Abstract
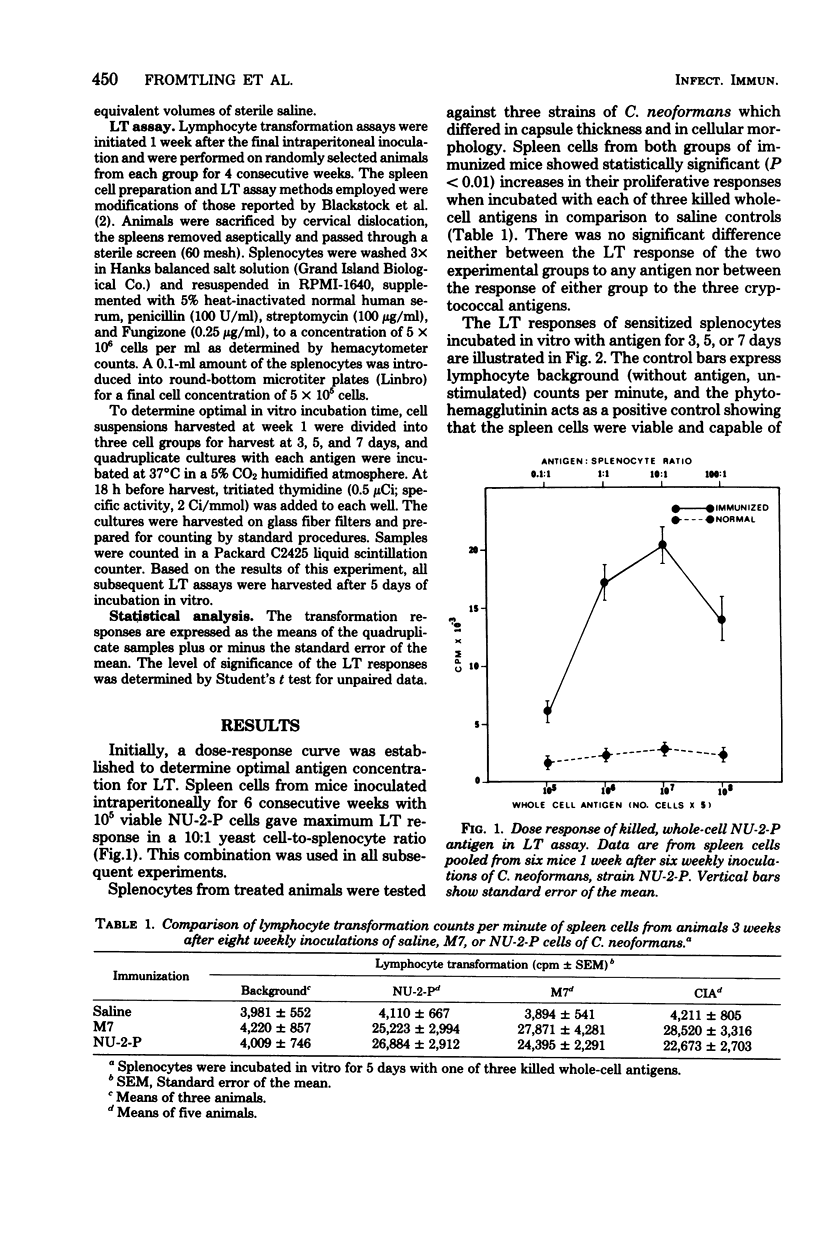
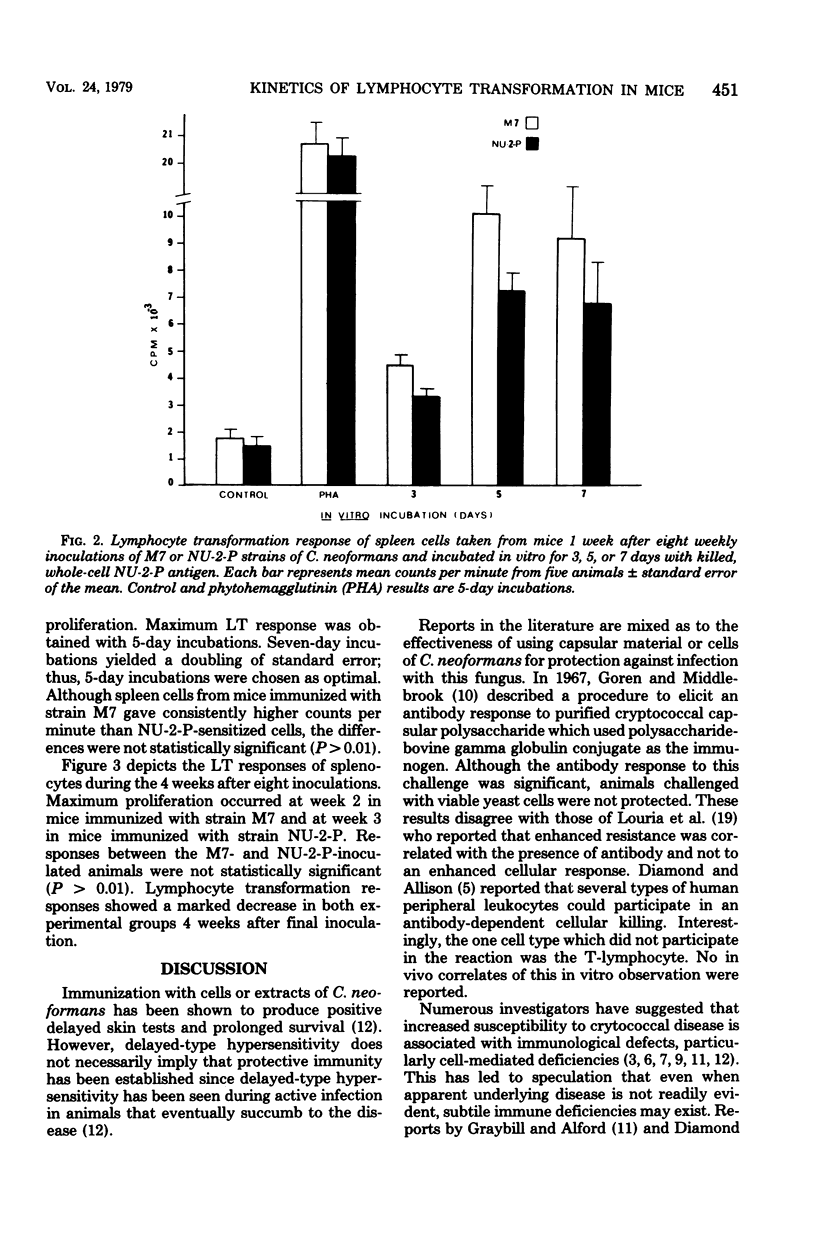
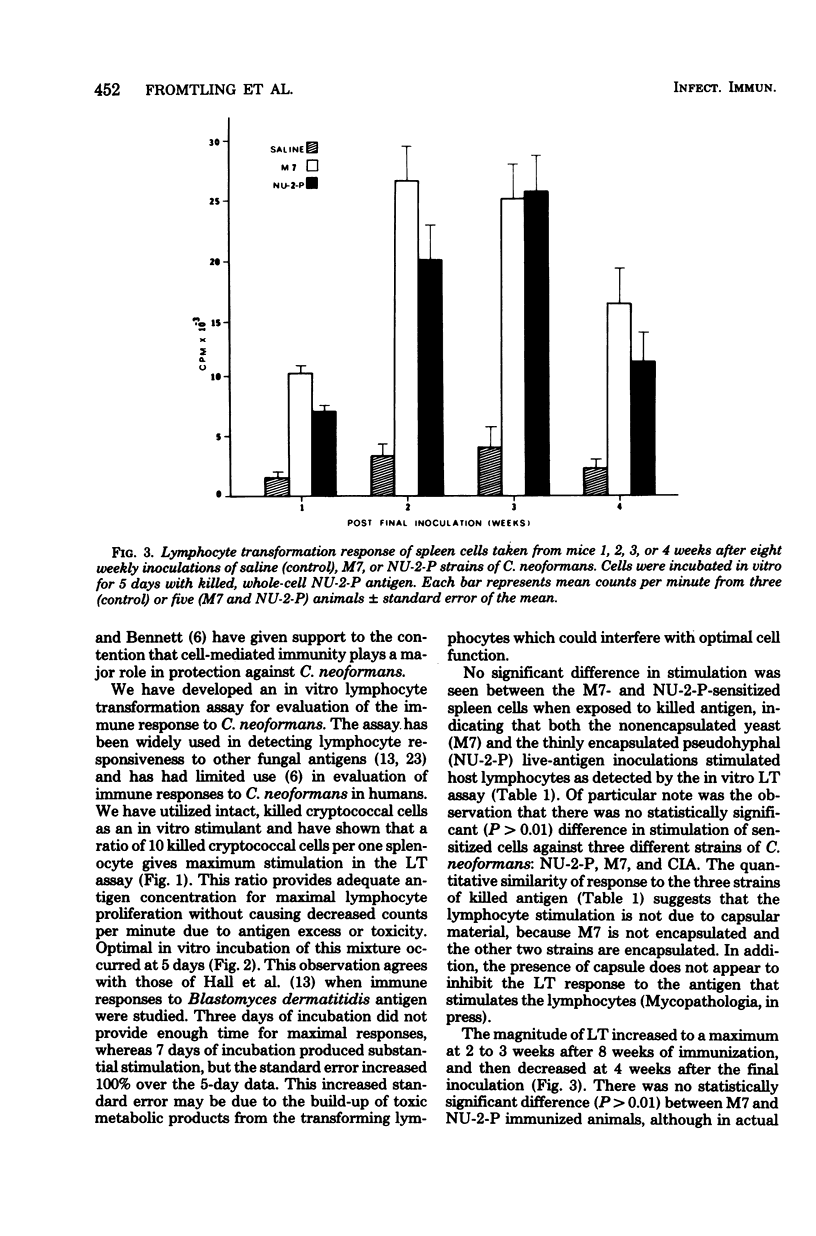
A murine model was developed to study the cell-mediated immune response of mice immunized with one of two live, avirulent forms of Cryptococcus neoformans: a nonencapsulated mutant and a thinly encapsulated pseudohyphal variant. A lymphocyte transformation assay was used to evaluate the cellular response of control and sensitized spleen cells after in vitro incubation with three merthiolate-killed whole-cell antigens of C. neoformans. An antigen-to-spleen cell ratio of 10:1 and 5 days of incubation of antigen-spleen cell mixtures were established as optimal conditions for maximum lymphocyte transformation. Maximum responses occurred from 2 to 3 weeks after the last of eight weekly intraperitoneal inoculations of C. neoformans. This assay provided an accurate, reproducible method of studying cell-mediated immunity to C. neoformans, and applications to the study of cryptococcal pathogenesis are proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bemmer A. M., Davidson W., Kuttin E. S., Zydon Y., Pinto M. Vaccine and Mycostatin in treatment of cryptococcosis of the respiratory tract. Sabouraudia. 1976 Jul;14(2):171–179. doi: 10.1080/00362177685190241. [DOI] [PubMed] [Google Scholar]
