Abstract
Purpose.
We evaluated the impact of glaucoma-related vision loss on reading ability and reading engagement in 10 reading activities.
Methods.
A total of 63 glaucoma patients and 59 glaucoma suspect controls self-rated their level of reading difficulty for 10 reading items, and responses were analyzed using Rasch analysis to determine reading ability. Reading engagement was assessed by asking subjects to report the number of days per week they engaged in each reading activity. Reading restriction was determined as a decrement in engagement.
Results.
Glaucoma subjects more often described greater reading difficulty than controls for all tasks except puzzles (P < 0.05). The most difficult reading tasks involved puzzles, books, and finances, while the least difficult reading tasks involved notes, bills, and mail. In multivariable weighted least squares regression models of Rasch-estimated person measures of reading ability, less reading ability was found for glaucoma patients compared to controls (β = −1.60 logits, P < 0.001). Among glaucoma patients, less reading ability was associated with more severe visual field (VF) loss (β = −0.68 logits per 5-dB decrement in better-eye VF mean deviation [MD], P < 0.001) and contrast sensitivity (β = −0.76 logits per 0.1-unit lower log CS, P < 0.001). Each 5-dB decrement in the better-eye VF MD was associated with book reading on 18% fewer days (P = 0.003) and newspaper reading on 10% fewer days (P = 0.008). No statistically significant reading restriction was observed for other reading activities (P > 0.05).
Conclusions.
Glaucoma patients have less reading ability and engage less in a variety of different reading activities, particularly those requiring sustained reading. Future work should evaluate the mechanisms underlying reading disability in glaucoma to determine how patients can maintain reading ability and engagement.
Keywords: glaucoma, reading, quality of life, low vision
Glaucoma and visual field loss are associated with less reading ability and engagement for a variety of reading tasks.
Introduction
Patients with low vision most often identify reading difficulty as their greatest vision-related concern and primary reason for seeking low vision services.1 Because reading is necessary for performing many daily tasks and achieving a number of common societal objectives (i.e., daily living, social interaction, recreation, and work), reading difficulty can cause substantial disability (defined as reduced ability compared to the norm to perform reading tasks that are important to an individual), and can be detrimental to quality of life (QOL).2–5
Previous studies that have evaluated reading in subjects with visual impairment almost exclusively have attributed reading disability to visual acuity (VA) loss due to refractive error, cataract, or macular disease.6–8 Therefore, there exists a common assumption (backed by some evidence) that reading is not affected in diseases of peripheral vision loss, such as glaucoma, especially when VA is normal.9 However, a few recent studies have suggested that glaucoma is associated with greater self-reported reading difficulty, and a recent paper has demonstrated modestly lower reading speeds in patients with glaucoma.4,5,10–13
These prior studies of reading in persons with glaucoma, however, were limited in their capacity to assess reading disability for a few reasons. First, most studies have been concerned more broadly with the impact of vision loss on overall QOL rather than specific effects on reading. Therefore, such works have lacked the proper instrumentation to evaluate reading alone. For example, the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25), which was developed by Mangione et al.11 to study the influence of vision impairment on QOL, contains only one item related to reading. Additionally, prior studies have used questions measured on a Likert-scale, which is flawed in its approach to measuring latent variables, such as reading ability.14 Finally, prior studies have not ventured to determine whether engagement in reading is impacted by glaucomatous vision loss.
Characterizing reading ability and reading engagement in patients with glaucoma would help us better understand how glaucoma disables individuals with the disease and better determine the best methods for enabling reading in patients with glaucoma. We sought to test the hypothesis that reading ability and engagement are less with more severe glaucomatous vision loss. Reading ability was judged by a subject's assessment of reading difficulty (i.e., the perceived difference between one's reading ability [a latent person trait] and the reading ability required to perform a certain reading task) using Rasch analysis.
Methods
The study protocol adhered to the tenets set forth by the Declaration of Helsinki, and all study procedures were approved by the Institutional Review Board of Johns Hopkins Medicine. All subjects gave written informed consent before all study procedures, which were completed between July 2009 and April 2011.
Study Subjects
The study sample consisted of subjects recruited from a convenience sample based on consecutive recruitment from the glaucoma clinic at the Wilmer Eye Institute at Johns Hopkins Hospital. Subjects were recruited for a broader study involving tests of reading speed as was previously published.13
Subjects for the control group were included in the study if they were at least 50 years old, had a chart diagnosis of glaucoma suspect or ocular hypertension, had a presenting Early Treatment Diabetic Retinopathy Study (ETDRS) VA of 20/40 or better in both eyes, and had right and left eye visual fields (VF) meeting the following criteria: mean deviation (MD) better than −3 dB in at least one eye and better than −4 dB in both eyes on a Swedish interactive thresholding algorithm (SITA) standard Humphrey 24-2 visual field test, and a glaucoma hemifield test (GHT) result of “within normal limits,” “borderline,” or “general reduction of sensitivity.”
