Abstract
Background
Oral multivitamins and minerals are often used in conjunction with ethylenediamine tetra acetic acid infusions to treat atherosclerotic disease. Whether high-dose multivitamins are effective as secondary prevention of atherosclerotic disease, however, has not been established.
Objective
The vitamin component of the Trial to Assess Chelation Therapy assessed whether oral multivitamins reduced cardiovascular events, and were safe.
Design
The Trial to Assess Chelation Therapy was designed as a double-blind placebo-controlled 2×2 factorial multicenter randomized trial.
Setting
134 US and Canadian academic and clinical sites participated.
Patients
1708 patients, age ≥50 years, ≥6 weeks post myocardial infarction, with creatinine level ≤ 176.8 µmol/L (2.0 mg/dL). (ClinicalTrials.gov: NCT00044213).
Intervention
Patients were randomly assigned to an oral 28-component high-dose multivitamin and multimineral mixture or placebo.
Measurements
Study results were analyzed per randomized group. The primary endpoint was time to total mortality, recurrent myocardial infarction, stroke, coronary revascularization, or hospitalization for angina. Limited secondary endpoints and subgroup analyses were also pre-specified.
Results
The median age was 65 years, 18% female. The qualifying myocardial infarction had occurred 4.6 (1.6, 9.2) years prior to enrollment. The median duration of follow-up was 55 months (IQR 26, 60) overall. The median number of months during which patients took their vitamins was 31 (13, 59) in the active treatment group, and 35 (13, 60) in the placebo group (p=0.65). There were 645 (76%) vitamin patients and 646 (76%) placebo patients who completed at least 1 year of oral therapy (p=0.98); and 400 (46.9%) vitamin patients and 426 (49.8%) placebo patients who completed at least 3 years of oral therapy (p=0.23). There were 783 (46%) of patients who discontinued their vitamin regimen (390 (46%) in placebo, 394 (46%) in active; p=0.67), and 17% of patients withdrew from the study. The primary endpoint occurred in 230 (27%) patients in the active vitamin group and 253 (30%) in the placebo group (hazard ratio 0.89, 95% CI 0.75–1.07, p=0.21). There was no evidence suggesting harm from vitamin therapy in any category of adverse events
Limitations
The study had considerable non-compliance and drop-out. Thus, the ability to draw firm conclusions (particularly regarding safety) is limited.
Conclusions
High-dose oral multivitamins and multiminerals did not produce a statistically significant reduction in cardiovascular events in post-myocardial infarction patients on standard medications, but this conclusion has to be tempered by the non-compliance rate.
Primary Funding Source
National Institutes of Health.
Introduction
Patients who maintain a diet rich in a highly complex mix of antioxidants and other micronutrients have lower rates of atherosclerosis (1,2,3). To date, clinical trials testing isolated and combination micronutrients taken orally have not replicated these benefits. Recent meta-analyses noted that only vitamins A, C, E, and the anti-oxidant mineral selenium had been tested in well-designed trials, with mixed results. The conclusion was that high doses of vitamins A and E might increase cancer risk in selected patients; vitamin C was inactive, and selenium might be beneficial (4,5). Yet studies of small numbers of vitamins and minerals are not fully reflective of the supplement use of a large segment of the US population, which increasingly favors multivitamin and multimineral supplements.
The Trial to Assess Chelation Therapy (TACT), a 2×2 factorial trial funded by the National Heart, Lung and Blood Institute and the National Center for Complementary and Alternative Medicine (6,7), assessed whether an ethylenediamine tetra acetic acid (EDTA)-based chelation regimen or an oral high-dose multivitamin and multimineral supplement improved cardiovascular outcomes in secondary prevention patients. The chelation results have been published (8). This is the report of the comparison of oral multivitamins and multiminerals with placebo.
Methods
Design
TACT was a double-blind two by two factorial trial in which patients were randomized to four groups:
Active oral vitamins + active IV chelation infusions
Placebo oral vitamins + active IV chelation infusions
Active oral vitamins + placebo IV chelation infusions
Placebo oral vitamins + placebo IV chelation infusions
The design and organizational aspects of TACT have been published (7). The National Heart, Lung, and Blood Institute and the National Center for Complementary and Alternative Medicine provided funding and oversight. The institutional review board at each clinical site approved the study, and patients provided written informed consent. A Data and Safety Monitoring Board (DSMB) monitored the study. No companies or commercial entities provided funding or had any role in the execution, interpretation or submission for publication of this work.