- Blackstock R., Schimpff R. D., Smith R. T. Heterogeneity of KCl-solubilized antigens of chemically induced sarcomas. Proc Soc Exp Biol Med. 1978 Mar;157(3):354–357. doi: 10.3181/00379727-157-40052. [DOI] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D., Gunn C. M. Cryptococcus neoformans. I. Nonencapsulated mutants. J Bacteriol. 1967 Nov;94(5):1475–1479. doi: 10.1128/jb.94.5.1475-1479.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- GADEBUSCH H. H. Passive immunization against Cryptococcus neoformans. Proc Soc Exp Biol Med. 1958 Jul;98(3):611–614. doi: 10.3181/00379727-98-24123. [DOI] [PubMed] [Google Scholar]
- Goodman J. S., Kaufman L., Koenig M. G. Diagnosis of cryptococcal meningitis. Value of immunologic detection of cryptococcal antigen. N Engl J Med. 1971 Aug 19;285(8):434–436. doi: 10.1056/NEJM197108192850804. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Middlebrook G. M. Protein conjugates of polysaccharide from Cryptococcus neoforman s. J Immunol. 1967 May;98(5):901–913. [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Taylor R. L. Host defense in cryptococcosis. I. An in vivo model for evaluating immune response. Int Arch Allergy Appl Immunol. 1978;57(2):101–113. [PubMed] [Google Scholar]
- Hall N. K., Deighton F., Larsh H. W. Use of an alkali-soluble water-soluble extract of Blastomyces dermatitidis yeast-phase cell walls and isoelectrically focused components in peripheral lymphocyte transformations. Infect Immun. 1978 Feb;19(2):411–415. doi: 10.1128/iai.19.2.411-415.