAB11 could be tracked visually, and quantitation of the signals for individual cisternae in each channel revealed similar profiles to those seen with mRFP-PHOSBP and GFP-RabERAB11 (Supplemental Figure S3). Moreover, 96% of n = 95 HypBSec7-GFP LGC (five different hyphae) were observed to mature to mCherry-RabERAB11. In summary, Golgi exit involves the gradual conversion of LGC into post-Golgi membranes by acquisition of RabERAB11 and loss of Golgi identity. The resulting RabERAB11 membranes undergo long-distance transport toward the apex, thus fulfilling the expected behavior for exocytic carriers.
The accumulation of RabERAB11 carriers at the SPK depends on HypBSec7
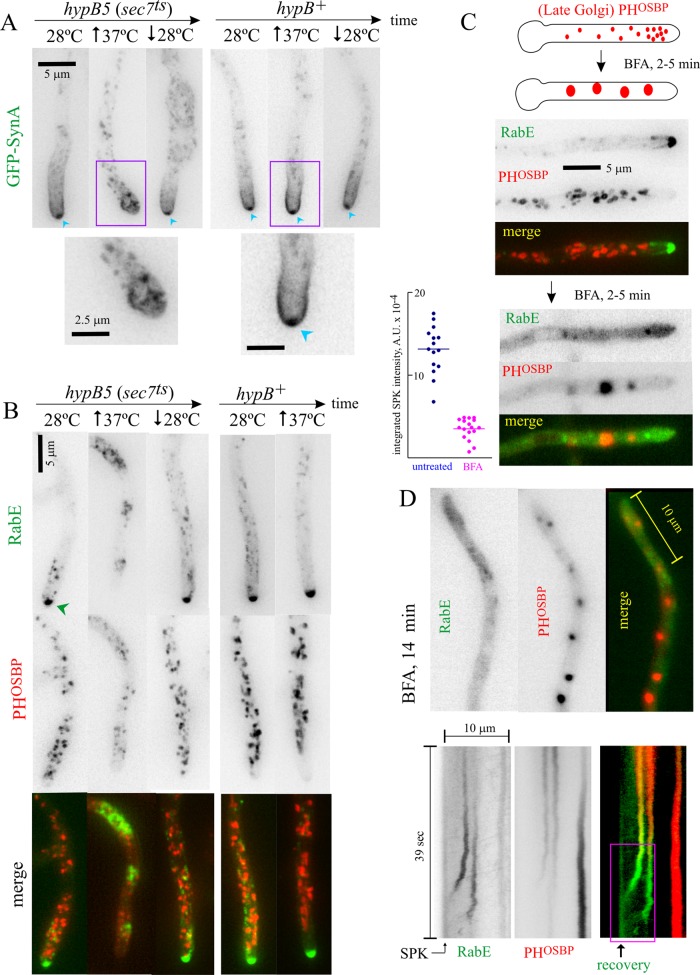
The foregoing results suggest that the SPK accumulation of RabERAB11 exocytic carriers reflects the balance between the biogenesis of these carriers from LGC, their transport to the apex, and their consumption by exocytosis. Thus impairment of Golgi exit should result in the reduction or dissipation of this structure. We tested this prediction by hindering the late Golgi activity of the key Golgi GTPase Arf1 with hypB5, a ts mutation in the gene encoding A. nidulans Sec7 (Yang et al., 2008; Pinar et al., 2013a), the late Golgi GEF of Arf1. In control experiments, we used the SynA synaptobrevin-like vesicle (v)-SNARE to label exocytic material. In the wild-type at both 28 and 37°C and in hypB5 cells at 28ºC, SynA, which is efficiently taken up from the plasma membrane by endocytosis, localizes to the apical crescent and to the SPK (Taheri-Talesh et al., 2008; Pantazopoulou and Peñalva, 2011; Figure 4A). In contrast, in hypB5 cells shifted to restrictive temperature (37ºC), the SPK accumulation of SynA dissipated (as did the plasma membrane pool; Pinar et al., 2013b; Figure 4A), as expected from impairment of Golgi exit. We next tested hypB5 cells coexpressing PHOSBP and RabERAB11. At 10–20 min after shifting hypB5 cells to the restrictive temperature, the LGC marker PHOSBP partially relocalized to the cytosol, consistent with impairment of late Golgi Arf1 activity (Figure 4B). Under these conditions, RabERAB11 was completely delocalized from the SPK to internal structures (the SPK was undetectable in n = 93 hypB5 hyphal tips photographed in two different experiments). These internal RabERAB11 structures did not overlap with LGC remnants, suggesting that they have post-Golgi identity (Figure 4B). Neither SynA nor RabERAB11 delocalization from the SPK reflected cell death because their normal distribution was restored when the temperature was shifted down subsequently (Figure 4, A and B). Thus late Golgi Arf1 GEF impairment delocalizes RabERAB11 but does not prevent RabERAB11 engagement with membranes.
FIGURE 4:
Membranes containing RabERAB11 have post-Golgi identity. (A) Temperature shift experiment comparing hypB5ts (sec7ts) and hypB+ strains (MAD3323 and MAD3320, respectively) expressing GFP-SynA to label the accumulation of exocytic membranes at the position of the SPK (indicated when present with blue arrowheads). Upward- and downward-pointing arrows indicate temperature shift up and down, respectively. The regions indicated by the violet boxes are displayed at the bottom at double magnification. (B) hypB5ts (sec7ts) and hypB+ strains (MAD3632 and MAD3167, respectively, expressing mRFP-PHOSBP and GFP-RabERAB11, the latter under the control of the alcAp on ethanol). In a control experiment with strain MAD5082 we confirmed that the same abnormal compartment was formed when GFP-RabERAB11expression was driven by the rabE promoter in a strain that did not express mRFP-PHOSBP. See also Supplemental Figure S4. (C) Top, aggregation of Golgi cisternae by BFA treatment. Bottom, mRFP-PHOSBP LGC aggregate with BFA, whereas GFP-RabE membranes delocalize to a haze (strain MAD4440). Left, SPK accumulation of RabERAB11 carriers is largely dissipated by BFA. The two sets of data are significantly different (p < 0.0001). (D) Two tip-proximal cisternae recovering from BFA treatment. The line depicts the region of interest used to plot the bottom kymograph. The two LGCs progressively acquire RabERAB11 and move toward the SPK, where their arrival results in a detectable increase in GFP fluorescence.
In addition to their secretory roles, yeast Ypt31/Ypt32 regulate endosome-to-Golgi transport in a process involving their F-box effector Rcy1 (Chen et al., 2005, 2011). Early endosomes of filamentous fungi are characteristically motile (Lenz et al., 2006; Abenza et al., 2009). Data to be described later established unambiguously that under normal circumstances, RabERAB11 moving structures and RabARAB5 early endosomes (EEs) are different populations. However, the acute perturbation of the Golgi by hypB5 likely results in severe disruption of endosome-to-Golgi traffic. Thus we considered the possibility that the abnormal hypB5 RabERAB11–containing structures might represent stranded membranes with some degree of endosomal identity. We tested this possibility with a hypB5 strain coexpressing GFP-RabERAB11 and mCherry-tagged early endosomal RabARAB5. RabERAB11 and RabARAB5 partially overlapped in this abnormal compartment (Supplemental Figure S4), which might be consistent with RabERAB11 playing a role in this recycling pathway. In any case, experiments with hypB5 demonstrated that the maintenance of the steady-state pool of RabERAB11 membranes at the SPK necessitates late Golgi Arf1 activity.