Setting and Participants
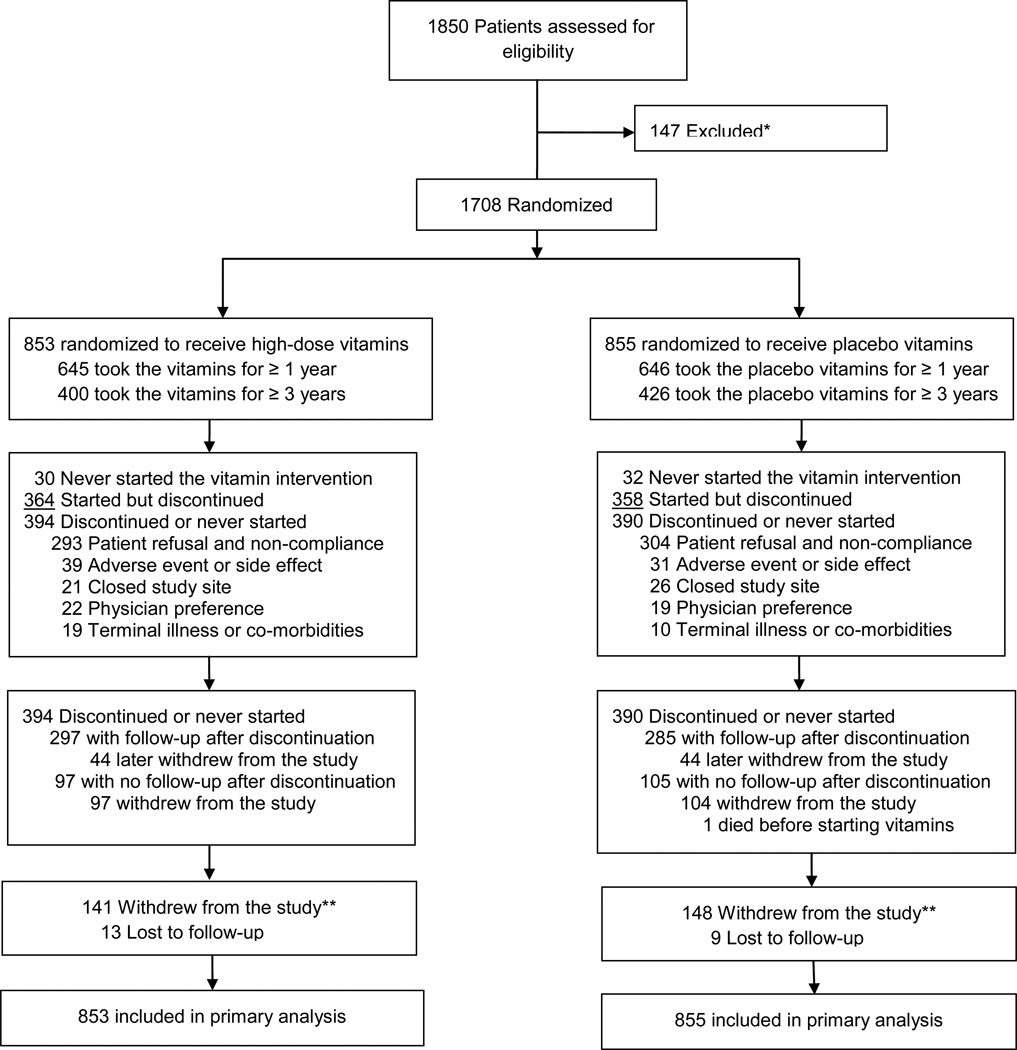
Eligible patients were at least 50 years of age and had sustained a myocardial infarction 6 weeks or more prior to enrollment. Patients were ineligible if they were women of childbearing potential, had a serum creatinine >176.8 µmol/L (2.0 mg/dL), or had other exclusion criteria as previously reported. Patients were enrolled at a total of 134 sites in the United States and Canada (Figure 1).
Figure 1.
Consort 2×2
Randomization and Interventions
Oral vitamins and placebo vitamins were prepared by the vitamin manufacturers and shipped to the central pharmacy for distribution to the sites. The active high-dose vitamin treatment was a 28-component mixture to be taken as 3 caplets twice daily, throughout the duration of the trial, designed to reflect the vitamin regimen commonly used by chelation practitioners (Table 1). The placebo caplets contained methylcellulose filler. Intravenous treatment consisted of 40 infusions of disodium EDTA-based chelation therapy, or a normal saline placebo (7,9). The study vitamins and infusion therapy were both double-blinded. During the infusion phase an open-label oral low-dose vitamin regimen was administered to all study participants.
Table 1.
TACT High Dose Vitamins & Mineral Supplements
| High-Dose Regimen (3 pills taken twice daily) |
Total Amount for 6 Pills |
% Daily Value |
|---|---|---|
| Vitamin A (as fish liver oil and beta-carotene) | 25,000 IU | 500% |
| Vitamin C (as calcium ascorbate, magnesium ascorbate and potassium ascorbate) | 1,200 mg | 2000% |
| Vitamin D3 (as cholecalciferol) | 100 IU | 25% |
| Vitamin E (as d-alpha tocopheryl succinate and d-alpha tocopheryl acetate) | 400 IU | 1333% |
| Vitamin K1 (as phytonadione) | 60 mcg | 75% |
| Thiamin (vitamin B1) (as thiamin mononitrate) | 100 mg | 6667% |
| Niacin (as niacinamide and niacin) | 200 mg | 1000% |
| Vitamin B6 (as pyridoxine hydrochloride) | 50 mg | 2500% |
| Folate (as folic acid) | 800 mcg | 200% |
| Vitamin B12 (as cyanocobalamin) | 100 mcg | 1667% |
| Biotin | 300 mcg | 100% |
| Pantothenic acid (as d-calcium pantothenate) | 400 mg | 4000% |
| Calcium (as calcium citrate and calcium ascorbate) | 500 mg | 50% |
| Iodine (from kelp) | 150 mcg | 100% |
| Magnesium (as magnesium aspartate, magnesium ascorbate and | 125% | |
| magnesium amino acid chelate) | 500 mg | |
| Zinc (as zinc amino acid chelate) | 20 mg | 133% |
| Selenium (as selenium amino acid chelate) | 200 mcg | 286% |
| Copper (as copper amino acid chelate) | 2 mg | 100% |
| Manganese (as manganese amino acid chelate) | 20 mg | 400% |
| Chromium (as chromium polynicotinate) | 200 mcg | 167% |
| Molybdenum (as molybdenum amino acid chelate) | 150 mcg | 200% |
| Potassium (as potassium aspartate and potassium ascorbate) | 99 mg | 3% |
| Choline (as choline bitartrate) | 150 mg | * |
| Inositol | 50 mg | * |
| PABA (as para-amino benzoic acid) | 50 mg | * |
| Boron (as boron aspartate and boron citrate) | 2 mg | * |
| Vanadium (as vanadyl sulfate) | 39 mcg | * |
| Citrus Bioflavonoids | 100 mg | * |
Daily Value not established. Other ingredients: Croscarmellose sodium, microcrystalline cellulose, magnesium stearate, hydroxypropyl cellulose, silicon dioxide.