Subjects for the glaucoma group were included in the study if they were at least 50 years old, had a chart diagnosis of primary open angle glaucoma, primary angle closure glaucoma, pseudoexfoliation glaucoma, or pigment dispersion glaucoma, had a presenting VA of 20/40 or better in at least one eye, and had right and left eye VFs meeting the following criteria: MD worse than −3 dB in the better-eye and a GHT result of “outside normal limits,” “generalized reduction of sensitivity,” or “borderline.”
Potential subjects in both groups were excluded if they had a VA worse than 20/40 in the better-seeing eye or had vision loss secondary to another eye condition, were illiterate by self-report, or had any laser procedure in the previous week or any ocular surgery in the previous 2 weeks. Based on prior research demonstrating that better-eye and integrated VF MD rarely differ and do not predict visual disability differently, VF severity was defined as the higher (less negative) MD between the 2 eyes taken from SITA standard 24-2 fields.15 All enrolled subjects participated in the same testing measures that are described below as well as tests of reading speed, which were analyzed in a separate study.13
Measurement of Reading Ability
Reading ability and reading engagement were assessed using a questionnaire administered orally to subjects during an in-person interview containing 10 different reading items: magazines, newspaper articles, bills, financial statements, handheld menus, religious texts, books, word puzzles, typed mail, and written notes or mail. The 10 reading items used in our questionnaire were taken from the Activity Inventory (AI), a visual function questionnaire developed and validated by Massof et al.16 to measure visual ability in patients with low vision. The 10 AI reading tasks were chosen to provide enough breadth and variety to cover the varying levels of reading ability among our subjects. For each activity, subjects first were asked how important the activity was to them. The importance response categories were: “not important,” “somewhat important,” “moderately important,” and “very important.” If the subject responded “not important,” then the interviewer moved on to the next task. If the task was rated with any other importance category, then the subject was asked to rate the difficulty of that reading task without the assistance of another person. The difficulty response categories were: “not difficult,” “slightly difficult,” “moderately difficult,” “very difficult,” “extremely difficult,” and “impossible.” Based on a previous Rasch analysis of the six rating categories used by a large sample of low vision patients,16 we merged two categories and assigned rank scores to the response categories as follows: 3, “not difficult”; 2, “slightly or moderately difficult”; 1, “very or extremely difficult”; and 0, “impossible.” When participants were asked to rate the difficulty of these reading tasks, they were assigning a rating to the magnitude of the difference between their reading ability and the ability level required by the task. Therefore, reading ability is a function of the difficulty ratings assigned to a series of different reading tasks, which was estimated by the Rasch analytical model.
Reading engagement was defined as the rate at which participants engaged in a particular reading activity in an average week. To evaluate reading engagement, subjects were asked to state how many days out of a typical week they perform each of the 10 reading activities that they deemed to be at least slightly important.
Measurement of Vision and Covariates
The VA was measured under binocular conditions with charts developed for the ETDRS.17 The VA was scored as the total number of letters read correctly and converted to the logMAR according to the method described previously.18 The VF MD was measured with the SITA standard 24-2 VF testing performed on a Humphrey Field Analyzer 2 (Carl Zeiss Meditec, Inc., Dublin, CA, USA). Contrast sensitivity (CS) was measured under binocular conditions as the number of letters read correctly on the Pelli-Robson chart and converted to a log scale (logCS).19 Lenticular changes, including nuclear sclerotic, cortical, and posterior subcapsular changes, as well as posterior capsular opacification (PCO), were graded as present or absent as described previously.9
Since several nonvisual age-related factors may decrease a person's ability to read with increasing age, such as decreased cognitive ability and depression,20–22 cognitive ability and depressive symptoms were measured in our subjects along with other general health-related variables. Cognitive ability was evaluated using the Mini-Mental State Exam (MMSE).23 The presence of depressive symptoms was assessed using part D of the General Health Questionnaire by Goldberg et al.,24 with a positive response to any question taken to indicate the presence of depressive symptoms. Sociodemographic variables, including age, race, education, and employment, were gathered using standardized questionnaires.
Statistical Methods and Programming
Group differences in demographic, health, and vision characteristics were analyzed using the Student's t-test for normally-distributed continuous variables and χ2 test for categorical variables.
Statistical Analyses of Reading Difficulty.
