Abstract
Recent ecological research has revealed that environmental factors can strongly affect insect immunity and influence the outcome of host–parasite interactions. To date, however, most studies examining immune function in mosquitoes have ignored environmental variability. We argue that one such environmental variable, temperature, influences both vector immunity and the parasite itself. As temperatures in the field can vary greatly from the ambient temperature in the laboratory, it will be essential to take temperature into account when studying vector immunology.
Over the past 20 years, our understanding of the physiological and molecular interactions between parasites, such as Plasmodium spp. and dengue virus, and their mosquito vectors1–3 has been radically transformed. Research has provided important insights into insect innate immunity4, identifying a multitude of immunity genes and immune system pathways that influence resistance to vector-borne parasites, and has also revealed potential targets for genetic manipulation5–7. However, this body of work is incomplete. In particular, mosquito resistance to infection tends to be viewed as a static phenotype consisting solely of standard immune responses measured under a narrow set of laboratory conditions1,8. But vectors and parasites associate in a variable world. From work in other invertebrate–parasite systems, we expect that mosquito susceptibility to vector-borne parasites depends not only on genetic factors, but also on a wide range of biotic and abiotic factors. To complete our understanding of immunity and resistance, we need to consider this environmental variation.
In this Opinion article, we argue that variation in ambient temperature markedly shapes mosquito immunity and, in turn, resistance to vector-borne pathogens and parasites. Although many aspects of the environment might be important in shaping vector resistance (for example, abiotic factors such as humidity9 and day length10, and biotic factors such as larval11 or adult nutrition12 and larval competition13), here we focus primarily on environmental temperature for four main reasons. First, although most mosquito insectaries are set at 26–28 °C, malaria parasites can be transmitted across a temperature range of 16–35 °C, and transmission of other vector-borne pathogens such as dengue virus, chikungunya virus and filarial nematodes occurs across a similar temperature range. Second, mosquitoes are small-bodied, ectothermic insects. Many studies have already demonstrated that temperature can markedly affect diverse aspects of mosquito physiology and ecology, including the rate and viability of egg hatching14, the rate of development15–17, the propensity to feed and the rate of feeding18, and survival15. It would be surprising if immune function were any different. Third, research on a wide range of invertebrates demonstrates that small, realistic changes in ambient temperature can significantly shape overall host resistance to a range of parasites by influencing, for example, parasite virulence, development rates, latency periods and clearance rates within the insect (see Supplementary information S1 (table)). Fourth, numerous studies have already found that the development rates of key vector-borne parasites are strongly temperature dependent; this has been shown for dengue virus19, West Nile virus20, yellow fever virus21, chikungunya virus22 and filarial nematodes23,24, as well as human25, rodent26,27 and avian28–30 malaria parasites. The extent to which temperature shapes vector resistance directly, through such effects on parasite biology, or indirectly, through effects on vector immunity, remains largely unexplored.
Below, we outline what is known about how temperature shapes insect immunity and physiology, and discuss how ambient temperature affects parasite fitness. We maintain that manipulating the environmental temperature is a tractable first step in incorporating important ecological realism into standard laboratory studies that characterize the vector–parasite interaction. A broader appreciation of the thermal ecology of vector–parasite interactions could have far reaching implications for understanding natural heterogeneity in mosquito resistance, modelling the transmission of vector-borne disease, predicting transmission ecology in a changing climate and evaluating the efficacy of novel control tools involving genetic manipulation of mosquito physiology.
Temperature and immunity
The insect immune system consists of both cellular and humoral responses that interact to control the spread of an infection31. These responses are tightly regulated and coordinated through signal transduction cascades that transmit the non-self recognition signal downstream. The Toll, immune deficiency (Imd) and the lesser studied janus kinase–signal transducer and activator of transcription (JAK–STAT) pathways are three characteristic insect immune pathways that respond to a suite of parasitic challenges32–34 (BOX 1). The end point of these signal cascades is typically the release of a transcription factor that triggers, for example, cell death or cell division, or the deployment of effector molecules such as antimicrobial peptides32,35 (BOX 2 provides an illustrative example for mosquitoes and malaria, which represents the best described vector–parasite system). Changes in body temperature owing to variation in ambient temperature will significantly shift the metabolic rates of both insect vectors and the parasites that they host36,37. The consequences for various immune processes and for the resistance phenotype have not been extensively examined.
Box 1. Standard insect immune pathways.
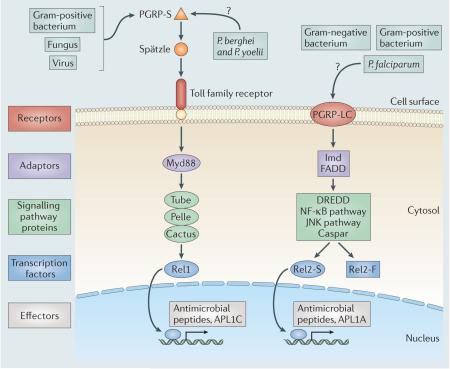
Different parasites and pathogens have antigens that are recognized as non-self by receptors on host cell surfaces, either directly or through intermediates (for example, Spätzle) (see the figure)31. This signal is transmitted into the interior of the cell through a cascade of adaptor and signalling proteins31. The Toll pathway is stimulated when a soluble peptidoglycan recognition protein (PGRP-S) binds either peptidoglycan, or an unknown ligand from rodent-infecting Plasmodium spp. such as Plasmodium berghei and Plasmodium yoelii, triggering a signal cascade that causes the ligand Spätzle to bind with the Toll family transmembrane receptor55. The immune deficiency (Imd) pathway is stimulated when the transmembrane protein PGRP-LC binds peptidoglycan or a Plasmodium falciparum ligand35,55. Both of these intracellular cascades, in turn, trigger the activation of transcription factors that pass through the nuclear membrane and initiate the upregulation of immunity genes and the synthesis of effector molecules that result in microbial killing31. In the mosquito, the Toll and Imd pathways end when the transcription factors Rel1 and Rel2-S, respectively, translocate into the nucleus and stimulate the expression of genes encoding antimicrobial peptides and genes involved in antimalarial defences (for example, the APL1 family in the Plasmodium resistance island)1,55.
Box 2. Mosquito–malaria parasite interactions.