Outcomes and Follow-up
The primary endpoint was a composite of time to death from any cause, reinfarction, stroke, coronary revascularization, or hospitalization for angina. The composite of time to cardiovascular death, reinfarction, or stroke was a prespecified secondary endpoint. A blinded independent clinical events committee at Brigham and Women’s Hospital adjudicated all non-procedural components of the primary end-point. The occurrence of coronary revascularizations was verified from the source medical record by the Duke Clinical Research Institute.
Patients were seen at baseline, and at each chelation infusion visit. Following the infusion phase, patients were called quarterly, attended annual clinic visits, and were seen at the end of the trial or at the 5 year follow-up, whichever was first. Vitamin/placebo caplets were distributed on a 3 to 6 monthly basis. Unused pills were returned to the site in order to assess compliance.
Safety monitoring included periodic physical examination and laboratory assessments. A blinded Medical Monitor at the Duke Clinical Research Institute reviewed all serious adverse events.
TACT pre-specified several subgroups for analyses based on assessing under-represented populations, subgroups of interest, and high-risk populations (7,8).
Statistical analysis
TACT originally planned to enroll 2372 patients over 3 years with a minimum follow-up of 1 year. This number provided 85% power for detecting a 25% relative reduction in the primary endpoint, assuming a 2.5-year event rate in the placebo arm of 20% and a significance level of 0.05. Due to difficulty enrolling patients, the blinded investigators requested, and the DSMB and sponsor granted, a reduction in sample size to 1700 coupled with an increase in follow-up time to preserve the 85% unconditional power (8).
Secure, web-based randomization was performed using permuted blocks stratified by clinical site. Treatment groups were compared as randomized (intention-to-treat) using two-sided significance tests. The log-rank test (10) was used for the statistical comparison of treatment with respect to clinical endpoints. Cumulative event rates were calculated according to the Kaplan-Meier method (11). Hazard ratios (HR) with associated confidence intervals (CI) were calculated using the Cox proportional hazards model (12). The Cox model was also used to assess the consistency of treatment effects by testing for interactions between treatment and the baseline characteristics pre-specified for subgroup analysis as well as assignment to active or placebo infusions. Continuous variables are expressed as medians and interquartile ranges (IQRs) unless otherwise specified. Group comparisons of simple proportions were performed using the Pearson Chi-square test. Final statistical analyses were performed using SAS software, versions 8.2 and 9.2 (SAS Institute, Cary NC).
Over the duration of the trial, the DSMB requested 11 interim analyses of the data. These interim reviews were performed primarily for safety and efficacy assessment of the EDTA chelation regimen and used a flexible alpha spending function approach with O’Brien-Fleming type monitoring boundaries (13,14). The level of significance required for the primary analysis at the completion of the study was 0.036.
This trial is registered at ClinicalTrials.gov, NCT00044213.
Results
Between September 10, 2003 and October 4, 2010, 1708 patients were randomized, 853 patients to the high-dose vitamin arm, and 855 patients to placebo. The last follow-up visit was completed October 31, 2011. The median duration of follow-up was 55 months (IQR 26, 60) overall. The trial was concluded upon achievement of the protocol-specified enrollment and follow-up period, and was not stopped based on any interim analyses.
Baseline characteristics
Baseline characteristics were similar between the randomized groups (Table 2). Patients were 65 (59,72) years old, 18% female, and 6% non-Caucasian. The qualifying myocardial infarction had occurred 4.6 (1.6, 9.2) years prior to enrollment. The study population had a high prevalence of diabetes (31%), of prior coronary revascularizations (83%), and guideline recommended medication use. Patients had a median fasting low-density lipoprotein level of 89 mg/dL (67, 115).
Table 2.