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaoui R. M., Hall N. K., Larsh H. W. Role of macrophages in immunity and pathogenesis of experimental cryptococcosis induced by the airborne route--Part II: Phagocytosis and intracellular fate of Cryptococcus neoformans. Mykosen. 1977 Nov;20(11):409–412. [PubMed] [Google Scholar]
- Karaoui R. M., Hall N. K., Larsh H. W. Role of macrophages in immunity and pathogenesis of experimental cryptococcosis induced by the airborne route.--Part I: Pathogenesis and acquired immunity of Cryptococcus neoformans. Mykosen. 1977 Oct;20(10):380–388. [PubMed] [Google Scholar]
- LOURIA D. B. Specific and non-specific immunity in experimental cryptococcosis in mice. J Exp Med. 1960 May 1;111:643–665. doi: 10.1084/jem.111.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson J. B., Ivey M. H., Bulmer G. S. Cryptococcus neoformans: pseudohyphal forms surviving culture with Acanthamoeba polyphaga. Infect Immun. 1978 Apr;20(1):262–266. doi: 10.1128/iai.20.1.262-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. T., Bulmer G. S. Tumor induction by Cryptococcus neoformans. Infect Immun. 1972 Aug;6(2):199–205. doi: 10.1128/iai.6.2.199-205.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T., Balish E. Experimental Candida albicans infection in conventional mice and germfree rats. Infect Immun. 1976 Jul;14(1):33–38. doi: 10.1128/iai.14.1.33-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