After a mosquito feeds on a vertebrate host infected with malaria parasites (Plasmodium spp.), gametocytes of all Plasmodium spp. enter first the anterior midgut and then the posterior midgut of the mosquito with the blood meal. Gametocytes become gametes shortly after entering the midgut, and male gametes fertilize female gametes to form zygotes58. Under standard laboratory conditions, zygotes become ookinetes within the first 12–24 hours post-infection and traverse the peritrophic matrix and midgut epithelium to form oocysts under the basal lamina58. Oocysts mature and rupture at approximately 14 days post-infection (under standard laboratory conditions), releasing sporozoites into the haemolymph; these sporozoites then migrate to the salivary glands58.
Ookinetes can be lysed inside the cytoplasm of the midgut epithelial cells45,103–105 or extracellularly in the basal lamina through the action of the mosquito compliment-like effector molecule TEP1 (REFS 33,45,56). In some strains of the mosquito vector Anopheles gambiae, the effector molecule phenoloxidase is released from circulating haemocytes and mediates the melanization of early oocysts that are established in the midgut epithelium106,107. Early oocysts can also be killed by nitric oxide produced by midgut epithelial cells, circulating haemocytes and potentially the fat body108.
In addition to these malaria parasite-specific responses, the mosquito can defend itself from invading bacteria and other pathogens via its complement system and through phagocytosis of invading bacteria by circulating haemocytes (granulocytes); both of these processes require the opsonin TEP1 (REFS 108–110). Finally, antimicrobial peptides are produced systemically by fat body cells and secreted into the haemolymph, and are also produced locally by barrier epithelial cells31.
It is clear that vector resistance is multifaceted and depends on many interacting traits of both the parasite (for example, growth rate, virulence and persistence strategies) and the vector (for example, immune pathways, stress responses and vector survival). Each trait potentially has a thermal performance curve. Furthermore, temperature can have an impact on parasite traits directly, indirectly (by affecting host defence mechanisms) or, most likely, a combination of both.
Effects of temperature on invertebrates in general
Invertebrates have been used widely by researchers in the field of ecological immunology to investigate the effects of environmental variation on the immune response and host fitness. Several studies have shown that temperature affects invertebrate immune traits. For example, following challenge with lipopolysaccharide (a bacterial product that activates immune responses), the larvae of the beetle Tenebrio molitor exhibit increases in phenoloxidase activity, antibacterial responses and metabolic rates, as well as weight loss, when maintained at a high temperature (30 °C) compared with when the larvae are maintained at lower temperatures of 10 °C or 20 °C38.
Similarly, compared with crickets (Gryllus texensis) grown at their preferred temperature, those that are exposed to a simulated heat wave (5 °C above preferred temperature) for 6 days have increased fitness (increased egg laying, egg development and mass gain), phenoloxidase activity and levels of lysozyme-like enzymes, and exhibit resistance to the Gram-negative bacterium Serratia marcescens39. Interestingly, the resistance and fitness of crickets infected with the Gram-positive bacterium Bacillus cereus is actually lower at temperatures that deviate from the average field temperature (26 °C), suggesting a complex interplay between temperature and resistance to different pathogens39. By contrast, fruitflies (Drosophila melanogaster) reared at cooler than normal temperatures (17 °C) have enhanced survival when infected with either Gram-positive or Gram-negative bacteria (Lactococcus lactis or Pseudomonas aeruginosa, respectively) and increased expression of a suite of immunity genes compared with fruitflies housed at standard and warmer rearing temperatures (25 °C and 29 °C, respectively)40. This boost in immune performance at cooler temperatures might seem counterintuitive, but heat shock proteins (in particular, HSP83) might boost immune function at cooler temperatures in this species36. To date, only one study reports that temperature can act in combination with other aspects of environmental variation, such as food availability and density of conspecifics, to affect phenoloxidase activity and total haemocyte counts41.
Finally, there is evidence that being kept at fluctuating temperatures can have an effect on immunity. Butterflies (Lycaena tityrus) exposed to temperatures fluctuating around cooler (17.7 °C) and warmer (23.7 °C) than normal temperatures experience significantly different development times, phenoloxidase activity, responses to hot and cold stressors, and total haemocyte numbers compared with butterflies housed at a constant 17.7 °C or 23.7 °C mean temperature42.
Effects of temperature on insect vectors
For insect vectors, we are aware of only two studies that specifically explore the effects of temperature on mosquito immunity43,44. The first study investigated how larval nutrition, adult body condition and ambient temperature affect the melanization response (an enzymatic cascade that results in encapsulation of parasites and pathogens with melanin) of adult Anopheles gambiae following challenge with Sephadex beads, and showed that melanization decreases progressively as temperature increases from 24 °C to 30 °C43.
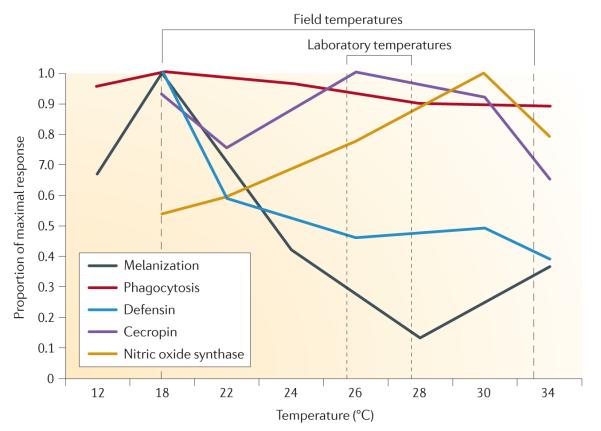
The second study measured how the performance of a suite of cellular and humoral immune responses varies in Anopheles stephensi across five different constant temperatures ranging from 12 °C to 34 °C44 (FIG. 1). Temperature was shown to significantly affect all immune responses measured, but in different ways. Unexpectedly, melanization, phagocytosis and expression of the antimicrobial peptide defensin were all highest at 18 °C (8 °C below the standard rearing temperature for this mosquito). Expression of nitric oxide synthase (a key enzyme involved in defence against a multitude of parasites and pathogens, including malaria parasites) peaked at 30 °C, whereas expression of the antimicrobial peptide cecropin was not directly affected by temperature44. Interestingly, the temporal dynamics of many of the immune responses studied changed depending on both the ambient temperature and the type of immune challenge administered. Thus, the immune profile described across the course of an infection at one temperature would potentially be completely different if the same experiment were run at a different temperature44.
Figure 1. Environmental temperature profoundly affects the rates of a range of humoral and cellular immune responses.