Baseline Characteristics
| High-dose Vitamins (N= 853) |
Placebo (N= 855) |
|
|---|---|---|
| Clinical Characteristics | ||
| Age-years | 65 (59, 72) | 65 (60, 72) |
| Female- (%) | 147 (17%) | 152 (18%) |
| Caucasian- (%) | 797 (93%) | 808 (95%) |
| Hispanic- (%) | 20 (2%) | 31 (4%) |
| BMI (kg/m2) | 29 (26, 33) | 30 (27, 34) |
| Blood Pressure (mmHg) | ||
| Systolic | 130 (118, 140) | 130 (120, 140) |
| Diastolic | 76 (70, 81) | 76 (70, 80) |
| History (%) | ||
| Hypercholesterolemia | 680 (81%) | 690 (82%) |
| Hypertension | 574 (67%) | 595 (70%) |
| Former cigarette smoker | 487 (57%) | 468 (55%) |
| Angina pectoris | 447 (52%) | 479 (56%) |
| Anterior MI | 341 (40%) | 333 (39%) |
| Diabetes | 282 (33%) | 256 (30%) |
| Congestive heart failure | 137 (16%) | 170 (20%) |
| Peripheral vascular disease | 125 (15%) | 143 (17%) |
| Valvular heart disease | 72 (9%) | 103 (12%) |
| Atrial fibrillation | 80 (10%) | 115 (14%) |
| Stroke | 56 (7%) | 55 (6%) |
| Time from qualifying MI to randomization-years | 4.5 (1.6, 9.5) | 4.6 (1.7, 9.0) |
| Coronary revascularizations | ||
| Either CABG or PCI | 705 (83%) | 709 (83%) |
| PCI | 484 (57%) | 523 (61%) |
| CABG | 390 (46%) | 384 (45%) |
| Concomitant Medications-(%) | ||
| Aspirin, warfarin or clopidogrel | 781 (92%) | 771 (90%) |
| Aspirin | 729 (85%) | 698 (82%) |
| Beta-blocker | 602 (71%) | 624 (73%) |
| Statin | 629 (74%) | 619 (72%) |
| ACE or ARB | 529 (62%) | 555 (65%) |
| Clopidogrel | 200 (24%) | 225 (27%) |
| Warfarin | 60 (7%) | 88 (11%) |
| Diabetes medication | ||
| Oral hypoglycemic | 207 (25%) | 173 (21%) |
| Insulin | 71 (9%) | 89 (11%) |
| Multivitamin* | 344 (42%) | 371 (45%) |
| Other vitamins/minerals* | 428 (52%) | 424 (51%) |
| Herbal products | 265 (32%) | 295 (36%) |
| Laboratory Examinations | ||
| Total cholesterol (mg/dL) | 164 (140, 193) | 166 (142, 197) |
| Triglycerides (mg/dL) | 141 (99, 206) | 138 (93, 202) |
| Glucose (mg/dL) | 102 (92, 122) | 103 (93, 120) |
| LDL (mg/dL) | 88 (67, 113) | 89 (68, 117) |
| HDL (mg/dL) | 43 (37, 51) | 42 (36, 50) |
| Creatinine (mg/dL) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.2) |
Participants were taking at baseline / prior to initiation of the study.
Continuous data are reported as median (IQR).
Abbreviations used: ACE = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Treatment compliance
There were 784 (46%) of patients who discontinued their vitamin regimen during the study (390 (46%) in placebo, 394 (46%) in active; p=0.67, eFigure 1). The median number of months during which patients took their vitamins was 31 (13, 59) in the active treatment group, and 35 (13, 60) in the placebo group (p=0.65). There were 645 (76%) vitamin patients and 646 (76%) placebo patients who completed at least 1 year of oral therapy (p=0.98); and 400 (47%) vitamin patients and 426 (50%) placebo patients who completed at least 3 years of oral therapy (p=0.23), (eTable 1). The most common reason for discontinuation was patient refusal (active: 74%, placebo: 78%, p=0.24). Vitamin/placebo discontinuations due to physician preference occurred in 5.6% in the active group, and 4.9% in the placebo group (p=0.65); and due to adverse events or side effects in 9.9% in the active group and 7.9% in the placebo group (p=0.34) (eTable 2). Women were more likely to discontinue vitamin therapy than men (eTable 3). The only baseline difference between treatment groups among patients who discontinued vitamins was a higher proportion of diabetes and greater use of oral hypoglycemic drugs among patients assigned to high-dose active vitamins (eTable 4). Withdrawal from the study was reported in 289 patients (17%, Figure 1), and was not different by vitamin treatment arm (p=0.69).