Rasch analysis was performed using Winsteps (Winsteps, Chicago, IL, USA) to estimate linear item measures for each reading task and linear person measures for each subject based upon their responses to the individual reading difficulty questions. Person measures were taken to be a measure of overall reading ability. Item measures and person measures were expressed in logits along the same scale. The zero value of the scale was defined as the mean of the item measures. Higher item measures corresponded to greater reading difficulty for the item. Higher person measures (indicating greater reading ability) corresponded to being able to perform more difficult reading tasks without difficulty. The item measures were anchored to item measures determined from a separate, larger cohort of glaucoma subjects seen at low vision clinics (n = 212) who were asked the same questions about reading difficulty.25 Analysis of differential item function (DIF) with the Mantel-Haenszel method was used to investigate each item for signs of dependencies on patient sample characteristics.26 We used χ2-type fit statistics to test the measurement validity of the observed item responses by evaluating the fit of the data to the expectations of the Rasch normative measurement model. The distributions of infit mean squares (the mean square residuals divided by the average expected variance of the responses) for the reading items were transformed to normal distributions with expected values of zero and unit standard deviations (SD).27
Unadjusted differences between mean control and glaucoma subjects' person measures was analyzed using the Welch-Satterthwaite equation,28 and the effect size was determined with Glass's Δ, which normalizes the magnitude of the difference to the SD of the control group.29 Normality of the distribution of person measures was verified by converting the cumulative frequencies of person measures to z-scores and confirming linearity.
Factors influencing the likelihood of reading disability with a certain reading task were evaluated in separate weighted least squares regression models for controls and glaucoma subjects using the Rasch-derived person measures (in logits) as the dependent variable, adjusting for age, sex, race, educational level, cognitive ability, and depressive symptoms. Since there is greater uncertainty at the high and low extremes of person measure, weighted least squares regression was used to effectively minimize the contribution of these subjects' measures to the regression. Each subject's person measure was weighted by the inverse of the squared standard error of the estimate (i.e., Fisher information) to correct for the heteroscedasticity introduced by the person measure estimates at the high or low extremes.
Statistical Analyses of Reading Engagement.
The effect of greater VF loss on reading restriction (defined as the decreased frequency of engagement in certain reading tasks) was analyzed using a negative binomial regression model controlling for age, sex, race, educational level, and cognitive ability. Outcomes were expressed as a rate ratio (RR), reflecting the ratio of days in which the reading task would be performed in two subjects with a 5-dB difference in their better-eye VF MD.
Analyses were performed using STATA 12 (STATA, College Station, TX, USA). Covariates were included in multivariable weighted least squares regression and negative binomial regression models if they were statistically significantly (P < 0.05) associated with the outcome of interest in univariable models.
Results
Description of Patients
A total of 59 glaucoma suspect controls and 63 glaucoma subjects completed all study procedures. Glaucoma subjects were older than controls (71.6 vs. 67.0 years, P < 0.01), but did not differ with regard to demographic or general health-related variables (Table 1). Compared to controls, glaucoma subjects had greater VF loss (better-eye MD −8.9 vs. +0.2 dB, P < 0.001), worse VA (binocular logMAR of 0.09 vs. 0.00, P < 0.001), and worse CS (logCS = 1.67 vs. 1.93, P < 0.001). The frequency of significant cataract or PCO did not differ by glaucoma status.
Table 1.
Characteristics of Subjects Completing Questionnaires by Glaucoma Status
|
Glaucoma Suspect Controls,
n
= 59 |
Glaucoma,
n
= 63 |
P
Value |
|
| Demographics | |||
| Age, y | 67.0 (8.5) | 71.6 (9.2) | <0.01 |
| Male sex, % | 37 | 42 | 0.58 |
| African-American race, % | 19 | 20 | 0.82 |
| Education, y | 15.4 (2.1) | 15.2 (2.4) | 0.59 |
| Employed, % | 47 | 42 | 0.56 |
| Health | |||
| MMSE score | 27.7 (1.5) | 27.4 (1.4) | 0.21 |
| Depressive symptoms, % | 4 | 5 | 0.81 |
| Vision | |||
| Visual field MD, better-eye | 0.2 (1.0) | −8.9 (6.8) | <0.001 |
| Binocular acuity, logMAR | 0.00 (0.11) | 0.09 (0.11) | <0.001 |
| Binocular log CS | 1.93 (0.13) | 1.67 (0.19) | <0.001 |
| Sig. cataract/PCO either eye, % | 4 | 7 | 0.41 |
Values shown for continuous variables reflect means with SD shown in parentheses. Sig., significant.