The accumulation of post-Golgi RabERAB11 carriers at the SPK is largely reduced by brefeldin A
If Golgi traffic is abrogated, the biogenesis of post-Golgi compartments should be abolished immediately. We used brefeldin A (BFA) to interfere with Golgi traffic. Unlike hypB5, BFA impairs both the early and late Golgi activity of Arf1. In A. nidulans this leads to disorganization of the Golgi network, resulting in almost immediate (within 5–10 min) coalescence of cisternae into large aggregates (Pantazopoulou and Peñalva, 2009, 2011; Figure 4C). Instead of behaving like Golgi cisternae, cytosolic RabERAB11 structures, including SPK accumulation, which was markedly reduced (Figure 4, C, and D, kymograph; note that the effect of BFA is incomplete and transient; see later discussion), rapidly delocalized to a cytosolic haze. These data imply that dysfunction of the Golgi network impairs the biogenesis of RabERAB11 carriers. Because Golgi-resident RabORAB1 and RabCRAB6, as well as Golgi cisternae labeled with resident SNAREs, rapidly aggregate with BFA (Pantazopoulou and Peñalva, 2011; Pinar et al., 2013a), we conclude that RabERAB11 membranes do not belong to the “Arf1 domain” (i.e., they lack Golgi identity), which strongly supports their post-Golgi nature.
After the initial ∼15-min period in the presence of BFA, the Golgi starts to recover spontaneously, albeit slowly (Pantazopoulou and Peñalva, 2009). Thus, at 15 min and later, cells that had been clearly affected by BFA (impaired exocytosis leads to tip swelling; Pinar et al., 2013a, b) displayed a gradual recovery of RabERAB11 at their apexes, with both the prominence of these “SPK primordia” and the number of hyphal tip cells showing them clearly increasing with time. This indicated that gradual recovery of the Golgi was paralleled by gradual recovery of the SPK. During the recovery phase, we were able to recognize isolated examples of maturation in which we tracked more easily the fate of post-Golgi RabERAB11 structures without the interference of the strong SPK signal. The kymograph in Figure 4D depicts a hyphal tip filmed during this phase. Two PHOSBP puncta mature into RabERAB11-positive membranes that undergo movement toward the apex. Their arrival at the environs of the plasma membrane is detectable by the increase in GFP-RabERAB11 fluorescence. The correlation between recovery of the apical accumulation of RabERAB11 and reassumption of Golgi function that occurs as cells recover spontaneously from BFA established beyond doubt that building the SPK requires Golgi exit.
The SPK cluster of RabERAB11 is rapidly replenished by carriers
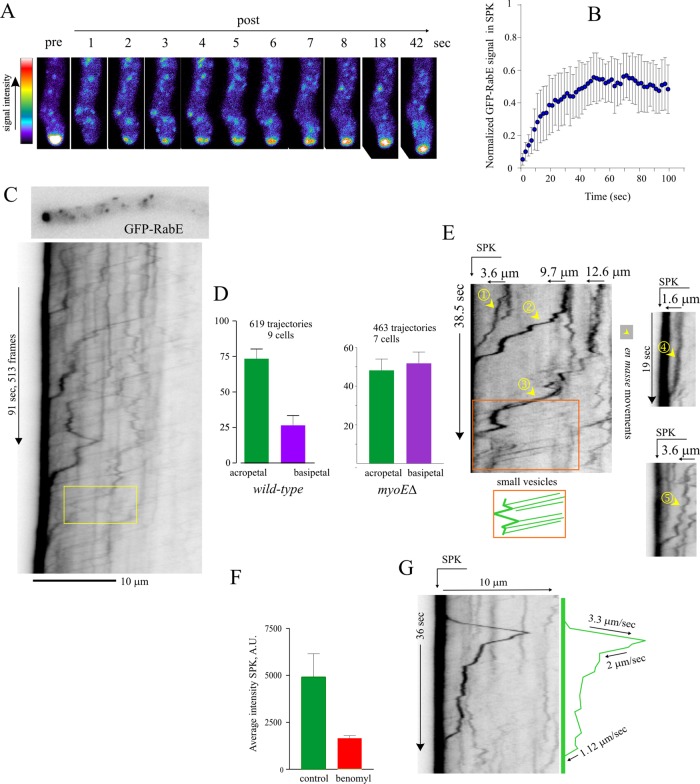
Fluorescence recovery after photobleaching (FRAP) experiments established that the SPK is indeed rapidly replenished with RabERAB11 (Figure 5, A and B; t1/2 ≈ 10 s). We attributed this rapid FRAP recovery to the strikingly fast and intense acropetal (i.e., toward the apex) traffic of RabERAB11 carriers bombarding the apex. This traffic was recorded by wide-field microscopy, streaming middle-plane sequences of 200–900 images acquired at 5–10 frames/s to the computer RAM (Supplemental Movie S1). Carrier trajectories were tracked with kymographs (Figure 5C). In the ∼3-min/900-frame sequence used for this kymograph, 47 such carriers actually hit the SPK, but this is an underestimation, as a proportion of carriers was inevitably lost by single-plane sequences taken with low exposure times to minimize photodamage. In n = 9 tips, 73% of RabERAB11 carriers moved toward the SPK, whereas 24% moved basipetally (Figure 5D).
FIGURE 5:
Intense bombardment of the apical region by exocytic RabERAB11 traffic. (A) Still-frame micrographs showing fast FRAP recovery of the SPK GFP-RabE signal. (B) Normalized GFP-RabE signal in the SPK after bleach event. Error bars are SD of n = 6 cells. (C) Kymograph traced across the axis of the depicted hyphal tip. Yellow box indicates an acropetally moving carrier that reverses direction. Derived from Supplemental Movie S1. (D) Percentage of acropetal and basipetal GFP-RabE trajectories in wild-type and myoE∆ cells. Error bars are SD. (E) Kymograph plots depicting five examples of large GFP-RabE carriers moving to the SPK and an area (boxed) depicting intense traffic of small carriers with uniform speeds. (F) Average intensity of the GFP-RabE SPK measured in sum projections of Z-stacks of images from cells treated or not with 10 μg/ml benomyl. (G) Kymograph depicting basipetal movement of a large GFP-RabE carrier. Experiments were carried out with strain MAD4120 and its myoE∆ derivative, MAD4409.
Acropetal trajectories were in two classes. Carriers with the strongest signal and most apical tracks moved intermittently and generally more slowly. Their trajectories reflect the movement en masse of large RabERAB11 structures to the SPK (Figure 5E, 1–5). The smallest carriers/weakest signals represented the majority of trajectories and involved structures moving several micrometers at uniform speeds (Figure 5E, orange box). They often moved acropetally for some distance before reversing direction (Figure 5C, box) or stopping in the tip at positions generally coinciding with larger RabERAB11 puncta (Figure 5E, box). This suggested that many such “small carriers” might not travel completely to the plasma membrane, contrasting with large RabERAB11 carrier movements, which would appear to represent the membrane-proximal exocytic transport step.