Primary and secondary outcomes
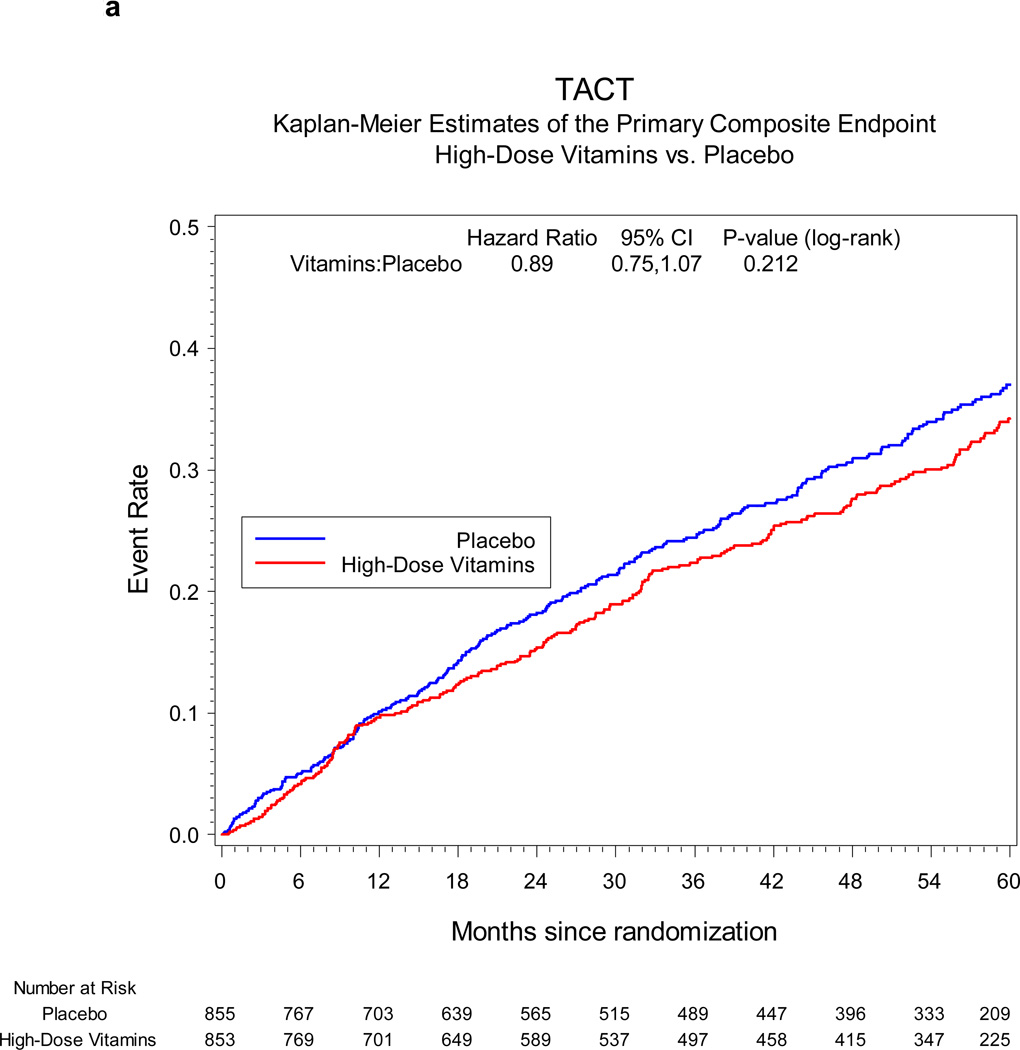
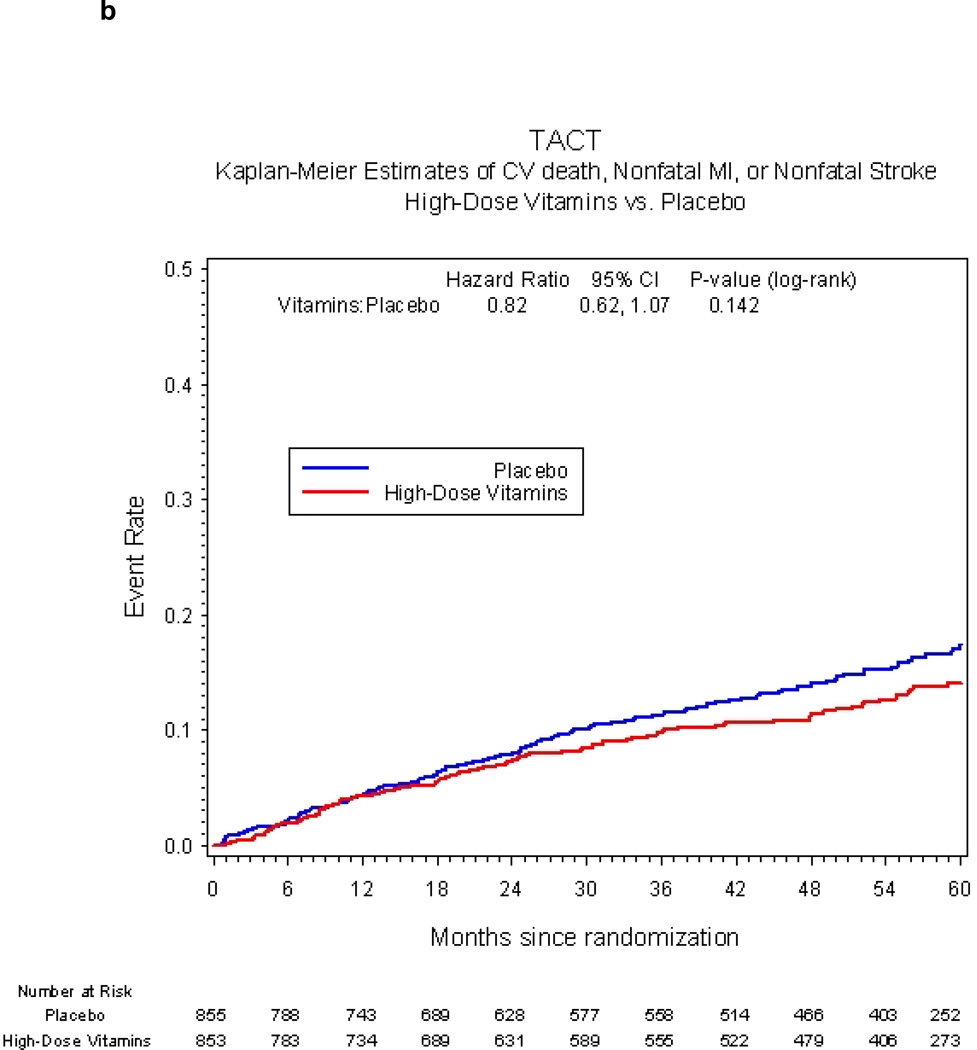
The primary endpoint occurred in 230 (27%) patients in the active vitamin group and 253 (30%) in the placebo group. The Kaplan-Meier 5-year event rate estimates were 34.2% (95% CI 30.5, 37.9) for the active vitamin group and 37.0% (95% CI 33.2, 40.8) for the placebo group (HR 0.89, 95% CI 0.75–1.07, p=0.21, Figure 2a). Treatment comparisons of the individual components of the primary endpoint were indeterminate due to the smaller numbers of events for each component (Table 3). The composite of cardiovascular death, non-fatal MI, or non-fatal stroke occurred in 94 (11%) patients in the active vitamin group and in 115 (13%) placebo patients (hazard ratio 0.82, 95% CI 0.62, 1.07, p=0.142, Figure 2b).