Self-Reported Difficulty With Specific Reading Tasks
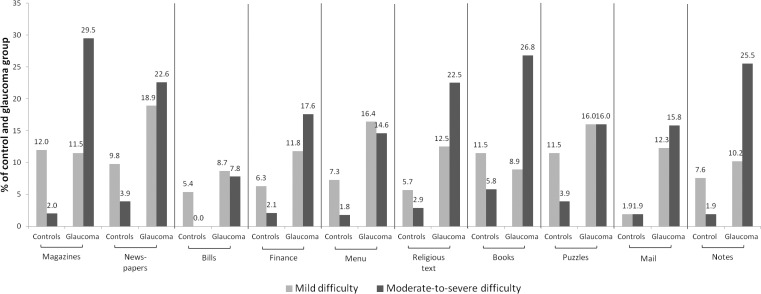
The percentage of glaucoma patients describing at least mild reading difficulty ranged from 16.5% to 41.5% across the spectrum of reading tasks. For each reading task, more glaucoma subjects described moderate-to-severe difficulty compared to controls (Fig. 1).
Figure 1.
Percentage of glaucoma and control subjects reporting difficulty for each of 10 different reading tasks. Responses were partitioned into three categories of difficulty: no difficulty (not shown), mild difficulty, and moderate-to-severe difficulty.
Estimation of Overall Reading Ability Using a Rasch Measurement Model
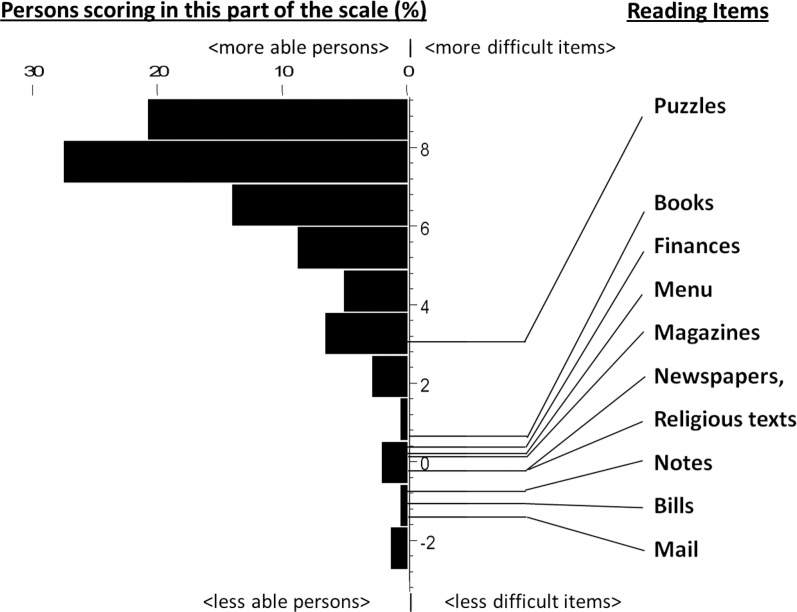
The item measures associated with the tasks and the distribution of person measures relative to these item measures are shown in Figure 2. Item and person measure separation reliabilities were 0.88 and 0.78, respectively, indicating that 88% and 78% of the variance in item and person measures were attributable to true differences in the items or people, and not estimation error. The DIF analysis showed that each of the items functions similarly for glaucoma subjects and control subjects, with no statistically significant differences in the way any of the items functioned except for religious texts (P > 0.1 for all items except religious texts). In determining the validity of applying the Rasch model, infit mean square residuals for each item measure were calculated, and they all fell within ± 2 SDs of the expected value, except for puzzles (>3 SDs). The misfit of puzzles to the model may be due to the infrequency with which subjects deemed puzzles to be important or due to individual variations in the interpretation of the term, “puzzles.”
Figure 2.
Distribution of reading ability from Rasch-estimated item measure and person measure scores (logits) mapped onto the same scale. Higher person measure scores indicate less reading ability in the individual. Higher item measure scores indicate less difficulty associated with the particular task. Item measure scores are found at the midpoint of the transition point from no difficulty to severe difficulty.
Predictors of Overall Reading Disability Based on Rasch Outcomes
Subjects with glaucoma and control subjects differed in their distribution of overall reading disability as defined by their person measures (Fig. 3, Glass's Δ = 1.13, P < 0.001).
Figure 3.