Rates of immune and humoral responses are represented as the mean proportion of the maximal response. Melanization and phagocytosis were measured as the proportion of Sephadex beads that were fully melanized or the proportion of haemocytes that had engulfed fluorescent beads, respectively, in mosquitoes housed at different constant temperatures. Expression levels (cDNA counts) of genes encoding two antimicrobial peptides, defensin and cecropin, and nitric oxide synthase are also shown for mosquitoes housed at different constant temperatures. These results clearly indicate that the immune phenotype described under laboratory conditions (26–28 °C) is not the same as the phenotype expressed across the range of possible field temperatures for parasite transmission (18–34 °C), and current laboratory studies on mosquito innate immune functions are missing potentially important biological complexity. Figure is modified from REF. 44.
Temperature and resistance
The overall effect of temperature on the ability of an insect to resist infection depends on not only the effects on elements of insect immune function and physiology, but also direct effects of temperature on the parasite itself. For example, recent research has shown that the effectiveness of mosquito immune responses against malaria parasites depends in part on the kinetics of host enzymes involved in the nitration and lysis of midgut cells infected with Plasmodium spp. parasites, and in part on the rate of parasite migration through the midgut epithelium45. Such host and parasite processes are not necessarily equally affected by temperature; the net effect of temperature on vector resistance and parasite fitness will depend on the relative thermal sensitivities of both host and parasite traits (BOX 2).
Temperature affects parasite physiology
Numerous studies have demonstrated the effects of temperature on diverse aspects of the host–parasite interaction, including host resistance, the latency period, the rate of parasite development, and parasite transmission in non-vector insect systems (see Supplementary information S1 (table)). For example, ambient temperature greatly shapes resistance of the crustacean Daphnia magna to infection with the bacterium Pasteuria ramosa46. When the host is housed under standard laboratory conditions (20–25 °C), the bacterium is highly virulent; by contrast, when the host is housed at lower temperatures (10–15 °C), it is much more resistant to infection. These lower temperatures, which are not optimal for the bacterium, represent thermal conditions that are commonly experienced by D. magna in the wild, a factor that might explain why this crustacean has not evolved resistance mechanisms against P. ramosa in the field46.
Similarly, a study on the resistance of pea aphids to a pathogenic fungus of insects revealed that temperature significantly influences the susceptibility of aphid clones to infection. Further, how susceptibility changed with ambient temperature varied among clones. Two of the tested clones became more susceptible at optimal temperatures for vegetative growth of the fungus in vitro, whereas two other clones deviated considerably from this expected response, becoming less susceptible to infection at these temperatures. This effect, again, has implications for the pattern of co-evolutionary dynamics that is played out in the field47. Both of the studies discussed above clearly demonstrate that traits which are strongly influenced by genetics (that is, resistance and susceptibility) can be greatly influenced by environmental variability.
Temperature and the physiology of vector-borne parasites
With respect to vector-borne parasites, research on the direct effects of temperature has been limited, and the primary focus has been on the effect of temperature on development rates within the insect vector. This has been studied for malaria parasites of rodents26,48, birds28–30, lizards49 and humans25,50,51; West Nile virus20; dengue virus19; and yellow fever virus21. In general, most of these studies show that warming temperatures are associated with a reduction in the development time of the parasite or pathogen. For example, the extrinsic incubation period of West Nile virus (strain NY99) in the mosquito Culex tarsalis decreases as ambient temperature warms from 10 °C to 30 °C52. Few studies have focused on how temperature shapes other aspects of parasite life history.
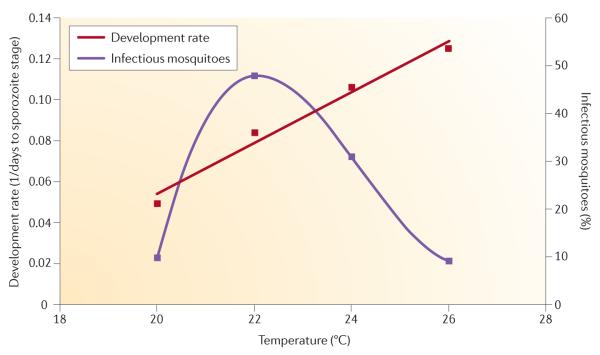
One recent study53 examined the effects of temperature on the development rate and survival of a malaria parasite of rodents in the vector A. stephensi. In line with previous work, this study showed that parasite development rates increase linearly with increasing temperatures over a 20–26 °C temperature range, suggesting that parasite transmission potential should increase with warming temperatures. However, the proportion of mosquitoes that actually became infectious (that is, had sporozoites in the salivary glands) followed a nonlinear response, being highest at around 22 °C and substantially declining at warmer temperatures (FIG. 2). This pattern might be due to the effects of warming temperatures on vector physiology — such as increased heart rate and circulation (resulting in sporozoites circulating past the salivary glands too quickly to penetrate) and boosted immune defences (for example, the production of nitric oxide) — or to direct negative effects of temperature on parasite survival (a development–survival trade-off). Whatever the mechanism, the result challenges the general assumption that ‘vector competence’ (the probability of a vector becoming infectious following an infectious blood meal) is temperature independent53, and shows that small changes in ambient temperature can profoundly affect the force of infection (the rate at which susceptible individuals become infected). Furthermore, it demonstrates that temperature can have complex effects on different components of the vector–parasite interaction54.
Figure 2. Changes in ambient temperature differentially affect two parasite traits.
The proportion of infectious mosquitoes (that is, the proportion with sporozoites in their salivary glands) can be used as a measurement of parasite survival, and parasite development rate is measured as the reciprocal of the number of days taken to reach the sporozoite stage. Although parasites develop to the sporozoite stage at faster rates with increasing ambient temperature, the proportion of infectious mosquitoes sharply declines as the ambient temperature rises above 22 °C. The data shown are for Plasmodium yoelii infecting Anopheles stephensi. Figure is modified from REF. 53.
