Abstract
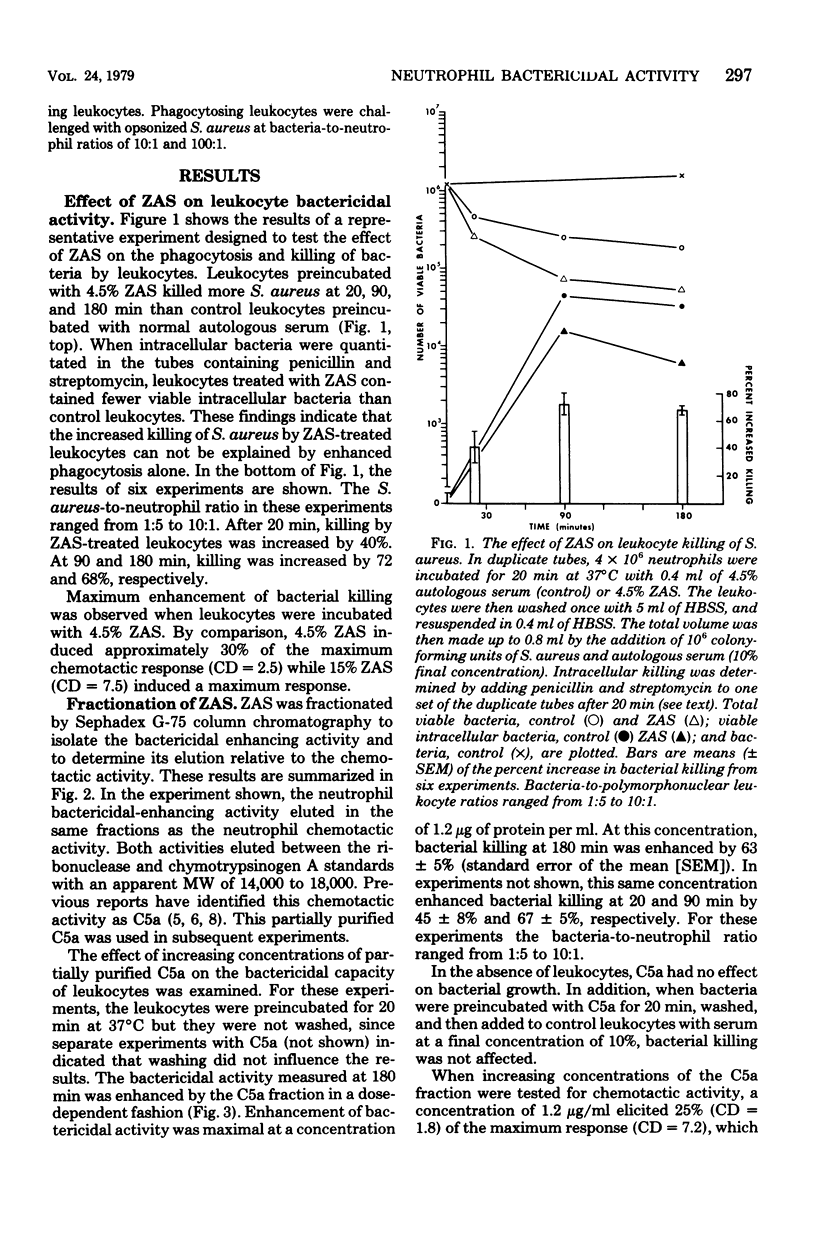
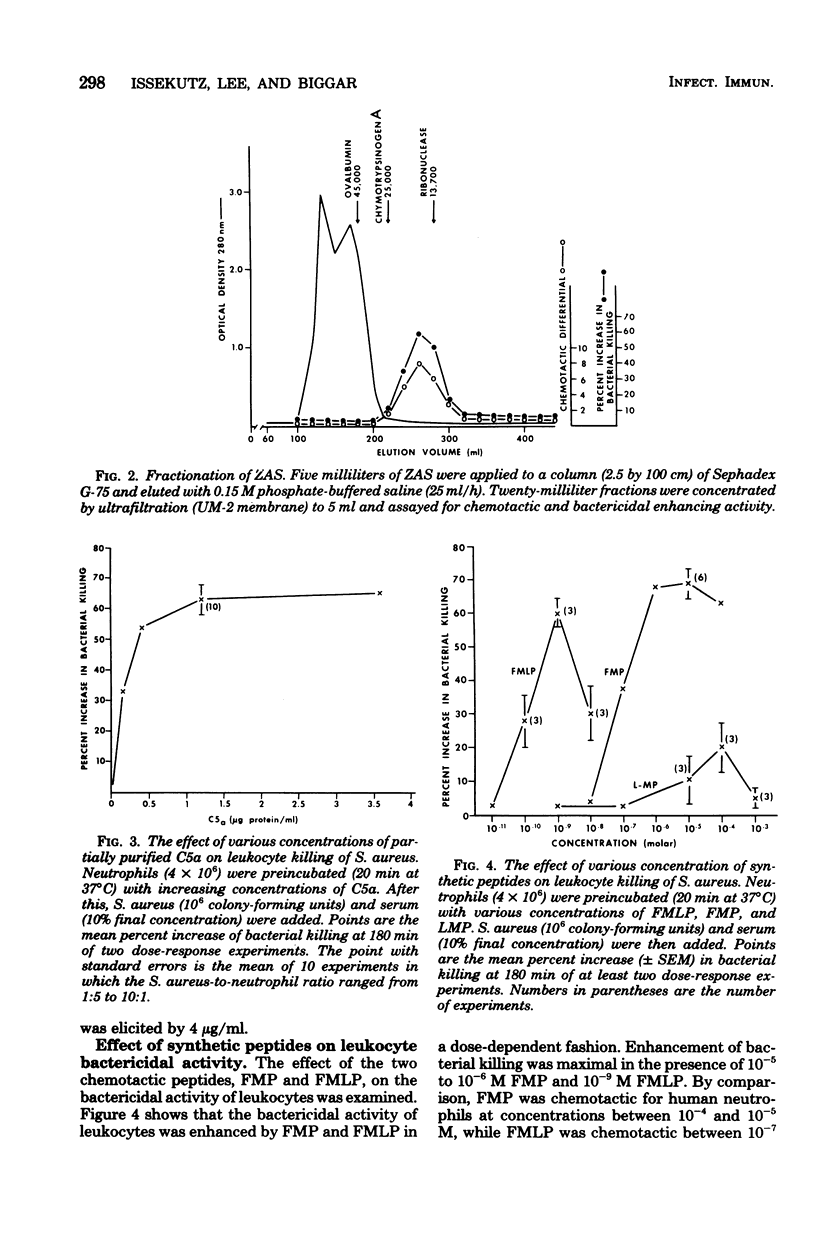
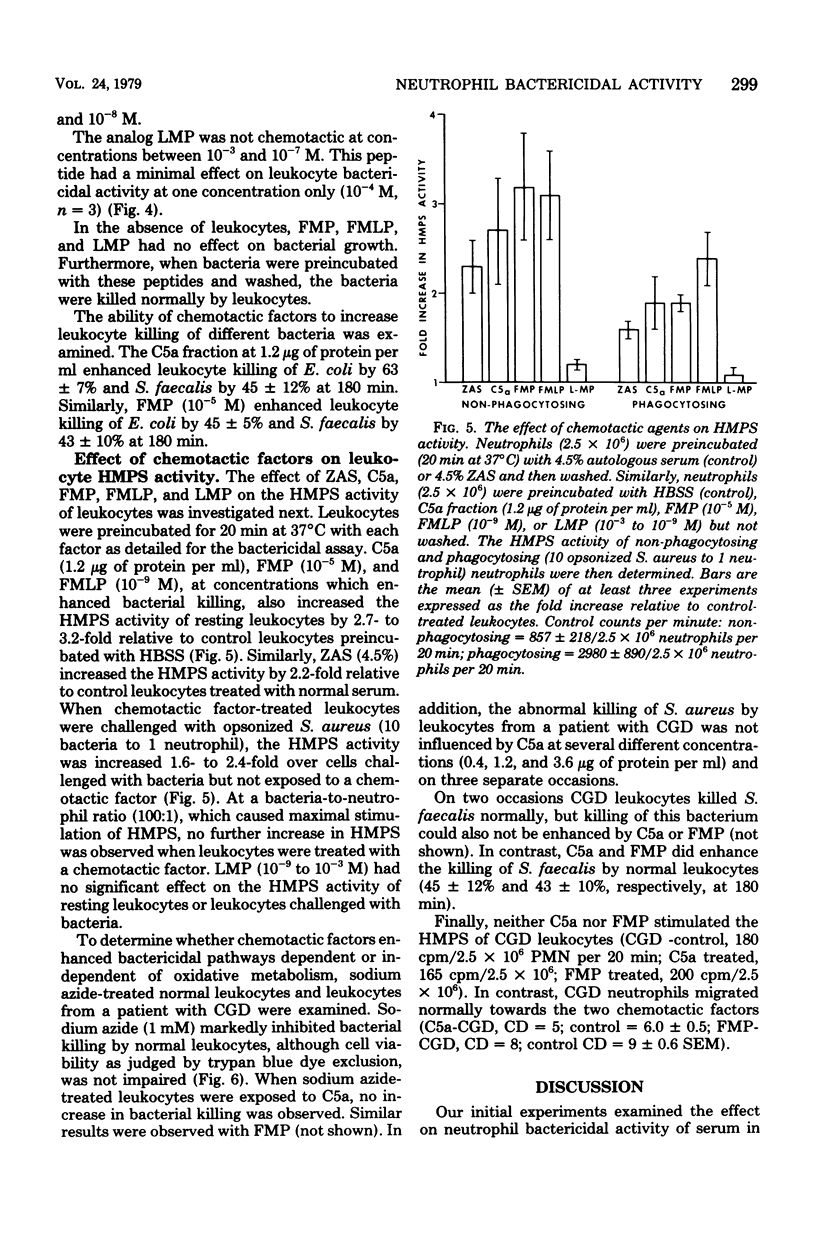
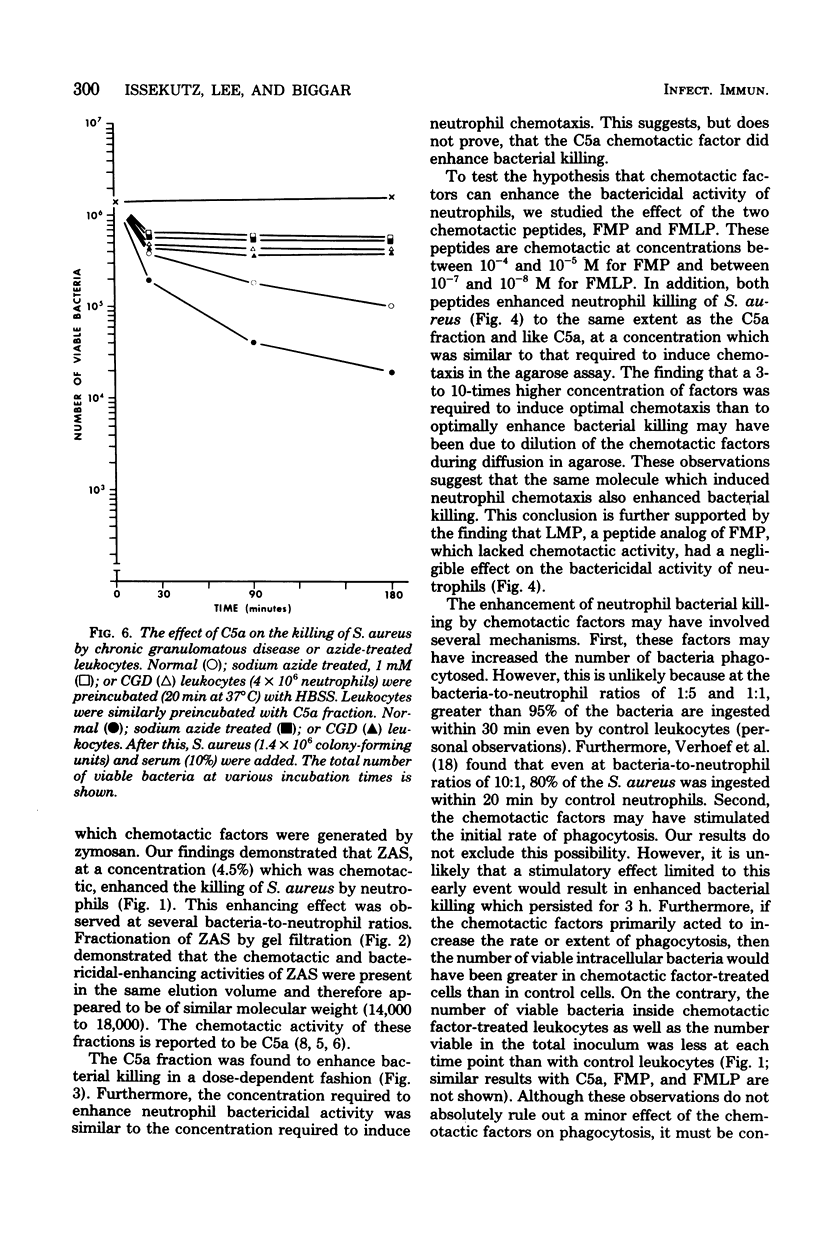
Neutrophils are important effector cells in the defense against microorganisms. They migrate into infected sites and then phagocytose and kill bacteria. Chemotactic factors may be important for initiating neutrophil migration. We investigated whether chemotactic factors might also influence an event subsequent to chemotaxis, namely bacterial killing. It was found that preincubation (20 min at 37°C) of human leukocytes with chemotactic substances such as zymosan-activated serum, a C5a-containing fraction of