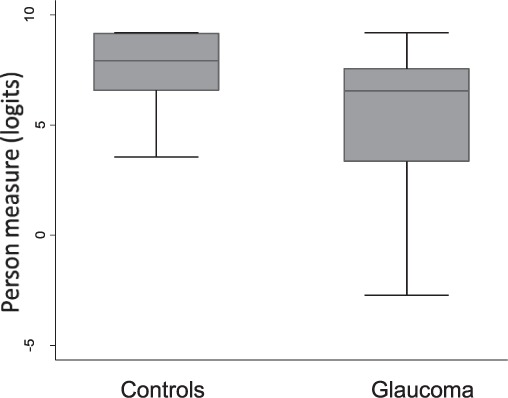
Box-whiskers plots of Rasch-estimated person measure score (logits) of control and glaucoma subjects, showing the 25th and 75th percentile range (box) and median values (transverse lines in the box).
In a weighted least squares regression model, subjects with glaucoma had significantly lower reading ability than control subjects (β = −1.60 logits, 95% CI = −2.54 to −0.66, P < 0.001, Table 2). In weighted least squares regression models of only glaucoma subjects, lower reading ability is associated with greater VF loss (β = −0.68 logits per 5-dB decrement in the better-eye VF MD, 95% CI = −1.03 to −0.33, P < 0.001) and lower CS (β = −0.48 logits per 0.1-unit lower binocular log CS, 95% CI = −0.76 to −0.20, P < 0.001). Worse VA was associated with less reading ability, but this association was not statistically significant (β = −0.17 logits per 0.1-logMAR increment, 95% CI = −0.59–0.24, P = 0.41). In multivariable models, African American subjects were less likely to have less reading ability compared to non-African American subjects, while subjects with lower educational attainment or depressive symptoms were more likely to have less reading ability. The presence of a visually-significant cataract or PCO in either eye was not a significant predictor of reading ability in multivariable models. No variable (visual or nonvisual) demonstrated a significant association with reading ability in models only containing control subjects.
Table 2.
Predictors of Reading Disability, Weighted Least Squares Regression Model
|
Variable |
Interval |
Δ Person Measure Score in Logits* (95% CI) |
P
Value |
| Visual† | |||
| Glaucoma | vs. no glaucoma | −1.60 (−2.54, −0.66) | <0.001 |
| VF loss MD, better eye | 5 dB worse | −0.68 (−1.03, −0.33) | <0.001 |
| Binocular log CS | 0.1 log units worse | −0.48 (−0.76, −0.20) | <0.001 |
| VA, binocular | 0.1 logMAR worse | −0.17 (−0.59, 0.24) | 0.41 |
| Nonvisual‡ | |||
| Age | 5 y older | 0.10 (−0.11, 0.30) | 0.36 |
| Male | vs. female | −0.83 (−1.68, 0.03) | 0.06 |
| African-American | vs. not African-American | 1.34 (0.39, 2.29) | <0.01 |
| Education | 4 y less | −0.89 (−1.53, −0.26) | <0.01 |
| MMSE score | 5 points lower | 1.22 (−0.19, 2.64) | 0.09 |
| Depressive symptoms | vs. no depressive symptoms | −8.29 (−10.07, −6.51) | <0.001 |
Person measure scores are derived from the Rasch analytic model. Higher scores indicate greater ability. Therefore, factors associated with a negative change in score are associated with greater reading disability.
The impact of visual metrics are from separate models in which only 1 visual metric was included along with all nonvisual metrics shown.
The impact of the metric is taken from a single model including 5 dB worse MD and all nonvisual metrics shown.
Self-Reported Engagement With Specific Reading Tasks
After controlling for age, sex, race, cognition, and education level, each 5-dB decrement in better-eye VF MD was associated with reading books on 18% fewer days (CI = 6%–28%, P = 0.003) and newspapers on 10% fewer days (CI = 3%–17%, P = 0.008, Fig. 4). Significant reading restriction with greater VF loss was not noted for any other reading tasks (P > 0.05 for all except books and newspapers).
Figure 4.
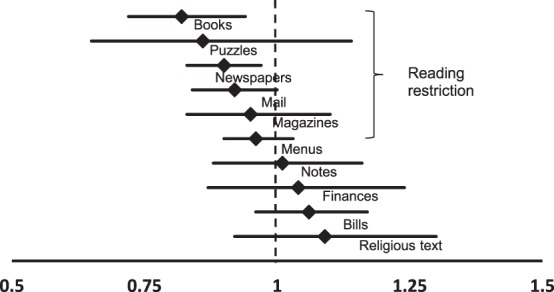
Task-specific rate ratios of reading engagement for each 5-dB decrement in the better-eye VF MD with horizontal lines indicating 95% CI. Statistically significant reading restriction was noted for books and newspapers (P < 0.05).