Implications
The complex effects of temperature on vector immune function have potentially far reaching implications. How and to what extent mosquitoes (species and populations) differ in their resistance to parasites is central to understanding natural variation in disease dynamics. Recent research indicates that Anopheles mosquitoes have the ability to finely discriminate between infection by Plasmodium falciparum, a malaria parasite of humans, and Plasmodium berghei, a malaria parasite of rodents, through the APL1 family of leucine-rich-repeat proteins, the encoding genes of which are located in a cluster of natural-resistance loci55. Specifically, APL1A is downstream of the Imd pathway, which recognizes P. falciparum, whereas APL1C is downstream of the Toll pathway, which recognizes P. berghei. These contrasting responses have been attributed to the different evolutionary histories of the mosquito–parasite associations55,56. However, these parasites have markedly different thermal sensitivities. Temperature differences in experimental conditions could themselves contribute to the differential expression of APL1C and APL1A, as well as to the variability in infection intensities among different Plasmodium spp.57,58. Although the development rates of P. falciparum and P. berghei are similar at their respective temperature optima50,59, the changes in mosquito physiology (rates of immune enzyme activity and the timing of Imd pathway activation60) that are seen as temperature increases from 20 °C to 27 °C, as well as the differences in the infection intensities associated with each parasite species60, could lead to differences in relative parasite growth rates.
At a more applied level, several of the current and prospective control tools being considered for use within integrated vector management (IVM) strategies61,62 could be affected by temperature. For example, temperature-dependent toxicity has been demonstrated for a wide range of chemicals targeting a variety of insect pests (for example, blattaria (cockroaches)63,64, lepidopterans (butterflies and moths)65,66, dipterans (true flies)67,68 and coleopterans (beetles and weevils)65,69). Although little work has been carried out to examine the effects of temperature on insecticide toxicity for disease vectors, some research suggests that the susceptibility of mosquito malaria vectors to pyrethroids, the most important class of insecticide in contemporary vector control, is affected by temperature70. Whether the temperature sensitivity of pyrethroid toxicity is due to changes in the expression or activity of detoxifying enzymes71,72 or perhaps to alterations in nerve sensitivity71 remains unknown.
Looking further forwards, it would be surprising if many of the novel transgenic technologies that are currently being developed in vectors to reduce parasite transmission were not sensitive to temperature changes. Such technologies include the transgenic upregulation of antimicrobial peptides73, the induction of Imd pathway transcription factors74 and the expression of other factors such as SM1 and haemolytic C-type lectins75,76. SM1 is an effector protein that is secreted into the mosquito midgut after a blood meal and binds to the surface of the midgut and salivary glands, interfering with Plasmodium spp. invasion75, whereas haemolytic C-type lectins lyse erythrocytes in the blood meal and inhibit the formation of Plasmodium spp. ookinetes76. As is the case for natural immunity, we anticipate that ambient temperature will have an impact on the effectiveness of these transgenic technologies, leading to variation in efficacy across space and time.
Moreover, the establishment and spread of transgenes through mosquito populations will be severely constrained if transgenic manipulation imposes any fitness costs to the vector. Two recent studies, which were carried out under standard insectary conditions, suggest that transgenic manipulation imposes minimal fitness costs to the mosquito vector74,77. However, physiologically mediated trade-offs between survival, reproduction and immunity often manifest under variable or stressful conditions (for example, heterogeneity in temperature and diet)78 and might therefore be easily missed in optimal laboratory environments. It has been suggested that transient74,79, rather than constitutive79, upregulation of effector molecules using transgenic technologies will impose minimal fitness costs. However, even transient changes in immunity can have long-term physiological consequences (as seen with immune priming80,81), and more work is needed to definitively determine the fitness costs and benefits of these technologies.
Similarly, the success of new vector control tools involving the transinfection of mosquitoes with Wolbachia spp. could also be affected by temperature. In natural insect systems, variation in ambient temperature has been shown to significantly influence Wolbachia spp. transmission82,83, dissemination84,85 and cytoplasmic incompatibility86,87, as well as their ability to shorten the life of their host88. Thus, the desirable transmission-blocking properties of vector infection with Wolbachia spp. (such as parasite interference and shortneing of the vector lifespan) that have so far been quantified under largely uniform laboratory conditions might vary considerably in vector populations distributed across thermally variable environments. It should be noted that the possible net outcomes could be very diverse, as they depend on interactions between the mosquito host, the parasite, Wolbachia spp. and the environment, all of which could respond in different ways to changes in ambient temperature.
Understanding the effects of temperature on parasite and vector fitness is also extremely relevant for modelling the transmission of vector-borne diseases and for predicting the consequences of future climate change. The risk of vector-borne disease transmission is frequently modelled using the ‘vectorial capacity’, which is a composite metric providing a measure of transmission intensity and is similar to the basic reproductive number, R0. All the mosquito and parasite life history traits that constitute the vectorial capacity are temperature sensitive, and, consequently, vector-borne diseases such as malaria are expected to be highly responsive to increases in temperature that occur as a result of climate change. However, these traits do not exhibit identical temperature profiles (FIG. 2), so warming temperatures might not necessarily correlate with an increase in malaria risk. More fundamentally, although numerous modelling studies (including some excellent recent work informing contemporary control strategies89,90) integrate aspects of the effects of environmental temperature, none includes the possible effects of temperature on vector resistance, which could be responsible for much of the variation in vector competence over time and space. Modelling work quantifying the spatial and temporal variation in the susceptibility of locusts and grasshoppers to fungal insect pathogens used in biological control provides an interesting illustration of the practical value of understanding temperature-dependent resistance and virulence91,92.
Conclusions and perspectives
The resistance phenotype, comprising both vector immunity and the fitness of the vector-borne pathogen, is shaped by variations in ambient temperature. The conventional approach of studying vector innate immunity under a narrow set of laboratory conditions overlooks important biological complexity and is unlikely to adequately explain the variation in natural or transgenic resistance of vectors to parasites under field conditions. To move forwards, we need to begin framing our mechanistic understanding of vector immune systems in the ecologically variable world in which vectors and parasites associate.
Further work is needed to establish whether vector immune profiles quantitatively and qualitatively change across different mean temperatures and, if so, to determine the implications for vector resistance. We know of only one study that has explicitly measured the performance of a suite of immune responses across a range of constant temperatures in a vector system44. A first basic step should be to integrate relevant environmental variability, such as differences in ambient temperature, into laboratory experiments to evaluate whether vector immune function is sensitive or robust to ecological change. Even just systematically exploring the effects of a range of constant temperatures on vector resistance and on a wider suite of immune parameters, rather than studying vectors at the standard 26 °C or 27 °C, would be informative.