Figure 2.
a Primary endpoint vitamin vs. placebo
b Secondary clinical endpoint vitamin vs. placebo
Table 3.
Clinical End Points*
| High-dose Vitamins (N= 853) |
Placebo (N= 855) |
Hazard Ratio (95% CI) |
P-Value | |
|---|---|---|---|---|
| Primary Endpoint | 230 (27%) | 253 (30%) | 0.89 (0.75, 1.07) | 0.212 |
| Death | 87 (10%) | 93 (11%) | 0.93 (0.69, 1.24) | 0.614 |
| Myocardial Infarction | 58 (7%) | 61 (7%) | 0.95 (0.66, 1.36) | 0.786 |
| Stroke | 8 (1%) | 15 (2%) | 0.53 (0.22, 1.25) | 0.139 |
| Coronary revascularization | 132 (15%) | 155 (18%) | 0.84 (0.66, 1.05) | 0.131 |
| Hospitalization for angina | 12 (1%) | 19 (2%) | 0.72 (0.35, 1.47) | 0.359 |
| Secondary Endpoint | 94 (11%) | 115 (13%) | 0.82 (0.62, 1.07) | 0.142 |
| Cardiovascular Death | 45 (5%) | 56 (7%) | 0.80 (0.54, 1.18) | 0.256 |
The percentages in each case are based on the number of patients experiencing the event at any time during follow-up (not first events) divided by the number of patients randomized. Primary endpoint = first occurrence of death from any cause, myocardial infarction, stroke, or hospitalization for unstable angina.
Secondary endpoint = first occurrence of death from a cardiovascular cause, myocardial infarction, or stroke.
Adverse events
Serious adverse events were documented in 124 (15%) of vitamin recipients and 103 (12%) placebo recipients (95% CI for between group difference=(−0.7, 5.7), eTable 5). Among the adverse events there were 12 (1.4%) incident neoplasms in the active group and 11 (1.3%) in the placebo group (95% CI for between group difference=(−0.8, 1.3)). There was no evidence suggesting harm from vitamin therapy in any category of adverse events (eTable 6).
Subgroup analyses
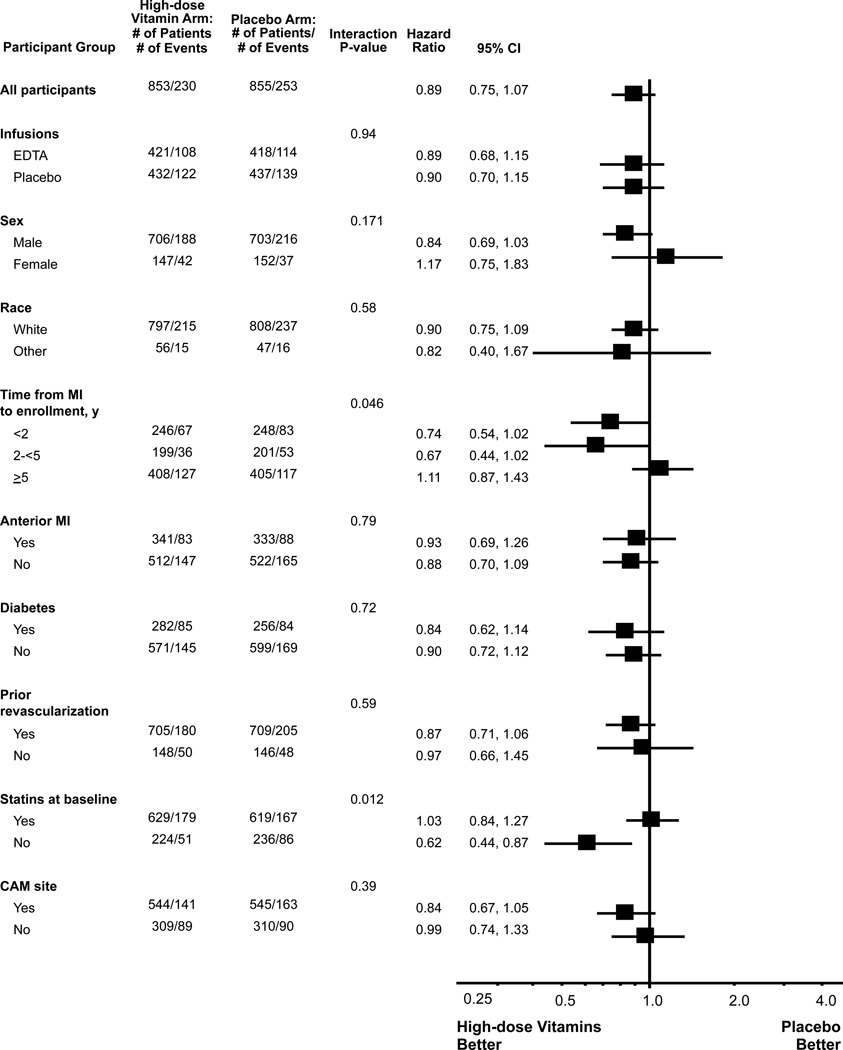
Pre-specified tests for treatment interactions (Figure 3) indicated that there was no statistically significant interaction (p=0.94) of oral vitamin therapy and EDTA chelation or placebo, nor between oral vitamin therapy and type of enrolling practice, defined as complementary or alternative medicine (CAM) or conventional medical practice (p=0.39). Statin therapy at baseline interacted with vitamin therapy (p for interaction=0.012).