Discussion
Having glaucoma makes reading more difficult across a broad range of reading tasks, and overall reading disability (as judged by Rasch-estimated reading ability person measures) is significantly affected by glaucoma. Patients with glaucoma also are more likely to avoid reading, with a trend showing that there is greater restriction of tasks involving sustained reading, such as books and newspapers. These findings are based upon a 10-item instrument, which was judged to be valid and reliable based upon high person and item measure reliability indices, the absence of significant DIF for 9 of 10 tasks, and the absence of misfitting items (with the exception of puzzles). Novel features of this study include the use of a Rasch measurement model for ordering task difficulty, the use of the Rasch model to measure overall reading ability, and the assessment of reading engagement in glaucoma. Central VA was well-preserved in the studied cohort, further reinforcing the concept that glaucomatous VF loss and CS loss can impair reading even in the presence of relatively normal VA.
Our findings support prior studies of reading disability in subjects with glaucoma which, through performance testing, have demonstrated that reading function is poorer in subjects with glaucoma. For example, Altangerel et al.12 tested the performance of glaucoma patients on a series of vision-related activities and identified reading small print as one of the tasks causing greatest difficulty among glaucoma patients. Similarly, using performance-based measures, Richman et al.30 found a significant correlation between binocular VF loss, CS, and VA, and reading in reduced illumination. Recent work has demonstrated that glaucoma is associated with decreased reading speed, particularly when reading is evaluated through sustained silent reading (as opposed to short-duration out-loud reading) and when individuals are asked to read low-contrast materials.13 While performance-based measures, such as those in prior works, can be made precisely and accurately, they typically are measured in tightly-controlled conditions, which may not fully reflect an individual patient's native environment.31,32 Additionally, performance-based testing does not elicit information about how well a respondent thinks he or she reads, which is information that more directly exemplifies the functional consequences of visual impairments. Therefore, we believe that self-rating of difficulty importantly assesses the individual's perception of functionality, which is a critical component of quality of life.
The findings of this current study also support the conclusions made from prior studies assessing reading disability using questionnaires or focus groups, which have found that over 40% of patients with glaucoma report problems with reading small print.4,10 However, these works evaluated reading only with a small number of questions imbedded within a larger questionnaire, and they did not have a proper instrument to measure disability properly in glaucoma subjects compared to control subjects. The present study added rigor to this previous work by using a validated Rasch analytical method to determine that overall reading disability is associated with glaucomatous vision loss. Our work also provided new evidence that different types of reading tasks can be affected differently by glaucoma.
Reading disability and reading restriction in patients with glaucoma may result from a number of mechanisms, including aberrant eye movements from VF defects, poorer detection of low contrast stimuli, poor acuity reserve, or inadequate lighting conditions. Data indicate that greater VF loss is associated with difficulty in finding the next line of text.33 Fewer saccades have been noted among patients with VF defects during various tasks, raising the possibility that abnormal eye movements may mediate reading impairment in glaucoma.34–37 Decreased CS also may mediate the impact of glaucoma on reading. Indeed, recent work found that patients with glaucoma are more challenged by decreased letter contrast than normally-sighted individuals.38 Glaucoma patients have worse distance- and near-vision, CS, and CS with glare when measured at home versus in the clinic, suggesting that CS in the native environment may be sufficiently low as to impair reading.39 Glaucoma patients may experience reading fatigue as a result of the exertion needed to overcome the above-mentioned visual and environmental factors, and low vision services should seek to address these factors to facilitate prolonged reading.
While reading is a common complaint among glaucoma patients,1 only a small percentage of glaucoma patients are referred to rehabilitative services. One barrier to referrals may be the fact that physicians may not view glaucoma patients as requiring visual rehabilitation services, as they most often refer patients with central vision deficits. An additional barrier to referral may be that glaucoma patients often do not express severe reading difficulty to the extent that reading would be impossible. Finally, rehabilitative services, including efforts to enable reading, are tailored primarily to serve patients with central vision loss and not those with VF loss.40 Additional work is necessary to define the best methods for enabling reading in patients with glaucoma, perhaps by creating proper lighting to optimize contrast and reduce glare, correcting aberrant eye movements, using visual aids to enlarge text, and/or teaching strategies to mitigate fatigue.39,41–43
One limitation of our study is that a large number of patients were at or near the maximum possible ability measure that could be estimated from our reading items because they responded “not difficult” to all of the queried tasks. We addressed this limitation by using weighted logistic regression to reduce the contributions of those who were at the extreme end of the scale, though future studies should consider adding more difficult items to extend the scale and more precisely estimate person measures scores in these individuals. Obtaining a greater number of subjects in future studies would be beneficial to resolve more precisely the magnitude of the association between VF loss and CS on reading disability, given the large confidence intervals (CI) surrounding those measures obtained in our study. While our study was able to comment on reading engagement, questioning subjects about the number of days they perform a task does not allow us to know precisely how much time is spent performing a certain task. To quantify reading engagement more accurately, future studies may take advantage of increasingly popular electronic-based reading technology to record time spent performing certain reading tasks more accurately. Finally, while reading disability and reading restriction were considered as independent outcomes here, further work is needed to determine whether they are indeed independent, or whether both decline together as vision declines.