Post-Golgi carriers travel toward and away from the apex on MTs
Evidence that many RabERAB11 carriers move acropetally on MTs is compelling. Benomyl markedly reduced the accumulation of carriers at the SPK (Figure 5F) and virtually abolished movement (Supplemental Figure S5). However, as we were concerned that benomyl might affect F-actin indirectly, we cofilmed GFP-RabERAB11 and GFP–α-tubulin (TubA)–labeled MTs. These movies, in which RabERAB11 carriers can be distinguished unambiguously from long, stiff MTs, established that the former move associated with the latter (Supplemental Movies S6 and S7).
RabERAB11 carriers were able to move basipetally (Figure 5G). Because the plus ends of tip MTs are oriented toward the apex (Konzack et al., 2005), these basipetal trajectories must reflect attachment to MTs undergoing catastrophe or dynein-dependent transport. Coimaging of TubA and RabERAB11 captured basipetal movements associated with MTs, strongly indicating that they are dynein mediated (Supplemental Movie S7). Physiologically, dynein engagement provides the secretory system with the capacity to drag post-Golgi membranes away from the tip region.
The average speed of RabERAB11 carriers (2.67 ± 0.04 SEM μm/s; n = 392) and their changes in direction resemble those of EEs moving on MTs (Lenz et al., 2006; Abenza et al., 2009; Schuster et al., 2011). We hypothesized that there might be a population of membranous carriers having both secretory and endocytic identity organized in mosaics of two different RAB domains. However, cofilming EEs labeled with RabARAB5 and exocytic carriers labeled with RabERAB11 simultaneously showed that EEs and RabERAB11 moving structures represent distinct entities (Supplemental Figure S6). In a second set of experiments, we labeled endosomes with FYVE2-GFP. This probe consists of a tandem duplication of the Vps27 FYVE domain fused to GFP (Abenza et al., 2010). FYVE2-GFP efficiently binds phosphatidylinositol 3-phosphate, which is present on the membranes of endosomes but absent from exocytic compartments. Cofilming of mCherry-RabERAB11 carriers with FYVE2-GFP endosomes revealed that the two populations of moving structures are clearly different: the number of RabERAB11 “movements” clearly outnumbers that of FYVE2-GFP endosomes, and the corresponding kymograph traces hardly, if at all, overlapped (Supplemental Figure S7).
Actin-dependent mechanisms are crucial for the apical delivery of RabERAB11 carriers
F-actin cooperates with MTs to maintain tip growth (Torralba et al., 1998; Sharpless and Harris, 2002; Virag et al., 2007; Taheri-Talesh et al., 2008, 2012; Zhang et al., 2011). In current models the F-actin role involves a dense mesh of actin cables that radiates from the SPK (Pearson et al., 2004; Taheri-Talesh et al., 2008). These cables are flexible, resembling the strings of a mop. They can penetrate as deeply as 25 μm into the hyphal cell (Taheri-Talesh et al., 2012), such that polarized LGC (Pantazopoulou and Peñalva, 2009) fall within their reach.
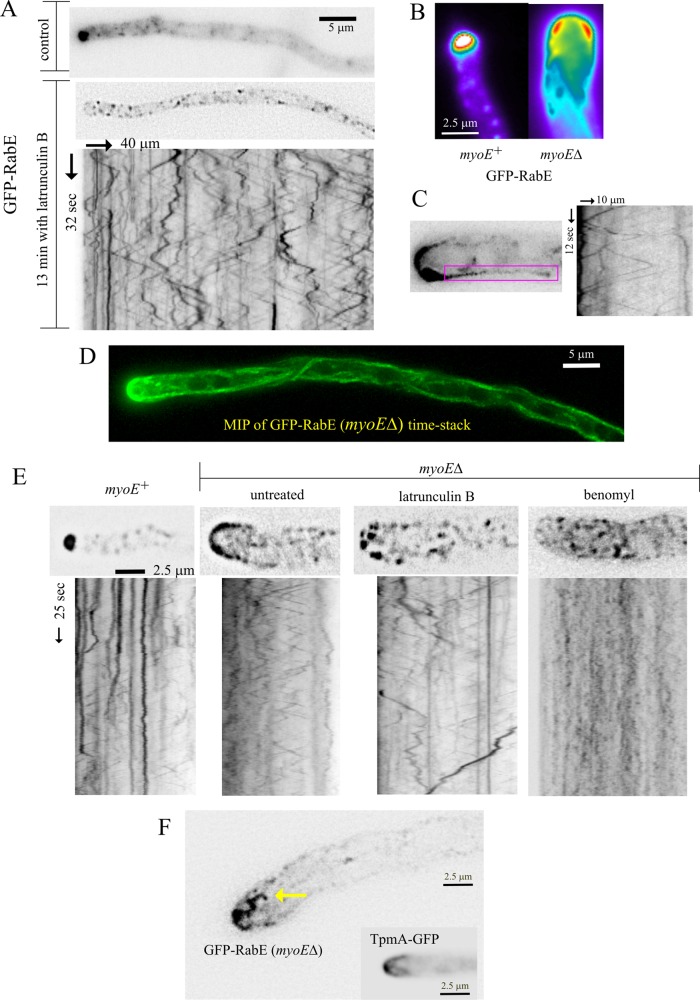
F-actin depolymerization with latB arrested growth and rapidly delocalized RabERAB11 from the SPK (Figure 6A) without noticeably affecting MT organization (Supplemental Figure S5), showing that exocytosis requires F-actin. The fact that exocytosis can proceed to some extent without MTs (Figure 5F; Horio and Oakley, 2005) but not without actin filaments indicates that actin and MTs cannot mediate redundant parallel transport pathways, suggesting instead that MTs are subordinated to actin.
FIGURE 6:
MyoE-dependent and -independent functions of F-actin. (A) Latrunculin B treatment delocalizes GFP-RabE–containing membranes at the SPK to the MT conveyor belt. Derived from Supplemental Movie S8. (B) An example of a myoE∆ tip in which the GFP-RabE crescent is thicker in a slightly subapical position. (C) Kymograph covering the position of a cortical MT, showing that it serves as track for RabERAB11 carriers moving in both directions and the uniform speeds characteristic of MT-dependent movements. (D) Maximal-intensity projection of a middle-plane time stack showing how moving GFP-RabE carriers delineate trajectories of MTs. (E) Micrographs from time stacks of images and the corresponding kymographs, showing effects of F-actin and MT depolymerization on the tip-and-apical crescent-associated GFP-RabE carriers. Both drugs delocalized these carriers from the tips of myoE∆ cells, but these carriers moved only when MTs were present. (F) Isolated frame from a time stack (Supplemental Movie S11) showing microfilament-like material decorated with GFP-RabE carriers. A hyphal tip in which actin cables are decorated with GFP-tropomyosin is shown for comparison. Experiments were carried out with strain MAD4120 and its myoE∆ derivative, MAD4409. The TpmA-GFP inset corresponds to MAD1750.