There is now strong evidence that, in addition to the mean temperature condition, the daily temperature fluctuation can be extremely important for a wide variety of ectotherm traits93–96. It will therefore be important to establish how fluctuations in daily temperature shape vector immune defences and resistance. Previous work48 has shown that diurnal temperature fluctuations can greatly shape the fitness of Plasmodium chabaudi (a malaria parasite of rodents) in the mosquito vector A. stephensi, and that these effects vary depending on the extent of the fluctuation and the baseline mean temperature. Given the dynamic nature of immune responses across a thermal gradient, it is likely that short-term variation in temperature is an extremely important determinant shaping the immune phenotype and mosquito resistance.
Genetic variation in immune defence mechanisms and vector resistance to infection might also be maintained through adaptation to geographically different environments. For example, D. melanogaster genotypes sampled from tropical Africa have significantly lower resistance to bacterial infection (by Providencia rettgeri) at a low constant temperature (18 °C) than at a high constant temperature (28 °C), suggesting that these fruitflies have undergone adaptation to warmer temperatures. However, in the North American fruitfly populations, there is no evidence for local adaptation to different thermal environments97. In a recent study, Raffel et al.98 demonstrated that thermal acclimation is important for resistance of the Cuban treefrog, Osteopilus septentrionalis, to the chytrid fungus Batrachochytrium dendrobatidis: unpredictable drops in ambient temperature slow down thermal acclimation and increase fungus-induced mortality. The extent to which mosquitoes or parasites become hot or cold adapted and what factors affect this remain largely unexplored.
One further challenge is to distinguish between the direct effects of temperature on parasite fitness and the indirect effects that are mediated through changes in vector physiology. One way to tease apart these effects is to compare parasite development in vitro with development in vivo across a variable thermal regime. For example, one study measured growth of a pathogenic bacterium both in vitro on nutritive plates and in vivo in D. melanogaster, and demonstrated that the increased resistance of D. melanogaster to bacterial infection at cooler temperatures is due to both slower bacterial growth and enhanced immune performance40. Similarly, studies on locusts and grasshoppers have shown that warmer body temperatures limit fungal infection owing to both direct negative effects of high temperatures on fungal development and enhanced host immune function99.
A final step is to validate insights from the laboratory through the study of vector immune function, competence and fitness in field settings. For example, the elucidation of immune mechanisms through gene expression data should, when possible, be validated with measures of functional resistance. Changes in ambient temperature might also affect post-transcriptional processes, which in turn could alter the downstream products of mRNAs so that the levels or activities of these products do not correlate with the gene expression data. Comparative experiments assessing the thermal dependence of innate immune parameters, resistance and fitness for a suite of vector populations sampled from different geographical regions and microclimates would be invaluable in extending our understanding of the heterogeneities in natural refractoriness, and of how a changing climate and landscape might mediate the susceptibility of insect vectors30,97.
As indicated previously, there are many sources of environmental variation (for example, diurnal and seasonal fluctuations in humidity100, as well as variation in day length10, pollution, altitude101 and the extent of vegetation cover102, and variation between indoor and outdoor environments101) that might interact with ambient temperature to mediate the range of actual temperatures experienced by an insect vector in nature. Predicting how these environmental variables combine to mechanistically influence the suite of factors involved in resistance is clearly a complex problem. That said, not all environmental parameters will have equal effects on vector fitness, physiology and resistance; evidence14–18 (Supplementary information S1 (table)) suggests that temperature is a strong driver, whereas other variables can have little or no effect. Unravelling patterns and mechanisms from the complexity of natural systems requires an interdisciplinary perspective drawing on insights from ecology, immunology and molecular biology. Adding environmental variability to an experimental system inevitably scales up the intricacies of that experimental work. But ignoring ecological realities does not make them go away.
Supplementary Material
Acknowledgements
The authors thank members of the Thomas, Read and Julian F. Hillyer laboratory groups for discussion, and D. Kroczynski and J. Teeple for insectary support. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institute of General Medical Sciences, the US National Institute of Allergy and Infectious Diseases or the US National Institutes of Health (NIH). Work in the authors’ laboratories is funded, in part, by a grant from the US Pennsylvania Department of Health using Tobacco Settlement Funds. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Work in the authors’ laboratories was also funded by the following: the US National Science Foundation (NSF)–NIH Ecology of Infectious Diseases programme (grant EF-0914384) and the NIH R21 programme (grant AI096036-01).
Footnotes
Competing interests statement The authors declare no competing financial interests.
FURTHER INFORMATION Courtney C. Murdock and Matthew B. Thomas’s homepage: http://www.thethomaslab.net
SUPPLEMENTARY INFORMATION See online article: S1 (table)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Courtney C. Murdock, Merkle Laboratory, Center for Infectious Disease Dynamics, Department of Entomology, Pennsylvania State University, Orchard Road, University Park, State College, Pennsylvania 16802, USA.
Krijn P. Paaijmans, Merkle Laboratory, Center for Infectious Disease Dynamics, Department of Entomology, Pennsylvania State University, Orchard Road, University Park, State College, Pennsylvania 16802, USA.
Diana Cox-Foster, Agricultural Sciences and Industries Building, Center for Infectious Disease Dynamics, Department of Entomology, Pennsylvania State University, Curtin Road, University Park, State College, Pennsylvania 16802, USA..
Andrew F. Read, Millennium Science Complex, Center for Infectious Disease Dynamics, Department of Biology, Pennsylvania State University, Pollock Road, University Park, State College, Pennsylvania 16802, USA.
Matthew B. Thomas, Merkle Laboratory, Center for Infectious Disease Dynamics, Department of Entomology, Pennsylvania State University, Orchard Road, University Park, State College, Pennsylvania 16802, USA.