zymosan-activated serum, N-formyl methionyl phenylalanine or N-formyl methionyl-leucine-phenylalanine, enhanced leukocyte killing of Staphylococcus aureus, Escherichia coli, and Streptococcus faecalis in a dose-dependent fashion. The concentration of chemotactic factor required to enhance killing was similar to that required to induce neutrophil chemotaxis. In addition, zymosan-activated serum, C5a fraction, and the two N-formyl methionyl peptides increased the hexose monophosphate shunt activity of resting and phagocytosing neutrophils by two- to threefold. In contrast, bacterial killing by sodium azide-treated neutrophils and neutrophils from a patient with chronic granulomatous disease was not increased by any chemotactic factor. These findings suggest that chemotactic factors stimulate neutrophil oxygen-dependent microbicidal pathways. These observations illustrate another important contribution of biologically active molecules to effector cell function and host defense.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Windhorst D. B., Good R. A. Improved tests for the evaluation of neutrophil function in human disease. J Lab Clin Med. 1968 Jul;72(1):136–148. [PubMed] [Google Scholar]
- Allred C. D., Hill H. R. Effect of chemoattractants on chemiluminescence. Infect Immun. 1978 Mar;19(3):833–838. doi: 10.1128/iai.19.3.833-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Cutler J. E., Munoz J. J. A simple in vitro method for studies on chemotaxis. Proc Soc Exp Biol Med. 1974 Nov;147(2):471–474. doi: 10.3181/00379727-147-38367. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Brai M., Osler A. G., Weissmann G. Lysosomal enzyme release from human leukocytes: mediation by the alternate pathway of complement activation. J Immunol. 1973 Jul;111(1):33–37. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Biggar W. D. Influence of serum-derived chemotactic factors and bacterial products on human neutrophil chemotaxis. Infect Immun. 1977 Jan;15(1):212–220. doi: 10.1128/iai.15.1.212-220.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]