The contention that F-actin acts downstream of MTs was strongly supported by the finding that latB displaced all RabERAB11 membranes, including those in the SPK, to a conspicuous population of rapidly moving structures showing bidirectional movement and average speed (3.15 ± 0.06 SEM μm/s; n = 231) consistent with MT motor powering (Figure 6A and Supplemental Movie S8). Coimaging of RabERAB11 and TubA-GFP in these latB-treated cells provided indisputable evidence that these structures move on MTs (Supplemental Movie S9). Indeed, when F-actin and MTs were depolymerized simultaneously, RabERAB11 membranes formed static aggregates that did not colocalize with PHOSBP (Supplemental Figure S8). Therefore, in the absence of F-actin, secretory carriers can arrive at the tip on MTs, but they are dragged out, presumably, by dynein. These findings indicate that MTs fuel the tip region with exocytic carriers, relaying them to an F-actin–dependent motor that mediates the last step in their transport to the SPK. MT transport works like a “conveyor belt” that shows up clearly if actin is depolymerized. This mechanism ensures that if the actin/MT “relay” is missed, RabERAB11-containing membranes are dragged out away from the tip by dynein (see later discussion)
Myosin V focuses RabERAB11 carriers at the apex, but F-actin plays roles beyond providing tracks
The single A. nidulans class V myosin, MyoE, colocalizes with the v-SNARE SynASnc1 (Taheri-Talesh et al., 2012) and RabERAB11 (Figure 1E) at the SPK, which is consistent with MyoE powering the actin-dependent step in exocytosis. myoE∆ markedly slows down, yet does not arrest, apical extension (Taheri-Talesh et al., 2012), showing that the MyoE role, albeit important, is not essential. In agreement, in myoE∆ cells, RabERAB11 carriers moved toward the tip and arrived at the plasma membrane. However, they could not be “focused,” spreading into an apical crescent instead of gathering at the apex (Figure 6B). Thus MyoE focuses RabERAB11 carriers at the apex via actin cables. Hardly any acropetal movements of the large RabERAB11 structures were seen in myoE∆ tips (Supplemental Movie S10), supporting the contention that these movements involve actomyosin.
RabERAB11 carriers must use MTs to arrive at the myoE∆ apical crescent. Within this crescent, the RabERAB11 signal appeared strongest in a ring immediately upstream from the apex (Figure 6B), as if this area, frequently contacted by MTs (Supplemental Figure S5), represented a preferred landing spot for carriers. Evidence that myoE∆ secretion is indeed MT dependent was compelling: movies captured carriers en route to the crescent, kymographs demonstrated that MT-like bidirectional transport was associated with cortical MTs (Figure 6C), and trajectories revealed by projecting time stacks onto a two-dimensional (2D) image clearly delineated MTs (Figure 6D).
myoE∆ markedly increased the number of basipetal movements in the tips (Figure 5D), showing that failure to engage MyoE increases the chance that RabERAB11 membranes will be dragged away from the tip by dynein. However, in contrast to actin depolymerization (Figure 6A), myoE∆ did not shift apical RabERAB11 material to the “MT conveyor belt,” indicating that F-actin is able to “capture”RabERAB11 carriers even when myosin V is completely absent. myoE∆ tips showed a tip haze of RabERAB11 (Figure 6, B–E) that movies resolved as string-like structures dangling from the apex, whose resemblance to tropomyosin-labeled structures indicated that they are microfilaments decorated with RabERAB11 (Figure 6F and Supplemental Movie S11). Hence secretory carriers cannot move on cables without MyoE but are capable of binding them, explaining the inability of myoE∆ to shift all of these carriers to the MT-based conveyor belt. Indeed, latB dispersed the crescent-and-string–associated RabERAB11 material of myoE∆ tips to moving structures (Figure 6E). Benomyl also dispersed this crescent-and-string–associated material, indicating that MTs supply the RabERAB11 membranes captured by F-actin. However, the resulting cytosolic structures were, as expected, immotile (Figure 6E and Supplemental Movie S11). We conclude that in myoE∆ cells, exocytosis is fueled by MTs with collaboration of a F-actin–dependent capture mechanism (the “actin mop”).
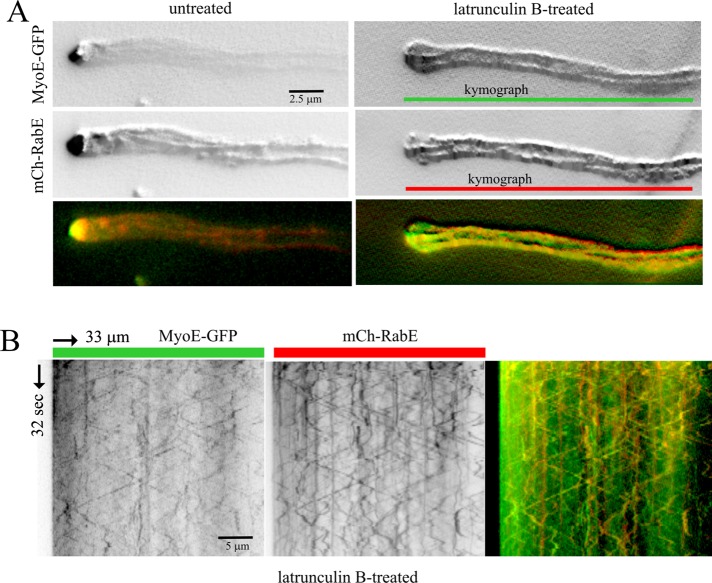
RabERAB11 carriers moving with the MT conveyor belt are loaded with MyoE
The behavior of RabERAB11 carriers displaced to the conveyor belt by latB implies that they are loaded with dynein and kinesin(s). The seamless relay between MTs and actin cables might be easier if RabERAB11 carriers were also loaded with MyoE. Under normal conditions, exocytic carriers labeled with MyoE-GFP can hardly be tracked by time-lapse imaging because in the steady state MyoE almost exclusively localizes to the SPK, resulting in faint signals elsewhere (Taheri-Talesh et al., 2012). To overcome this obstacle, we depolymerized F-actin with latB before cofilming MyoE-GFP and mCherry-RabERAB11 with a beam splitter and maximized detection by drawing 2D maximal-intensity projections of the resulting “time stacks.” In untreated cells, projections revealed, besides the SPK, very faint signals for MyoE and, as described earlier, MT tracks for RabERAB11 (Figure 7A). Latrunculin B shifted both MyoE-GFP and mCherry-RabERAB11 from the SPK to the population of bidirectionally moving structures. The 2D projections of MyoE and RabERAB11 trajectories in latB cells colocalized, clearly delineating MTs (Figure 7A). The resulting increase of MyoE-GFP signal in the moving population sufficed to detect MyoE trajectories with kymographs, which established that MyoE-GFP and mCherry-RabERAB11 actually colocalize on the same moving structures (Figure 7B). Therefore MyoE is a passenger of RabERAB11 carriers relocalizing to the “MT conveyor belt” after F-actin depolymerization. These carriers move in both acropetal and basipetal directions (Figure 7B), implying that they are loaded with one (or more) kinesins, dynein, and MyoE riding as passenger. Figure 8 shows a model (see Discussion) depicting how maturation of post-Golgi carriers is integrated with transport to the apex by a MT-based conveyor belt that concentrates carriers in the tip region before they are relayed to MyoE transport.
FIGURE 7:
Latrunculin B displaces RabERAB11 carriers to MTs. (A) Maximal-intensity projection of middle-plane time stack showing trajectories of mCherry-RabE and MyoE-GFP. The finding that latB displaced carriers from the SPK to the MT conveyor belt allowed us to determine that the trajectories of MyoE and RabE colocalize, delineating MTs. (B) Kymograph of the latrunculin B–treated cell, showing how MyoE-GFP and mCherry-RabE colocalize on moving structures. These experiments were carried out with strain MAD4403 cultured on fructose, with MyoE-GFP being expressed from an endogenously tagged myoE allele (Taheri-Talesh et al., 2012).