References
- 1.Cirimotich CM, Dong YM, Garver LS, Sim SZ, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev. Comp. Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magalhaes T, et al. Expression of defensin, cecropin, and transferrin in Aedes aegypti (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae), and the abnormal development of nematodes in the mosquito. Exp. Parasitol. 2008;120:364–371. doi: 10.1016/j.exppara.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Steinert S, Levashina EA. Intracellular immune responses of dipteran insects. Immunol. Rev. 2011;240:129–140. doi: 10.1111/j.1600-065X.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- 4.Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol. Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- 5.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Crampton JM. Approaches to vector control: new and trusted prospects for genetic manipulation of insect vectors. Trans. R. Soc. Trop. Med. Hyg. 1994;88:141–143. doi: 10.1016/0035-9203(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 7.Speranca MA, Capurro ML. Perspectives in the control of infectious diseases by transgenic mosquitoes in the post-genomic era - a review. Mem. Inst. Oswaldo Cruz. 2007;102:425–433. doi: 10.1590/s0074-02762007005000054. [DOI] [PubMed] [Google Scholar]
- 8.Jaramillo-Gutierrez G, et al. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009;9:154. doi: 10.1186/1471-2180-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson RCM. The reactions of mosquitoes to temperature and humidity. Bull. Entomol. Res. 1938;29:125–140. [Google Scholar]
- 10.Rund SSC, Hou TY, Ward SM, Collins FH, Duffield GE. Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc. Natl Acad. Sci. USA. 2011;108:E421–E430. doi: 10.1073/pnas.1100584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okech BA, Gouagna LC, Yan G, Githure JI, Beier JC. Larval habitats of Anopheles gambiae s. s. (Diptera: Culicidae) influences vector competence to Plasmodium falciparum parasites. Malaria J. 2007;6:50. doi: 10.1186/1475-2875-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okech BA, et al. Influence of sugar availability and indoor microclimate on survival of Anopheles gambiae (Diptera: Culicidae) under semifield conditions in western Kenya. J. Med. Entomol. 2003;40:657–663. doi: 10.1603/0022-2585-40.5.657. [DOI] [PubMed] [Google Scholar]
- 13.Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc. R. Soc. B. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Impoinvil DE, Cardenas GA, Gihture JI, Mbogo CM, Beier JC. Constant temperature and time period effects on Anopheles gambiae egg hatching. J. Am. Mosq. Control Assoc. 2007;23:124–130. doi: 10.2987/8756-971x(2007)23[124:ctatpe]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyimo EO, Takken W, Koella JC. Effect of rearing temperature and larval density on larval survival, age at pupation, and adult size of Anopheles gambiae. Entomol. Exp. Appl. 1992;63:265–271. [Google Scholar]
- 16.Shelton RM. Effect of temperatures on development of eight mosquito species. Mosq. News. 1973;33:1–12. [Google Scholar]
- 17.Zakharova NF, Losev GI, Yakubovich VY. The effect of density and temperature on larval populations of the malaria vector Anopheles sacharovi. Med. Parazitol. (Mosk.) 1990;1990:3–7. (in Russian) [PubMed] [Google Scholar]
- 18.Lardeux FJ, Tejerina RH, Quispe V, Chavez TK. A physiological time analysis of the duration of the gonotrophic cycle of Anopheles pseudopunctipennis and its implications for malaria transmission in Bolivia. Malaria J. 2008;7:141. doi: 10.1186/1475-2875-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambrechts L, et al. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl Acad. Sci. USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Staples JE. Incubation periods of yellow fever virus. Am. J. Trop. Med. Hyg. 2010;83:183–188. doi: 10.4269/ajtmh.2010.09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westbrook CJ, Reiskind MH, Pesko KN, Greene KE, Lounibos LP. Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to chikungunya virus. Vector Borne Zoonotic Dis. 2010;10:241–247. doi: 10.1089/vbz.2009.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devaney E, Lewis E. Temperature-induced refractoriness of Aedes aegypti mosquitoes to infection with the filaria Brugia pahangi. Med. Vet. Entomol. 1993;7:297–298. doi: 10.1111/j.1365-2915.1993.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 24.Lardeux F, Cheffort J. Temperature thresholds and statistical modelling of larval Wuchereria bancrofti (Filariidea: Onchocercidae) developmental rates. Parasitology. 1997;114:123–134. doi: 10.1017/s0031182096008359. [DOI] [PubMed] [Google Scholar]
- 25.Okech BA, et al. Resistance of early midgut stages of natural Plasmodium falciparum parasites to high temperatures in experimentally infected Anopheles gambiae (Diptera: Culicidae) J. Parasitol. 2004;90:764–768. doi: 10.1645/GE-135R1. [DOI] [PubMed] [Google Scholar]
- 26.Vanderberg JP, Yoeli M. Effects of temperature on sporogonic development of Plasmodium berghei. J. Parasitol. 1966;52:559–564. [PubMed] [Google Scholar]
- 27.Sato Y, Matsuoka H, Araki M, Ando K, Chinzei Y. Effect of temperature to Plasmodium berghei and P. yoelii on mosquito stage in Anopheles stephensi. Jpn J. Parasitol. 1996;45:98–104. [Google Scholar]
- 28.Ball GH, Chao J. Temperature stresses on mosquito phase of Plasmodium relictum. J. Parasitol. 1964;50:748–752. [PubMed] [Google Scholar]
- 29.Chao J, Ball GH. Effect of temperature on Plasmodium relictum in Culex tarsalis. J. Parasitol. 1962;49:28. [PubMed] [Google Scholar]
- 30.LaPointe DA, Goff ML, Atkinson CT. Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai’i. J. Parasitol. 2010;96:318–324. doi: 10.1645/GE-2290.1. [DOI] [PubMed] [Google Scholar]
- 31.Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. [DOI] [PubMed] [Google Scholar]
- 32.Dimopoulos G. Insect immunity and its implication in mosquito–malaria interactions. Cell. Microbiol. 2003;5:3–14. doi: 10.1046/j.1462-5822.2003.00252.x. [DOI] [PubMed] [Google Scholar]
- 33.Yassine H, Osta MA. Anopheles gambiae innate immunity. Cell. Microbiol. 2010;12:1–9. doi: 10.1111/j.1462-5822.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 34.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 35.Garver LS, Dong YM, Dimopoulos G. Casper controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chown SL, Nicolson SW. Insect Physiological Ecology: Mechanisms and Patterns. Oxford Univ. Press; 2004. [Google Scholar]
- 37.Angilletta MJ, Huey RB, Frazier MR. Thermodynamic effects on organismal performance: is hotter better? Physiol. Biochem. Zool. 2010;83:197–206. doi: 10.1086/648567. [DOI] [PubMed] [Google Scholar]
