Abstract
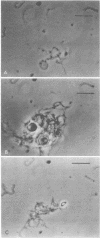
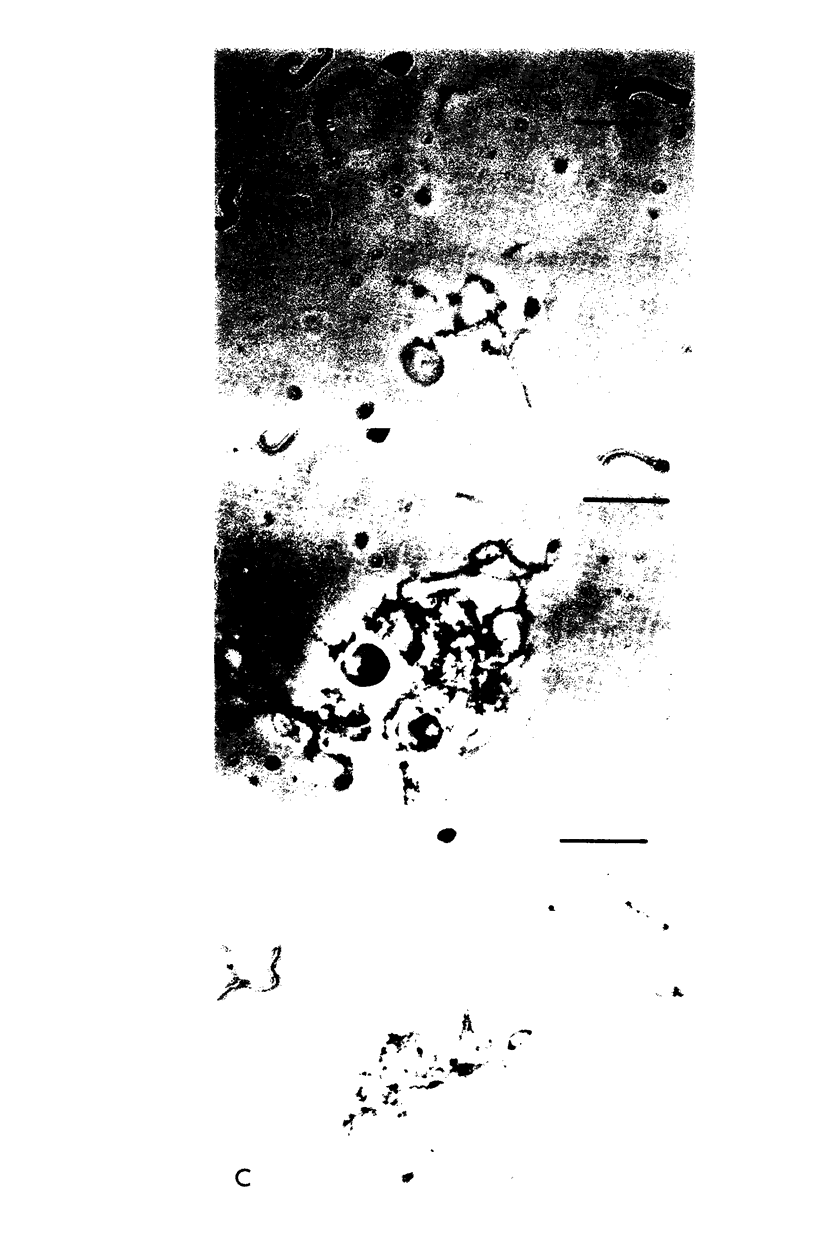
The viscous mucoid fluid that accumulates within syphilitic lesions may be due to breakdown of host tissue during infection, or may be synthesized by Treponema pallidum. Experiments were performed to investigate the acidic mucopolysaccharides that occur at the surface of T. pallidum (Nichols strain). These mucopolysaccharides were demonstrated by reaction with acidified bovine serum albumin and by agglutination with wheat germ agglutinin and soybean agglutinin. The polycations ruthenium red and toluidine blue influenced treponemal survival. Concentrations of both compounds at 200 μg/ml inhibited survival, whereas concentrations at 0.1μg/ml enhanced survival. The mucopolysaccharide concentration within the mucoid fluid that accumulates during intratesticular infection was determined by reaction with acidified bovine serum albumin; it ranged from 10,000 μg/ml to less than 8 μg/ml. The addition of this mucoid fluid to treponemal suspensions resulted in differing effects on T. pallidum survival. Some preparations were inhibitory, and others were stimulatory. Commercial preparations of hyaluronic acid and chondroitin sulfate at 400, 200, 100, and 50 μg/ml were detrimental to treponemal survival. The organisms exhibited pronounced clumping in the presence of the higher concentrations of hyaluronic acid. These clumps of treponemes were comprised of mucopolysaccharides as shown by acidified bovine serum albumin and toluidine blue reactions and by hyaluronidase degradation. Results are discussed in terms of the derivation and potential role of acidic mucopolysaccharides at the surface of T. pallidum.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELANGER L. F., HARTNETT A. Persistent toluidine blue metachromasia. J Histochem Cytochem. 1960 Jan;8:75–75. doi: 10.1177/8.1.75. [DOI] [PubMed] [Google Scholar]
- BELANGER L. F., MIGICOVSKY B. B. A comparison between different mucopolysacchwride stains as applied to chick epiphyseal cartilage. J Histochem Cytochem. 1961 Jan;9:73–78. doi: 10.1177/9.1.73. [DOI] [PubMed] [Google Scholar]
- BLUMENKRANTZ N. Microtest for mucopolysaccharides by means of toluidine blue: with special reference to hyaluronic acid. Clin Chem. 1957 Dec;3(6):696–702. [PubMed] [Google Scholar]
- CHRISTIANSEN S. Protective layer covering pathogenic treponemata. Lancet. 1963 Feb 23;1(7278):423–425. doi: 10.1016/s0140-6736(63)92309-2. [DOI] [PubMed] [Google Scholar]