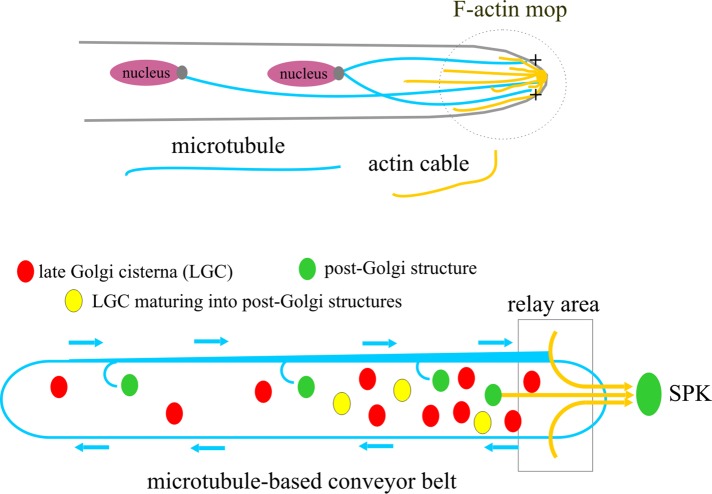
FIGURE 8:
Model for the biogenesis and delivery to the apex of post-Golgi carriers. The biogenesis of RabERAB11 carriers from LGC is coupled to their long-distance transport to the apex by the concerted action of F-actin and a MT conveyor belt. The MT-based conveyor belt relays these carriers to the tip mop of microfilaments. See Discussion for details.
DISCUSSION
How traffic traverses and exits the Golgi is a fundamental problem. The finding that nonstacked fungal Golgi cisternae are resolvable by fluorescence microscopy (Wooding and Pelham, 1998) paved the way for studies revealing that cisternae are transient entities undergoing changes in protein content by which early components are substituted by late components over time (Losev et al., 2006; Matsuura-Tokita et al., 2006). These observations met key predictions of the cisternal maturation model (Glick and Luini, 2011). In this model, Golgi cisternae forming by coalescence of ER-derived vesicles become progressively enriched in cargo as they advance across a maturation pathway until they reach a stage in which membranes and cargo exit the Golgi as carriers destined to the plasma membrane or endosomes (Glick and Nakano, 2009). The transient nature of late Golgi cisternae has been experimentally observed (Losev et al., 2006; Matsuura-Tokita et al., 2006). Recently the PtdIns4P-coupled biogenesis of endosome-destined Gga2p- and AP-1-enriched carriers from LGC (representing another maturation process at the exit of the Golgi) has been thoroughly documented (Daboussi et al., 2012). In contrast, the transition between LGC membranes and exocytic carriers had not been imaged in vivo.
Maturation of LGC into exocytic carriers with post-Golgi identity
A. nidulans LGC are separated from the site of exocytic carrier delivery by a few-micrometer gap, facilitating the filming of the Golgi–to–post-Golgi transition during exocytosis. We predicted that the key determinant of post-Golgi identity would be RabERAB11 because Ypt31 (yeast RAB11) activation is critical for Golgi exit (Jedd et al., 1997; Morozova et al., 2006) and Ypt31 contributes to recruitment of Myo2 (the only motor mediating yeast exocytosis) to post-Golgi carriers (Lipatova et al., 2008; Jin et al., 2011; Santiago-Tirado et al., 2011). In rapidly growing hyphal tip cells, the lifespan of LGC, as determined with PHOSBP, is ∼2 min. At the end of this period, LGC gradually lose Golgi identity as RabERAB11 is progressively recruited until it nearly becomes their only detectable marker. The peak of RabERAB11 content coincides with the engagement of motors, resulting in rapid movement to the apex. The average time of residence of RabERAB11 in association with LGC that precedes transport is ∼20 s. The sharp transition between late Golgi and post-Golgi identity might be consistent with RabERAB11 “negating” Golgi identity. For example, yeast RAB11 homologue Ypt32 negates RAB6/Ypt6 activity by recruiting the Ypt6 GAP, Gyp6 (Suda et al., 2013), and RAB1/Ypt1 activity by recruiting the “anti-current” Ypt1 GAP, Gyp1 (Rivera-Molina and Novick, 2009). The domain of action of Ypt1 (Sclafani et al., 2010) and A. nidulans RabORAB1 (Pinar et al., 2013a) possibly includes the late Golgi. Thus our data can be easily reconciled with previous work by expanding the regulatory function of RabORAB1 to the whole Golgi, leaving for RabERAB11 the role of mediating exocytic Golgi exit.
Golgi exit and subsequent transport of RabERAB11 carriers
The diffraction-limited spatial resolution of our time-resolved series precluded us from reaching a firm conclusion on whether the exit of carriers involves budding of one or more vesicles from the same LGC or transport to the SPK of the whole membrane content of the LGC. However, in some cases in which the maturing LGC appeared as an optically resolvable hollow circle, as in the example of Figure 3, the shape of the resulting RabERAB11 structure precisely matched that of the parental LGC, indicating that at least in these cases the whole cisterna is involved in the biogenesis of the post-Golgi carrier. The fact that observations were reproduced when we used endogenously tagged HypBSec7 instead of PHOSBP as LGC marker indicates that a major portion of the parental LGC is involved in the biogenesis of the post-Golgi carrier, which would meet one important prediction that derives from the cisternal maturation model, namely that after maturation, Golgi cisternae dissipate (Matsuura-Tokita et al., 2006). However, the persistence of a weak signal in the position of “parental” LGCs (as in the Figure 3 example) suggests that not all the membrane of any given LGC is incorporated en bloc into a RabERAB11 carrier. This is not unexpected, given that LGC, in addition to generating exocytic carriers, deliver membrane vesicles toward the endosomes (Daboussi et al., 2012) and that some membrane could be contributed to another cisterna via intra-Golgi retrograde transport. Although this work describes the overall landscape of the dynamic transition between the Golgi and exocytic carriers in A. nidulans, future work involving a wider set of markers to define more precisely both the composition and the fate of LGC and exocytic carriers, as well as superresolution to try to resolve membrane subdomains within them, will be required to delineate more precisely the mechanisms by which LGC give rise to exocytic membranes.
Direct imaging established that exocytosis requires cooperation of MT and F-actin, in agreement with genetic data (Zhang et al., 2011; Taheri-Talesh et al., 2012). MyoE focuses RabERAB11 carriers at the main site of exocytosis, the apex (Taheri-Talesh et al., 2008), via actin filaments, whereas MT transport bulldozes post-Golgi structures originating away from the tip toward the tip mesh of actin. Large RabERAB11 carriers show irregular speeds in kymographs and generally reach the apex. The movement of these large carriers possibly reflects MyoE powering, since similar tracks are not seen in myoE∆ kymographs. Irregular speeds might result from low myosin V processivity (Reck-Peterson et al., 2001) and/or might be only apparent, reflecting the curling trajectories of actin cables in three dimensions (kymographs are plotted with 2D images). Small RabERAB11 carriers show uniform (2.5 μm/s on average) speeds, may reverse direction before they arrive to the apex, and follow regular and recurrent trajectories attributable to stiff MTs. The multiplicity of kinesins, the potential side effects of kinesin-null mutations on dynein (Zhang et al., 2003) and MT stability (Requena et al., 2001), and the likely MyoE assumption of kinesins’ roles in the corresponding null mutants hindered the assignment of these trajectories to single kinesins.