- 38.Catalan T, Wozniak A, Niemeyer HM, Kalergis AM, Bozinovic F. Interplay between thermal and immune ecology: effect of environmental temperature on insect immune response and energetic costs after an immune challenge. J. Insect Physiol. 2011;58:310–317. doi: 10.1016/j.jinsphys.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Adamo SA, Lovett MME. Some like it hot: the effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. J. Exp. Biol. 2011;214:1997–2004. doi: 10.1242/jeb.056531. [DOI] [PubMed] [Google Scholar]
- 40.Linder JE, Owers KA, Promislow DEL. The effects of temperature on host–pathogen interactions in D. melanogaster: who benefits? J. Insect Physiol. 2008;54:297–308. doi: 10.1016/j.jinsphys.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Triggs A, Knell RJ. Interactions between environmental variables determine immunity in the Indian meal moth Plodia interpunctella. J. Anim. Ecol. 2012;81:386–394. doi: 10.1111/j.1365-2656.2011.01920.x. [DOI] [PubMed] [Google Scholar]
- 42.Fischer K, Koelzow N, Hoeltje H, Karl I. Assay conditions in laboratory experiments: is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia. 2011;166:23–33. doi: 10.1007/s00442-011-1917-0. [DOI] [PubMed] [Google Scholar]
- 43.Suwanchaichinda C, Paskewitz SM. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J. Med. Entomol. 1998;35:157–161. doi: 10.1093/jmedent/35.2.157. [DOI] [PubMed] [Google Scholar]
- 44.Murdock CC, et al. Complex effects of temperature on mosquito immune function. Proc. R. Soc. B. 2012;279:3357–3366. doi: 10.1098/rspb.2012.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira G, Lieberman J, Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–859. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell SE, Rogers ES, Little TJ, Read AF. Host-parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution. 2005;59:70–80. [PubMed] [Google Scholar]
- 47.Stacey DA, et al. Genotype and temperature influences pea aphid resistance to a fungal entomopathogen. Physiol. Entomol. 2003;28:75–81. [Google Scholar]
- 48.Paaijmans KP, et al. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl Acad. Sci. USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fialho RF, Schall JJ. Thermal ecology of a malarial parasite and its insect vector: consequences for the parasites transmission success. J. Anim. Ecol. 1995;64:553–562. [Google Scholar]
- 50.Noden BH, Kent MD, Beier JC. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology. 1995;111:539–545. doi: 10.1017/s0031182000077003. [DOI] [PubMed] [Google Scholar]
- 51.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan GY. Deforestation and vectorial capacity of Anopheles gambiae giles mosquitoes in malaria transmission, Kenya. Emerg. Infect. Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J. Med. Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 53.Paaijmans KP, Blanford S, Chan BHK, Thomas MB. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol. Lett. 2012;8:465–468. doi: 10.1098/rsbl.2011.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kutz SJ, et al. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet. Parasitol. 2009;163:217–228. doi: 10.1016/j.vetpar.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Mitri C, et al. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 2009;5:e10000576. doi: 10.1371/journal.ppat.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong YM, et al. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:513–525. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poudel SS, Newman RA, Vaughan JA. Rodent Plasmodium: population dynamics of early sporogony within Anopheles stephensi mosquitoes. J. Parasitol. 2008;94:999–1008. doi: 10.1645/GE-1407.1. [DOI] [PubMed] [Google Scholar]
- 58.Vaughan JA. Population dynamics of Plasmodium sporogony. Trends Parasitol. 2007;23:63–70. doi: 10.1016/j.pt.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Rastogi M, Pal NL, Sen AB. Effect of variation in temperature on development of Plasmodium berghei (NK-65 strain) in Anopheles stephensi. Folia Parasitol. 1987;34:289–297. [PubMed] [Google Scholar]
- 60.Garver LS, et al. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS Pathog. 2012;8:e1002737. doi: 10.1371/journal.ppat.1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas MB, et al. Lessons from agriculture for the sustainable management of malaria vectors. PLoS Med. 2012;9:e1001262. doi: 10.1371/journal.pmed.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Berg H, Cham MK, Ichimori K. Handbook for Integrated Vector Management. WHO; 2012. [Google Scholar]
- 63.Vinson EB, Kearns CW. Temperature and the action of DDT on the American roach. J. Econ. Entomol. 1952;45:484–496. [Google Scholar]
- 64.Blum MS, Kearns CW. Temperature and the action of pyrethrum in the American cockroach. J. Econ. Entomol. 1956;49:862–865. [Google Scholar]
- 65.Sparks TC, Pavloff AM, Rose RL, Clower DF. Temperature-toxicity relationships of pyrethroids on Heliothis virescens (F) (Lepidoptera, Noctuidae) and Anthonomus grandis grandis Boheman (Coleoptera, Curculionidae) J. Econ. Entomol. 1983;76:243–246. [Google Scholar]
- 66.Sparks TC, Shour MH, Wellemeyer EG. Temperature-toxicity relationships of pyrethroids on three lepidopterans. J. Econ. Entomol. 1982;75:643–646. [Google Scholar]
- 67.Cutkomp LK, Subramanyam B. Toxicity of pyrethroids to Aedes aegypti larvae in relation to temperature. J. Am. Mosq. Control Assoc. 1986;2:347–349. [PubMed] [Google Scholar]
- 68.Devries DH, Georghiou GP. Influence of temperature on the toxicity of insecticides to susceptible and resistant house flies (Diptera, Muscidae) J. Econ. Entomol. 1979;72:48–50. [Google Scholar]
- 69.Watters FL, White NDG, Cote D. Effect of temperature on toxicity and persistence of three pyrethroid insecticides applied to fir plywood for the control of the red flour beetle (Coleoptera, Tenebrionidae) J. Econ. Entomol. 1983;76:11–16. [Google Scholar]
- 70.Hodjati MH, Curtis CF. Effects of permethrin at different temperatures on pyrethroid-resistant and susceptible strains of Anopheles. Med. Vet. Entomol. 1999;13:415–422. doi: 10.1046/j.1365-2915.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 71.Harwood AD, You J, Lydy MJ. Temperature as a toxicity identification evaluation tool for pyrethroid insecticides: toxicokinetic confirmation. Environ. Toxicol. Chem. 2009;28:1051–1058. doi: 10.1897/08-291.1. [DOI] [PubMed] [Google Scholar]
- 72.Miller TA, Adams ME. In: Insecticide Mode of Action. Coats JR, editor. Academic; 1982. pp. 3–27. [Google Scholar]