In summary, individuals with glaucoma experience greater reading disability than individuals with normal vision, as evidenced by the greater levels of reading difficulty and restriction of specific reading tasks. Sustained reading tasks may be affected particularly by glaucoma, but further studies will be needed to confirm these findings. Given the importance of reading to performing numerous daily tasks and meeting many societal objectives, further work is necessary to define methods to optimize reading in this group of patients.
Acknowledgments
Supported by National Institutes of Health (NIH; Bethesda, MD, USA) Grants EY018595 and EY022976, Pradeep Y. Ramulu (Financial Support), and Research to Prevent Blindness.
Disclosure: A.M. Nguyen, None; S.W. van Landingham, None; R.W. Massof, None; G.S. Rubin, None; P.Y. Ramulu, None
References
- 1. Brown JC, Goldstein JE, Chan TL, Massof R, Ramulu PY. the Low Vision Research Network Study Group. Description of functional complaints in patients seeking outpatient low vision services in the United States [published online ahead of print April 23, 2014]. Ophthalmology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008; 49: 1907–1915 [DOI] [PubMed] [Google Scholar]
- 3. Sewo Sampaio PY, Ito E. Activities with higher influence on quality of life in older adults in Japan. Occup Ther Int. 2012; 20: 1–10 [DOI] [PubMed] [Google Scholar]
- 4. Mangione CM, Berry S, Spritzer, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998; 116: 227–233 [DOI] [PubMed] [Google Scholar]
- 5. Burr JM, Kilonzo M, Vale L, Ryan M. Developing a preference-based Glaucoma Utility Index using a discrete choice experiment. Optom Vis Sci. 2007; 84: E797–E809 [DOI] [PubMed] [Google Scholar]
- 6. Legge GE, Ross JA, Isenberg LM, Lamay JM. Psychophysics of reading. Clinical predictors of low-vision reading speed. Invest Ophthalmol Vis Sci. 1992; 33: 677–687 [PubMed] [Google Scholar]
- 7. Whittaker SG, Lovie-Kitchin JAN. Visual requirements for reading. Optom Vis Sci. 1993; 70: 54–65 [DOI] [PubMed] [Google Scholar]
- 8. Fine SL, Berger JW, Maguire MG, Ho AC. Age-related macular degeneration. N Engl J Med. 2000; 342: 483–492 [DOI] [PubMed] [Google Scholar]
- 9. Ramulu PY, West SK, Munoz B, Jampel HD, Friedman DS. Glaucoma and reading speed: the Salisbury Eye Evaluation project. Arch Ophthalmol. 2009; 127: 82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson P, Aspinall P, O'Brien C. Patients' perception of visual impairment in glaucoma: a pilot study. Br J Ophthalmol. 1999; 83: 546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001; 119: 1050–1058 [DOI] [PubMed] [Google Scholar]
- 12. Altangerel U, Spaeth GL, Steinmann WC. Assessment of Function Related to Vision (AFREV). Ophthalmic Epidemiol. 2006; 13: 67–80 [DOI] [PubMed] [Google Scholar]
- 13. Ramulu PY, Swenor BK, Jefferys JL, Friedman DS, Rubin GS. Difficulty with out-loud and silent reading in glaucoma. Invest Ophthalmol Vis Sci. 2013; 54: 666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massof RW. The measurement of vision disability. Optom Vis Sci. 2002; 79: 516–52 [DOI] [PubMed] [Google Scholar]
- 15. Arora KS, Boland MV, Friedman DS, Jefferys JL, West SK, Ramulu PY. The relationship between better-eye and integrated visual field mean deviation and visual disability. Ophthalmology. 2013; 120: 2476–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massof RW, Ahmadian L, Grover LL, et al. The Activity Inventory (AI): an adaptive visual function questionnaire. Optom Vis Sci. 2007; 84: 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferris FL III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982; 94: 91–96 [PubMed] [Google Scholar]
- 18. Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991; 32: 422–443 [PubMed] [Google Scholar]
- 19. Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clin Vision Sci. 1991; 6: 471–475 [Google Scholar]
- 20. Weiss BD, Reed RL, Kligman EW. Literacy skills and communication methods of low-income older persons. Patient Educ Couns. 1995; 25: 109–119 [DOI] [PubMed] [Google Scholar]
- 21. Blazer DG. Depression in the elderly. N Engl J Med. 1989; 320: 164–166 [DOI] [PubMed] [Google Scholar]