RabERAB11 carriers bind actin cables without MyoE. Thus the F-actin tip “mop” actually “wipes” carriers. Potential candidates tethering carriers to actin might be nonmotile myosin domain–containing proteins, such as U. maydis Mcs1 (Schuster et al., 2012), or even kinesin-1 itself (Hodges et al., 2009). Actin depolymerization displaces exocytic RabERAB11 carriers to a kinesin/dynein-powered conveyor belt, an observation that we exploited to demonstrate that these carriers are loaded with MyoE, dynein, and one or more kinesins. RabERAB11, in concert with PsdIns4P, is the almost-certain MyoE recruiter, but the adaptors for kinesin and dynein are unknown. The presence of kinesins and MyoE on the same cargo might enhance each other's processivity (Ali et al., 2008; Hodges et al., 2009), as might tropomyosin in the tip actin cables enhance the processivity of MyoE during the final run (Hodges et al., 2012).
A model of post-Golgi exocytic traffic: the conveyor belt relays carriers to actin cables
Post-Golgi structures (Figure 8, green ovals) originate anywhere within the cell by maturation of Golgi cisternae (red ovals and yellow ovals indicate cisternae before and during maturation, respectively) and are loaded with motors as post-Golgi identity is acquired, thus becoming exocytic carriers. A carrier arising distally is transported by kinesin(s) to the tip, where the actin cable mop collects it. Then MyoE engages a cable and completes the delivery of the carrier to the apex. If myosin engagement fails, dynein drags the carrier away from the tip. Kinesins eventually counteract dynein, such that any carrier traveling basipetally may reverse direction to return to the tip, giving the carrier another chance to engage actin. Globally this results in MT-based bidirectional conveyor belt-like movement that becomes very conspicuous if F-actin is depolymerized. Given that the most apical Golgi equivalents are within reach of the actin cables (Figure 8), some RabERAB11 structures might be transported directly to the apex, and hence the relay mechanism may coexist with direct runs. MyoE is not essential because tip MTs are capable of delivering carriers directly to the plasma membrane. In the absence of MTs, those cisternae reached by actin cables maintain exocytosis with direct runs. This work also clarifies the evasive nature of the SPK. The vesicles that it contains are, at least in part, exocytic RabERAB11 carriers derived from the Golgi (Figure 8).
RAB11, hyphal cells, and melanocytes
The kinesin/myosin V relay mechanism operating in hyphal tips is conceptually similar to the “myosin V capture coupled to MT conveyor belt transport” proposed by Hammer and colleagues to explain the accumulation of melanosomes at the dendritic tips of melanocytes (Wu et al., 1998). Indeed, our findings reveal that melanosome transport involving an exocytic organelle derived from the lysosome and the transport of exocytic post-Golgi carriers derived from Golgi cisternae evolved to use cooperation of cytoskeletal elements and their motors.
MATERIALS AND METHODS
Nomenclature
RabERAB11 and RabDRAB8 are AN0347 and AN6974, respectively, in the AspGD database (www.aspgd.org).
Aspergillus strains, culture media, and genetic manipulation
A. nidulans standard growth media were used (Cove, 1966). For microscopy experiments, hyphae from strain MAD4120 or its derivatives (Supplemental Figure S2) were cultured in liquid “watch minimal medium” (Peñalva, 2005) containing 0.1% (wt/vol) glucose and 5 mM ammonium tartrate. In experiments involving strains carrying alcAp -driven transgenes (indicated in the corresponding figure legends and described in what follows), we used 0.05–0.1% fructose (low expression levels) or 1% ethanol (high expression levels) instead of 1% glucose (which represses the promoter; Supplemental Figure S2). Strains carried markers in standard use. Strains are listed in Supplemental Table S1. Genetic crosses were performed with standard methods (Clutterbuck, 1993), and transformation was previously described (Tilburn et al., 1983). The heterokaryon rescue technique for assessment of lethality associated with rabE∆ was used as described (Osmani et al., 2006)
Gene expression constructs
These constructs are represented schematically in Supplemental Figure S2. For expressing GFP-RabE under the control of its natural promoter, we constructed by PCR fusion (Szewczyk et al., 2006) a rabEp::gfp-rabE::pyrGAf::3′-UTRrabE DNA molecule containing, from 5′ to 3′, the 5′-untranslated region (UTR) of rabE amplified from genomic DNA with primers 7 and 8 (Supplemental Table S2); the gfp-rabE transgene amplified from p1979 with primers 9 and 2; and a pyrGAf::3′UTRrabE fragment previously generated by fusion PCR between the Aspergillus fumigatus pyrG gene (pyrGAf; amplified from p1530 (Osmani et al., 2006) with primers 10 and 11) with the 3′-UTR of rabE, amplified from genomic DNA with primers 12 and 13. The assembled molecule was cloned in pGEM t-easy to generate plasmid p2089, which was used to transform MAD1739. Southern blot analysis of transformants was used to select MAD4120, resulting from a single crossover event at the 3′-UTR of rabE. Thus this strain carries an in locus tandem duplication of rabE with one of the copies tagged with GFP.
Plasmids p1979 and p2027 (plasmid numbers correspond to our −20ºC collection of DNA molecules) drive expression of GFP- and mCherry-tagged RabERAB11, respectively, under the control of the alcAp. These plasmids carry a nonfunctional mutant argB* allele targeting integration to the argB locus on chromosome III (Calcagno-Pizarelli et al., 2007). The rabE open reading frame was amplified from a complete cDNA clone (p1815 in our plasmid collection) with primers 1 and 2 (Supplementary Table S2) and cloned into the BamHI/XhoI 6.92-kb fragment of p1398. Next coding regions of GFP (from p1929; Pantazopoulou and Peñalva, 2011; primers 3 and 4) or mCherry (from p1920; Abenza et al., 2012) were inserted into the BamHI site. The constructs were used to transform MAD540 (Supplemental Table S1). Single-copy integration events at argB were identified by Southern blot (Supplemental Figure S2). Transformants MAD3069 and MAD3575 were used in subsequent genetic crosses. Using p1979 as template, we constructed a plasmid expressing mutant GDP-locked S23N-RabE (p2109), using Stratagene's site-directed mutagenesis kit with mutagenic primers 5 and 6 (Supplemental Table S2). MAD4271 carrying a single-copy integration of p2109 was selected after transformation of MAD1117.
To obtain rabE∆::pyrGAf nuclei, the open reading frame of rabE was substituted by the pyrGAf selection marker using a fragment obtained by fusion PCR (primers 15–20). The resulting linear 5′-UTRrabE::pyrGAf::3′-UTRrabE molecule was transformed into nkuAΔ::bar strain MAD1739, which carries pyrG89, resulting in pyrimidine auxotrophy. Viability could be rescued only in heterokaryosis with a rabE+ allele. The rabD∆ mutation was constructed by the same approach. rabD∆ was recovered in homokaryosis (strain MAD3584 represents one of the multiple transformants obtained carrying the deletion allele, as determined by Southern blotting).