- 73.Kokoza V, et al. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc. Natl Acad. Sci. USA. 2010;107:8111–8116. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong Y, et al. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog. 2011;7:1–12. doi: 10.1371/journal.ppat.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li CY, Marrelli MT, Yan GY, Jacobs-Lorena M. Fitness of transgenic Anopheles stephensi mosquitoes expressing the SM1 peptide under the control of a vitellogenin promoter. J. Hered. 2008;99:275–282. doi: 10.1093/jhered/esn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida S, et al. Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development. PLoS Pathog. 2007;3:1962–1970. doi: 10.1371/journal.ppat.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isaacs AT, et al. Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathog. 2011;7:e1002017. doi: 10.1371/journal.ppat.1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferguson HM, Read AF. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18:256–261. doi: 10.1016/s1471-4922(02)02281-x. [DOI] [PubMed] [Google Scholar]
- 79.Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFκB. signaling. Aging Cell. 2006;5:533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 80.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329:1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roth O, et al. Transgenerational immune priming as cryptic parental care. J. Anim. Ecol. 2010;79:722–722. doi: 10.1111/j.1365-2656.2009.01649.x. [DOI] [PubMed] [Google Scholar]
- 82.Hurst GDD, Jiggins FM, Robinson SJW. What causes inefficient transmission of male-killing Wolbachia in Drosophila? Heredity. 2001;87:220–226. doi: 10.1046/j.1365-2540.2001.00917.x. [DOI] [PubMed] [Google Scholar]
- 83.Guruprasad NM, Mouton L, Puttaraju HP. Effect of Wolbachia infection and temperature variations on the fecundity of the Uzifly Exorista sorbillans (Diptera: Tachinidae) Symbiosis. 2011;54:151–158. [Google Scholar]
- 84.Mouton L, Henri H, Charif D, Bouletrea M, Vavre F. Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol. Lett. 2007;3:210–213. doi: 10.1098/rsbl.2006.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wiwatanaratanabutr I, Kittayapong P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J. Invertebr. Pathol. 2009;102:220–224. doi: 10.1016/j.jip.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 86.Mouton L, Henri H, Bouletreau M, Vavre F. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology. 2006;132:49–56. doi: 10.1017/S0031182005008723. [DOI] [PubMed] [Google Scholar]
- 87.Clancy DJ, Hoffmann AA. Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia-infected Drosophila simulans. Entomol. Exp. Appl. 1998;86:13–24. [Google Scholar]
- 88.Reynolds KT, Thomson LJ, Hoffmann AA. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics. 2003;164:1027–1034. doi: 10.1093/genetics/164.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gething P, et al. Modelling the global constraints of temperature on transmission of Plasmodium falciparum and P. vivax. Parasit. Vectors. 2011;4:92. doi: 10.1186/1756-3305-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith DL, Smith TA, Hay SI. In: Shrinking the Malaria Map: a Prospectus on Malaria Elimination. Feachem RGA, Phillips AA, Targett GA, editors. The Global Health Group; 2009. pp. 108–126. [Google Scholar]
- 91.Klass JI, Blanford S, Thomas MB. Use of a geographic information system to explore spatial variation in pathogen virulence and the implications for biological control of locusts and grasshoppers. Agric. Forest Entomol. 2007;9:201–208. [Google Scholar]
- 92.Klass JI, Blanford S, Thomas MB. Development of a model for evaluating the effects of environmental temperature and thermal behaviour on biological control of locusts and grasshoppers using pathogens. Agric. Forest Entomol. 2007;9:189–199. [Google Scholar]
- 93.Ratte HT. In: Environmental Physiology and Biochemistry of Insects. Hoffmann KH, editor. Springer; 1985. pp. 31–66. [Google Scholar]
- 94.Cloudsley-Thompson JL. The significance of fluctuating temperatures on the physiology and ecology of insects. Entomologist. 1953;86:183–189. [Google Scholar]
- 95.Eubank WP, Atmar JW, Ellington JJ. The significance and thermodynamics of fluctuating versus static thermal environments on Heliothis zea egg development rates. Environ. Entomol. 1973;2:491–496. [Google Scholar]
- 96.Humpesch UH. Effect of fluctuating temperature on the duration of embryonic development in two Ecdyonurus spp. and Rhithrogena cf hybrida (Ephemeroptera) from Austrian streams. Oecologia. 1982;55:285–288. doi: 10.1007/BF00376913. [DOI] [PubMed] [Google Scholar]
- 97.Lazzaro BP, Flores HA, Lorigan JG, Yourth CP. Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathog. 2008;4:e1000025. doi: 10.1371/journal.ppat.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raffel TR, et al. Disease and thermal acclimation in a more variable and unpredictable climate. Nature Clim. Change. 2012 Aug 12; (doi:10.1038/nclimate1659) [Google Scholar]
- 99.Ouedraogo RM, Cusson M, Goettel MS, Brodeur J. Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var acridum. J. Invertebr. Pathol. 2003;82:103–109. doi: 10.1016/s0022-2011(02)00185-4. [DOI] [PubMed] [Google Scholar]
- 100.Parham P, et al. Modeling the role of environmental variables on the population dynamics of the malaria vector Anopheles gambiae sensu stricto. Malaria J. 2012;11:271. doi: 10.1186/1475-2875-11-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae giles mosquitoes in malaria transmission, Kenya. Emerg. Infect. Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paaijmans KP, Thomas MB. The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malaria J. 2011;10:183. doi: 10.1186/1475-2875-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peterson TML, Gow AJ, Luckhart S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Rad. Biol. Med. 2007;42:132–142. doi: 10.1016/j.freeradbiomed.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luckhart S, Vodovotz Y, Cui LW, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl Acad. Sci. USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar S, Barillas-Mury C. Ookinete-induced midgut peroxidases detonate the time bomb in anopheline mosquitoes. Insect Biochem. Mol. Biol. 2005;35:721–727. doi: 10.1016/j.ibmb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 106.Collins FH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 107.Gorman MJ, Cornel AJ, Collins FH, Paskewitz SM. A shared genetic mechanism for melanotic encapsulation of CM-Sephadex beads and a malaria parasite, Plasmodium cynomolgi B, in the mosquito, Anopheles gambiae. Exp. Parasitol. 1996;84:380–386. doi: 10.1006/expr.1996.0126. [DOI] [PubMed] [Google Scholar]
- 108.Hillyer JF, Estevez-Lao TY. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev. Comp. Immunol. 2010;34:141–149. doi: 10.1016/j.dci.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 109.Hillyer JF, Barreau C, Vernick KD. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. Int. J. Parasitol. 2007;37:673–681. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dimopoulos G, Richman A, Muller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc. Natl Acad. Sci. USA. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.