Figure 3.
Forest plot vitamin vs. placebo
Discussion
The TACT vitamin study found that, in a secondary prevention population of post-MI patients, the use of a 28-component high-dose multivitamin and multimineral regimen did not statistically significantly reduce cardiovascular events. The complex mixture of oral multivitamins and multiminerals appeared safe. Because of a high rate of withdrawals and non-compliance, however, both of these conclusions have to be interpreted cautiously.
The present study adds to the existing literature on vitamin therapy in three ways. First, complementary and alternative medicine practitioners, rather than clinical researchers or supplement companies, designed the specific components of the active oral regimen, leading to a unique high-dose mixture (eTable 7). Secondly, an English-language MEDLINE search up to August 2013 demonstrated only 1 other large-scale trial of a multivitamin preparation focusing on cardiovascular outcomes (15) that tested more than 4 components. That trial, the Physicians Health Study 2, included only 5.1% (n=754) of patients with self-reported vascular disease. Thus, the present multivitamin study, with its multiple high-dose components, and its enrollment of 1708 post-myocardial infarction patients, adds to the knowledge base of multivitamin therapy as secondary prevention. Finally, and most relevant to the complementary and alternative medicine community, the two by two factorial design permitted the determination that vitamin therapy did not interact with intravenous chelation, an intervention shown to have a modestly positive effect on cardiovascular outcomes (8).
Cardiovascular disease remains the principal cause of death and disability in the US. Among the treatments used by patients to treat or prevent heart disease are those supported by an evidence base and prescribed by physicians, and over-the counter nutritional supplements, vitamins, and minerals advertised by the vitamin and supplement industry and purchased by many patients. Clinical trials have randomized thousands of patients into trials testing small numbers of antioxidant vitamins and minerals, typically vitamin C, vitamin E, beta carotene, and selenium; alone, or in in different factorial groups and combinations. The systematic analyses of trials testing small numbers of vitamins and minerals to prevent cardiovascular disease can be characterized as negative, with a suggestion that some supplements, in high doses and for some patients, may be harmful (4, 5, 16). The US public, however, has progressively increased its adoption of more complex multivitamin and multimineral supplements to prevent cardiac disease and maintain health (17). The use of multi-combination supplements has grown from 30% of the overall supplement market in 1988–1994 to 39% in 2003–2006. The supplement industry has grown from $4 billion in 1994 to $23.7 billion in 2008 (18, 19). And clinical trials have not kept pace with the use of these multiple-agent supplements.
Admittedly, there are observations that correlate diets rich in varied micronutrients to cardiovascular health, and plausible mechanisms by which complex mixtures of vitamins, minerals, and micronutrients could improve cardiovascular outcomes (1,2,3). Micronutrients, including vitamin C (20), some bioflavonoids, and others (21), may improve endothelial function. Vitamin E is an antioxidant vitamin and may even repair iron handling within the atherosclerotic plaque, thereby influencing oxidant damage (22). Many other mechanisms have been described that are beyond the scope of this discussion. Moreover, the safety concerns raised by clinical trials with single anti-oxidant vitamins have not been addressed with complex multivitamin and multimineral mixtures (4,23,24). Thus, it is reasonable to expand the reach of vitamin trials and test complex mixtures with the hope of elucidating efficacy and safety.
The TACT high-dose vitamins showed an 11% relative reduction in the primary composite endpoint relative to the placebo vitamin group that was not statistically significant. This difference was substantially smaller than the trial was powered to measure. Thus, although this trial does not support the routine use of this high dose oral multivitamin regimen for all post-myocardial infarction patients, the reduced statistical power due to a small difference between groups, as well as non-compliance with the study regimen, limits the conclusion of non-efficacy. Future studies of this particular regimen would have to take into account a smaller effect size than we estimated, as well as the barriers to compliance that were identified.