- DeLAMATER E. D., SAURINO V. R., URBACH F. Studies on the immunology of spirochetoses. I. Effect of cortisone and experimental spirochetosis. Am J Syph Gonorrhea Vener Dis. 1952 Mar;36(2):127–139. [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Mucopolysaccharidase of Treponema pallidum. Infect Immun. 1979 Apr;24(1):261–268. doi: 10.1128/iai.24.1.261-268.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Wolff E. T. Mucopolysaccharide material resulting from the interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells. Infect Immun. 1978 Nov;22(2):575–584. doi: 10.1128/iai.22.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSFELD H. Studies on production of hyaluronic acid in tissue culture; the presence of hyaluronidase in embryo extract. Exp Cell Res. 1958 Feb;14(1):213–216. doi: 10.1016/0014-4827(58)90231-3. [DOI] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Ibrahim A. N., Streitfeld M. M. The microassay of hyaluronic acid concentration and hyaluronidase activity by capillary turbidity (CT) and capillary turbidity reduction (CTR) tests. Anal Biochem. 1973 Dec;56(2):428–434. doi: 10.1016/0003-2697(73)90208-x. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., OGSTON A. G. THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. 4. THE OSMOTIC PRESSURE OF MIXTURES OF SERUM ALBUMIN AND HYALURONIC ACID. Biochem J. 1963 Nov;89:249–253. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. B., DORFMAN A. Inhibition of hyaluronidase. Physiol Rev. 1955 Apr;35(2):381–402. doi: 10.1152/physrev.1955.35.2.381. [DOI] [PubMed] [Google Scholar]
- METZGER M., HARDY P. H., Jr, NELL E. E. Influence of lysozyme upon the treponeme immobilization reaction. Am J Hyg. 1961 Mar;73:236–244. doi: 10.1093/oxfordjournals.aje.a120182. [DOI] [PubMed] [Google Scholar]
- Miller J. N. Development of an experimental syphilis vaccine. Med Clin North Am. 1972 Sep;56(5):1217–1220. doi: 10.1016/s0025-7125(16)32347-1. [DOI] [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Hyaluronidase and experimental syphilis. III. Metachromasia in syphilitic orchitis and its relationship to hyaluronic acid. Am J Syph Gonorrhea Vener Dis. 1950 Nov;34(6):501–514. [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Morphologic and histochemical sequences in syphilitic and in tuberculous orchitis in the rabbit. Am J Syph Gonorrhea Vener Dis. 1954 May;38(3):189–202. [PubMed] [Google Scholar]
- SYKES J. A., MOORE E. B. A simple tissue culture chamber. Tex Rep Biol Med. 1960;18:288–297. [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Cortisone in experimental syphilis; a preliminary note. Bull Johns Hopkins Hosp. 1950 Nov;87(5):505–509. [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Studies on the mechanism of action of cortisone in experimental syphilis. Am J Syph Gonorrhea Vener Dis. 1954 Sep;38(5):371–387. [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]