Plasmid p2090 drives expression of GFP-RabD under the control of the alcA promoter. The plasmid carries a truncated, nonfunctional pyroA* allele targeting integration to chromosome IV pyroA4 locus after selecting for pyridoxine auxotrophy (Calcagno-Pizarelli et al., 2007). For the construction of p2090, the GFP coding region was amplified from p1979 with primers 3 and 21 and cloned as a BamHI/EcoRI-digested fragment into p1920. Next the coding region of rabD was amplified from a cDNA (p1783) clone with primers 22 and 23 and inserted as an EcoRI/XmaI fragment. p2090 was used to transform MAD1741. MAD4033 is a single-copy transformant.
Microscopy and quantitative analyses
We used a Leica DMI6000B inverted fluorescence microscope coupled to a Hamamatsu ORCA ERII camera with a Dual View beam splitter and the HCX 63×/1.4 numerical aperture objective. The microscope was driven by MetaMorph (Molecular Devices). Microscopy was in all cases made in vivo, using Lab-Tek chambers (Nalge Nunc International, Rochester, NY). Culture temperature control, other aspects of the microscopy setup, and the methodology for the “on-stage” temperature shift experiments have been described in detail (Pinar et al., 2013a). For the quantitation of the GFP-RabERAB11 signal at the SPK we used sum projections derived from Z-stacks of images acquired under the same conditions to calculate the overall signal in equivalent circular regions of interest. BFA, benomyl, and latB were used at 200, 5–10, and 40 μg/ml, respectively.
For multidimensional sequences (x, y, z, four to six planes every 0.35 μm; t, 3- to 4-s intervals; w, two channels acquired simultaneously with a Dual View beam splitter) were deconvolved with the CMLE algorithm of Huygens Professional (www.svi.nl). Maximal-intensity projections of the two channels were aligned with MetaMorph and used to measure the integrated fluorescence intensity of a circular region containing a cisterna, whose position was manually adjusted for each time point to correct short-range movement. Measurements were carried out in time sequences that covered the complete maturation cycle. These values were normalized and plotted against time. Graphs corresponding to different maturation events were aligned relative to the time points showing the maximum mRFP-PHOSBP or HypBSec7-GFP channel value, which was set as 100%. For measuring the lifetime of PHOSBP (Figure 2) or HypBSec7 (Supplemental Figure S3) LGC, we considered the time between the earliest detection of an increase in mRFP-PHOSBP fluorescence and the time at which fluorescence dropped to near background. For RabE, we determined the “residence time” associated with a LGC as the period between the frame at which GFP-RabE was first detected in an mRFP-PHOSBP LGC and the time at which the RabE-enriched membranes underwent movement. All measurements were made in hyphae maintaining apical extension.
FRAP experiments used a Leica SP5 confocal microscope and a 488-nm argon laser, using the Leica LAS FRAP wizard. Photobleaching (five cycles) with 60% of the laser power bleached the SPK GFP-RabE signal in middle hyphal tip planes to background levels. From 5 to 10 prebleaching and from 50 to 90 postbleaching frames were acquired every 2 s (4% laser power). These conditions were compatible with hyphal growth (thus with exocytosis). The integrated intensity values of the region of interest were background subtracted and normalized, setting the maximal prebleach value as 100%. Curve fitting used Prism, version 6 (GraphPad), using the one-phase association equation to calculate recovery t1/2.
Supplementary Material
Acknowledgments
We thank H. Arst for critical reading of the manuscript, A. Galindo for cDNA clones, N. Taheri-Talesh and B. Oakley for strains, E. Reoyo for technical assistance, and two anonymous referees for useful comments. Supported by Ministerio de Economía y Competitividad (Spain) Grant BIO2012-30965, Comunidad de Madrid Grant S2010/BMD-2414, National Institutes of Health Grant GM097580, and Uniformed Services University of the Health Sciences intramural funds.
Abbreviations used:
- latB
latrunculin B
- LGC
late Golgi cisterna(e)
- MT
microtubule
- SPK
Spitzenkörper
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-02-0710) on June 18, 2014.
REFERENCES
- Abenza JF, Galindo A, Pantazopoulou A, Gil C, de los Ríos V, Peñalva MA. Aspergillus RabBRab5 integrates acquisition of degradative identity with the long-distance movement of early endosomes. Mol Biol Cell. 2010;21:2756–2769. doi: 10.1091/mbc.E10-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza JF, Galindo A, Pinar M, Pantazopoulou A, de los Ríos V, Peñalva MA. Endosomal maturation by Rab conversion in Aspergillus nidulans is coupled to dynein-mediated basipetal movement. Mol Biol Cell. 2012;23:1889–1901. doi: 10.1091/mbc.E11-11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza JF, Pantazopoulou A, Rodríguez JM, Galindo A, Peñalva MA. Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic. 2009;10:57–75. doi: 10.1111/j.1600-0854.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- Ali MY, Lu H, Bookwalter CS, Warshaw DM, Trybus KM. Myosin V and Kinesin act as tethers to enhance each others’ processivity. Proc Natl Acad Sci USA. 2008;105:4691–4696. doi: 10.1073/pnas.0711531105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol. 2013;202:191–199. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Cabrera M, Ungermann C. Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases. J Biol Chem. 2013;288:28704–28712. doi: 10.1074/jbc.M113.488213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno-Pizarelli AM, et al. Establishment of the ambient pH signaling complex in Aspergillus nidulans: PalI assists plasma membrane localization of PalH. Eukaryot Cell. 2007;6:2365–2375. doi: 10.1128/EC.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Chen S, Tokarev AA, Liu F, Jedd G, Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol Biol Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Shah AH, Segev N. Ypt31/32 GTPases and their F-Box effector Rcy1 regulate ubiquitination of recycling proteins. Cell Logist. 2011;1:21–31. doi: 10.4161/cl.1.1.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutterbuck AJ. Aspergillus nidulans. In: O'Brien SJ, editor. Genetic Maps, Vol. 3: Locus Maps of Complex Genomes. 6th ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. 3.71–3.84. [Google Scholar]
- Cove DJ. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Daboussi L, Costaguta G, Payne GS. Phosphoinositide-mediated clathrin adaptor progression at the trans-Golgi network. Nat Cell Biol. 2012;14:239–248. doi: 10.1038/ncb2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KW, Bretscher A. Myosin-V is activated by binding secretory cargo and released in coordination with Rab/exocyst function. Dev Cell. 2012;23:769–781. doi: 10.1016/j.devcel.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Luini A. Models for Golgi traffic: a critical assessment. Cold Spring Harb Perspect Biol. 2011;3:a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BS, Nakano A. Membrane traffic within the Golgi apparatus. Annu Rev Cell Dev Biol. 2009;25:113–132. doi: 10.1146/annurev.cellbio.24.110707.175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SD, Read ND, Roberson RW, Shaw B, Seiler S, Plamann M, Momany M. Polarisome meets Spitzenkörper: microscopy, genetics, and genomics converge. Eukaryot Cell. 2005;4:225–229. doi: 10.1128/EC.4.2.225-229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges AR, Bookwalter CS, Krementsova EB, Trybus KM. A nonprocessive class V myosin drives cargo processively when a kinesin-related protein is a passenger. Curr Biol. 2009;19:2121–2125. doi: 10.1016/j.cub.2009.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges AR, Krementsova EB, Bookwalter CS, Fagnant PM, Sladewski TE, Trybus KM. Tropomyosin is essential for processive movement of a class V myosin from budding yeast. Curr Biol. 2012;22:1410–1416. doi: 10.1016/j.cub.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann-Marriott MF, Uchida M, van de Meene AM, Garret M, Hjelm BE, Kokoori S, Roberson RW. Application of electron tomography to fungal ultrastructure studies. New Phytol. 2006;172:208–220. doi: 10.1111/j.1469-8137.2006.01868.x. [DOI] [PubMed] [Google Scholar]