Among the minority of patients not taking statins at baseline, there was an interaction of oral vitamin therapy with statin use. This finding, which addressed a pre-specified subset of patients intolerant to statins or self-selected to be off statins, should not be interpreted as evidence that vitamin therapy can safely be substituted for statins in post-myocardial infarction patients. This finding requires additional mechanistic research and independent replication before the clinical implications can be understood. We also did not replicate the results of Brown et al (25), who observed that a reduction in clinical events associated with simvastatin was attenuated by the concomitant use of vitamin E, C, beta-carotene, and selenium.
In spite of our conclusions that high-dose oral vitamins and minerals do not appear, by themselves, to have a role in the management of the post MI patient, it is likely that patients will continue using vitamins for cardiovascular health. It is, therefore, important to comment further on the safety of the TACT vitamins. In spite of the doses used, higher in most components than those used by Sesso et al (15), there were no differences in serious adverse events compared with placebo and certainly no difference in incident cancers between groups. This conclusion however, must be tempered by a high discontinuation rate for study therapy.
Study limitations
There are a number of important limitations in this study. The study’s statistical plan was based on an effect size (25% reduction) that may have been overly optimistic for the oral vitamins. The TACT vitamin regimen, requiring 6 large caplets daily, imposed a barrier to patient compliance. In addition, the patient burden of the chelation component of the factorial trial was high. Thus, combining an oral vitamin regimen with a study of an intravenous therapy likely increased the non-compliance rate for the oral therapy reported here. This non-compliance rate reduced the ability to make definitive comments about the potential toxicity of such a high-dose vitamin and mineral mixture. Nevertheless, the loss of outcomes data, at least, is partially mitigated because all patients had their death status checked at the end of the study through the Social Security Death Index and the Canadian death registry. In addition, although there were a higher than expected number of patients that withdrew from the study, some patients did so after having sustained a primary endpoint. Patients who discontinued vitamins or placebo continued to be followed (unless, of course, they withdrew from the study), so for the patients who discontinued vitamins/placebo but remained in the trial, we obtained follow-up information beyond the time of the therapy discontinuation.
Conclusions
In stable patients with a history of MI on appropriate evidence based medical therapy, the use of high-dose oral multivitamins and multiminerals appeared safe, but did not produce a statistically significant reduction in cardiovascular events. These conclusions must be interpreted cautiously due to a high rate of non-compliance with the study regimen.
Supplementary Material
Acknowledgements
The National Heart, Lung, and Blood Institute and the National Center for Complementary and Alternative Medicine provided funding and oversight, grant # U01AT001156 and U01HL092607. The authors gratefully acknowledge the organizational skills of Ana Mon MPH Project Leader at the Clinical Coordinating Center, Alyssa Cotler at the National Center for Complementary and Alternative Medicine, Susan Dambrauskas (formerly at the National Heart, Lung, and Blood Institute), and Vivian Thompson at the Duke Clinical Research Institute for their competent professional assistance, and the Florida Heart Research Institute for supporting the pilot study. The above-mentioned contributors received no compensation for their work other than their usual salary. Gervasio A. Lamas MD had full access to all the data in the study had final responsibility for the decision to submit for publication. All statistical analyses were performed by Kerry Lee PhD, and Lauren Lindblad MS. Gervasio A. Lamas MD reports that from 2000 to 2003 he served as a consultant to OmniComm, the electronic data capture company used in the trial. No funds were received, and all ties were severed as of 09/10/2003. No other disclosures are reported from the authors.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Center for Complementary and Alternative Medicine, or the National Institutes of Health.
Reproducible Research Statement: Study protocol, statistical code and data set: Not available.
References
- 1.Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the 'antioxidant hypothesis'. Public Health Nutr. 2004;7:407–422. doi: 10.1079/phn2003543. [DOI] [PubMed] [Google Scholar]
- 2.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshipura KJ, Hung HC, Li TY, et al. Intakes of fruits, vegetables and carbohydrate and the risk of CVD. Public Health Nutr. 2009;12:115–121. doi: 10.1017/S1368980008002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 5.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. EDTA Chelation Therapy for Coronary Artery Disease. 2011 Apr; RFA: AT-01-004. [Google Scholar]
- 7.Lamas GA, Goertz C, Boineau R, et al. Design of the trial to assess chelation therapy (TACT) Am Heart J. 2012;163:7–12. doi: 10.1016/j.ahj.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241–1250. doi: 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozema TC. Special issue: protocols for chelation therapy. J Adv Med. 1997;10:5–100. [Google Scholar]
- 10.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Second Edition. New York, NY: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 12.Cox DR. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 13.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 14.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 15.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2012;308:1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virtamo J, Pietinen P, Huttunen JK, ATBC Study Group, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 17.Lonn EM. Multivitamins in prevention of cardiovascular disease. JAMA. 2012;308:1802–1803. doi: 10.1001/jama.2012.28259. [DOI] [PubMed] [Google Scholar]
- 18.Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994) NCHS Data Brief. 2011;61:1–8. [PubMed] [Google Scholar]
- 19.Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 21.Galleano M, Pechanova O, Fraga CG. Hypertension, nitric oxide, oxidants, and dietary plant polyphenols. Curr Pharm Biotechnol. 2010;11:837–848. doi: 10.2174/138920110793262114. [DOI] [PubMed] [Google Scholar]
- 22.Blum S, Vardi M, Brown JB, et al. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2–2 genotype. Pharmacogenomics. 2010;11:675–759. doi: 10.2217/pgs.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 24.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 25.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.