- 22. Lewinsohn PM, MacPhillamy DJ. The relationship between age and engagement in pleasant activities. J Gerontol. 1974; 29: 290–294 [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. ”Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189–198 [DOI] [PubMed] [Google Scholar]
- 24. Goldberg DP, Hillier VF. A scaled version of the general health questionnaire. Psychol Med. 1979; 9: 139–145 [DOI] [PubMed] [Google Scholar]
- 25. Goldstein JE, Chun MW, Fletcher DC, Deremeik JT, Massof RW. Low Vision Research Network Study Group. Visual ability of patients seeking outpatient low vision services in the United States [published online ahead of print June 19, 2014]. JAMA Ophthalmol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959; 2: 719–748 [PubMed] [Google Scholar]
- 27. Wright B, Masters GN. Rating Scale Analysis: Rasch Measurement. Chicago, IL: Mesa Press; 1982. [Google Scholar]
- 28. Ruxton GD. The unequal variance t-test is an underused alternative to Student's t-test and the Mann–Whitney U test. Behav Ecol. 2006; 17: 688–690 [Google Scholar]
- 29. McGaw B, Glass GV. Choice of the metric for effect size in meta-analysis. Am Educ Res J. 1980; 17: 325–337 [Google Scholar]
- 30. Richman J, Lorenzana LL, Lankaranian D, et al. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol. 2010; 128: 1576–1582 [DOI] [PubMed] [Google Scholar]
- 31. Bhorade AM, Perlmutter MS, Wilson B, et al. Differences in vision between clinic and home and the effect of lighting in older adults with and without glaucoma. JAMA Ophthalmol. 2013; 131: 1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman SM, Munoz B, Rubin GS, West SK, Bandeen-Roche K, Fried LP. Characteristics of discrepancies between self-reported visual function and measured reading speed. Salisbury Eye Evaluation Project Team. Invest Ophthalmol Vis Sci. 1999; 40: 858–864 [PubMed] [Google Scholar]
- 33. Viswanathan AC, McNaught AI, Poinoosawmy D, et al. Severity and stability of glaucoma: patient perception compared with objective measurement. Arch Ophthalmol. 1999; 117: 450–454 [DOI] [PubMed] [Google Scholar]
- 34. Crabb DP, Smith ND, Rauscher FG, et al. Exploring eye movements in patients with glaucoma when viewing a driving scene. PLoS One. 2010; 5: e9710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith ND, Crabb DP, Garway-Heath DF. An exploratory study of visual search performance in glaucoma. Ophthalmic Physiol Opt. 2011a; 31: 225–232 [DOI] [PubMed] [Google Scholar]
- 36. Smith ND, Crabb DP, Glen FC, Burton R, Garway-Heath DF. Eye movements in patients with glaucoma when viewing images of everyday scenes. Seeing Perceiving. 2011b; 25: 471–492 [DOI] [PubMed] [Google Scholar]
- 37. Smith ND, Glen FC, Crabb DP. Eye movements during visual search in patients with glaucoma. BMC Ophthalmol. 2012; 12: 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burton R, Crabb DP, Smith ND, Glen FC, Garway-Heath DF. Glaucoma and reading: exploring the effects of contrast lowering of text. Optom Vis Sci. 2012; 89: 1282–1287 [DOI] [PubMed] [Google Scholar]
- 39. Bowers AR, Meek C, Illumination Stewart N. and reading performance in age-related macular degeneration. Clin Exp Optom. 2001; 84: 139–147 [DOI] [PubMed] [Google Scholar]
- 40. Owsley C, McGwin G Jr, Lee PP, Wasserman N, Searcey K. Characteristics of low-vision rehabilitation services in the United States. Arch Ophthalmol. 2009; 127: 681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seiple W, Szlyk JP, McMahon T, Pulido J, Fishman GA. Eye-movement training for reading in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005; 46: 2886–2896 [DOI] [PubMed] [Google Scholar]
- 42. Kerkhoff G, Münßinger U, Haaf E, Eberle-Strauss G, Stögerer E. Rehabilitation of homonymous scotomata in patients with postgeniculate damage of the visual system: saccadic compensation training. Restor Neurol Neurosci. 1992; 4: 245–254 [DOI] [PubMed] [Google Scholar]
- 43. Virgili G, Acosta R, Grover LL, Bhentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013; 10:CD003303 [DOI] [PMC free article] [PubMed] [Google Scholar]