- Horio T, Oakley BR. The role of microtubules in rapid hyphal tip growth of Aspergillus nidulans. Mol Biol Cell. 2005;16:918–926. doi: 10.1091/mbc.E04-09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Sultana A, Gandhi P, Franklin E, Hamamoto S, Khan AR, Munson M, Schekman R, Weisman LS. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell. 2011;21:1156–1170. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konzack S, Rischitor PE, Enke C, Fischer R. The role of the kinesin motor KipA in microtubule organization and polarized growth of Aspergillus nidulans. Mol Biol Cell. 2005;16:497–506. doi: 10.1091/mbc.E04-02-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JH, Schuchardt I, Straube A, Steinberg G. A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J. 2006;25:2275–2286. doi: 10.1038/sj.emboj.7601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Lipatova Z, Tokarev AA, Jin Y, Mulholland J, Weisman LS, Segev N. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol Biol Cell. 2008;19:4177–4187. doi: 10.1091/mbc.E08-02-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell. 2010;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD, Segev N. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol. 2006;8:1263–1269. doi: 10.1038/ncb1489. [DOI] [PubMed] [Google Scholar]
- Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20:1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani AH, Oakley BR, Osmani SA. Identification and analysis of essential Aspergillus nidulans genes using the heterokaryon rescue technique. Nat Protoc. 2006;1:2517–2526. doi: 10.1038/nprot.2006.406. [DOI] [PubMed] [Google Scholar]
- Pantazopoulou A, Peñalva MA. Organization and dynamics of the Aspergillus nidulans Golgi during apical extension and mitosis. Mol Biol Cell. 2009;20:4335–4347. doi: 10.1091/mbc.E09-03-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulou A, Peñalva MA. Characterization of Aspergillus nidulans RabCRab6. Traffic. 2011;12:386–406. doi: 10.1111/j.1600-0854.2011.01164.x. [DOI] [PubMed] [Google Scholar]
- Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CL, Xu K, Sharpless KE, Harris SD. MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Mol Biol Cell. 2004;15:3658–3672. doi: 10.1091/mbc.E03-11-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva MA. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet Biol. 2005;42:963–975. doi: 10.1016/j.fgb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Peñalva MA, Galindo A, Abenza JF, Pinar M, Calcagno-Pizarelli AM, Arst HN, Jr, Pantazopoulou A. Searching for gold beyond mitosis: mining intracellular membrane traffic in Aspergillus nidulans. Cell Logist. 2012;2:2–14. doi: 10.4161/cl.19304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell. 2007;12:739–750. doi: 10.1016/j.devcel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Pinar M, Pantazopoulou A, Arst HN, Jr, Peñalva MA. Acute inactivation of the Aspergillus nidulans Golgi membrane fusion machinery: correlation of apical extension arrest and tip swelling with cisternal disorganization. Mol Microbiol. 2013a;89:228–248. doi: 10.1111/mmi.12280. [DOI] [PubMed] [Google Scholar]
- Pinar M, Pantazopoulou A, Peñalva MA. Live-cell imaging of Aspergillus nidulans autophagy: RAB1 dependence, Golgi independence and ER involvement. Autophagy. 2013b;9:1–20. doi: 10.4161/auto.24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck-Peterson SL, Tyska MJ, Novick PJ, Mooseker MS. The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors. J Cell Biol. 2001;153:1121–1126. doi: 10.1083/jcb.153.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena N, Alberti-Segui C, Winzenburg E, Horn C, Schliwa M, Philippsen P, Liese R, Fischer R. Genetic evidence for a microtubule-destabilizing effect of conventional kinesin and analysis of its consequences for the control of nuclear distribution in Aspergillus nidulans. Mol Microbiol. 2001;42:121–132. doi: 10.1046/j.1365-2958.2001.02609.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci USA. 2009;106:14408–14413. doi: 10.1073/pnas.0906536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell. 2011;20:47–59. doi: 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Lipowsky R, Assmann MA, Lenz P, Steinberg G. Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc Natl Acad Sci USA. 2011;108:3618–3623. doi: 10.1073/pnas.1015839108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Treitschke S, Kilaru S, Molloy J, Harmer NJ, Steinberg G. Myosin-5, kinesin-1 and myosin-17 cooperate in secretion of fungal chitin synthase. EMBO J. 2012;31:214–227. doi: 10.1038/emboj.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Chen S, Rivera-Molina F, Reinisch K, Novick P, Ferro-Novick S. Establishing a role for the GTPase Ypt1p at the late Golgi. Traffic. 2010;11:520–532. doi: 10.1111/j.1600-0854.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless KE, Harris SD. Functional characterization and localization of the Aspergillus nidulans formin SEPA. Mol Biol Cell. 2002;13:469–479. doi: 10.1091/mbc.01-07-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y, Kurokawa K, Hirata R, Nakano A. Rab GAP cascade regulates dynamics of Ypt6 in the Golgi traffic. Proc Natl Acad Sci USA. 2013;110:18976–18981. doi: 10.1073/pnas.1308627110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc. 2006;1:3111–3120. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- Taheri-Talesh N, Horio T, Araujo-Bazán LD, X, Espeso EA, Peñalva MA, Osmani SA, Oakley BR. The tip growth apparatus of Aspergillus nidulans. Mol Biol Cell. 2008;19:1439–1449. doi: 10.1091/mbc.E07-05-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri-Talesh N, Xiong Y, Oakley BR. The functions of myosin II and myosin V homologs in tip growth and septation in Aspergillus nidulans. PLoS One. 2012;7:e31218. doi: 10.1371/journal.pone.0031218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburn J, Scazzocchio C, Taylor GG, Zabicky-Zissman JH, Lockington RA, Davies RW. Transformation by integration in Aspergillus nidulans. Gene. 1983;26:205–211. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Torralba S, Raudaskoski M, Pedregosa AM, Laborda F. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology. 1998;144:45–53. doi: 10.1099/00221287-144-1-45. [DOI] [PubMed] [Google Scholar]
- Virag A, Lee MP, Si H, Harris SD. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol Microbiol. 2007;66:1579–1596. doi: 10.1111/j.1365-2958.2007.06021.x. [DOI] [PubMed] [Google Scholar]
- Wooding S, Pelham HRB. The dynamics of Golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JA., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, El Ganiny AM, Bray GE, Sanders DAR, Kaminskyj SGW. Aspergillus nidulans hypB encodes a Sec7-domain protein important for hyphal morphogenesis. Fungal Genet Biol. 2008;45:749–759. doi: 10.1016/j.fgb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li S, Fischer R, Xiang X. Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent. Mol Biol Cell. 2003;14:1479–1488. doi: 10.1091/mbc.E02-08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan K, Wu X, Chen G, Sun J, Reck-Peterson SL, Hammer JA, 3rd, Xiang X. Aspergillus myosin-V supports polarized growth in the absence of microtubule-based transport. PLoS One. 2011;6:e28575. doi: 10.1371/journal.pone.0028575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.