Abstract
Background
Risk for drug abuse (DA) is strongly associated with neighborhood social deprivation (SD). However, the causal nature of this relationship is unclear.
Method
Three Swedish population-based cohorts were followed up over 5 years for incident registration of DA in medical, legal or pharmacy records. In each cohort, we examined the SD–DA association, controlling carefully for individual socio-economic status (SES) with multiple measures, in the entire sample and among pairs of first cousins, paternal and maternal half-siblings, full siblings and monozygotic (MZ) twins discordant for SD exposure. The number of informative relative pairs ranged from 6366 to 166208.
Results
In all cohorts, SD was prospectively related to risk for incident DA. In relative pairs discordant for SD exposure, the SD–DA association was similar to that seen in the entire population in cousins, half-siblings, full siblings and MZ twins. Eliminating subjects who were residentially unstable or had DA in the first two follow-up years did not alter this pattern. When divided by age, in the youngest groups, the SD–DA association was weaker in siblings than in the entire population.
Conclusions
Across three cohorts, controlling for individual SES and confounding familial factors, SD prospectively predicted risk for incident DA registration. These results support the hypothesis that the SD–DA association is in part causal and unlikely to result entirely from personal attributes, which both increase risk for DA and cause selection into high SD environments. At least part of the SD–DA association arises because exposure to SD causes an increased risk of DA.
Keywords: Causal inference, co-relative design, drug abuse, social deprivation, socio-economic status
Introduction
In one of the founding works of psychiatric epidemiology, Faris & Dunham (1939) observed that individuals admitted to hospitals for drug abuse (DA) in Chicago resided disproportionally in deprived parts of the city. They write that, for DA:
almost 50 per cent of the cases are in the hobo and rooming-house areas at the center of the city … drug addicts come mainly from the zone of transition where it is easier to obtain an in-group solidarity and maintain contacts with other addicts and ‘dope’ peddlers (Faris & Dunham, 1939, p. 122)
Since this seminal observation, subsequent research has generally verified the association between individual low social class and risk for DA (Warner et al. 1995; Muntaner et al. 1998; Compton et al. 2007). We recently showed, in a nationwide Swedish cohort, a robust association in adolescents between a neighborhood-level measure of social deprivation (SD) and subsequent risk for DA (Kendler et al. 2012).
However, the degree to which this association results from causation (or ‘stress’) versus selection (or ‘drift’) is less clear. In their well-known study of Israelis of European and North African background, Dohrenwend et al. (1992) concluded that the association of low socio-economic status (SES) and substance use disorders, especially in men, arose largely as a result of ‘social causation’. In the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), Sareen et al. (2011) used a prospective design to show that incident cases of substance use disorders increased following a decline in income.
The attribution of causal relationships between putative risk factors and outcomes that cannot be experimentally manipulated is among the most challenging problems facing epidemiological research. There are two broad approaches to this problem: statistical methods and natural experiments (Rutter 2007a,b). In the current study we used an extension of a natural experiment: the co-twin control design. Traditionally, in this approach, the association between an exposure and an outcome is compared in the general population and in monozygotic (MZ) and dizygotic (DZ) twin pairs discordant for the exposure (Kendler et al. 1993). From the pattern of the associations in these three groups, it is possible to assess the degree to which the association observed in the population may be causal versus due to confounding from genetic and/or familial-environmental factors. Co-relative designs have been used to distinguish stress versus drift models for the association between SES and schizophrenia (Goldberg & Morrison, 1963; Turner & Wagenfeld, 1967).
If the association between low SES and DA is truly causal, the expectation would be that the SES-DA association would be of similar strength in the general population as in relative pairs discordant for their level of SES. However, if the SES-DA association results partly or entirely from familial confounding factors (e.g. poor rearing environment, genetically influenced personality traits), then the association should be weaker or disappear entirely when assessed in discordant twins. This design should be especially robust for DA because of the strength of familial/genetic factors in its etiology (Rounsaville et al. 1991; Tsuang et al. 1996; Bierut et al. 1998; Kendler & Prescott, 1998; Merikangas et al. 1998; van den Bree et al. 1998).
In this study, we explored the association between neighborhood-level measures of SD and subsequent risk for DA as assessed from medical, legal and pharmacy records in three consecutive nationwide Swedish cohorts. We examined the association in the entire population and then in an expanded set of relatives pairs: first-cousins, paternal and maternal half-siblings, full siblings and MZ twins discordant for their level of SD. Our aim was to gain insights into the nature of the SD–DA relationship and the degree to which it might be causal (i.e. SD→DA) versus a result of familial confounding factors.
Method
Our study used linked data from multiple Swedish nationwide registries. Linkage was achieved through the unique individual Swedish 10-digit personal identification (ID) number assigned to all Swedish residents at birth or immigration. This ID number was replaced by a serial number to preserve confidentiality.
The following sources were used to create our database: the Total Population Register, containing annual data on family and geographical status; the Multi-Generation Register, providing information on family relationships; the Swedish Hospital Discharge Register, containing all hospitalizations for all Swedish inhabitants between 1964 and 2009; the Swedish Prescribed Drug Register, containing all prescriptions in Sweden picked up by patients from July 2005 to 2009; the Out-patient Care Register, containing information from all out-patient clinics between 2001 and 2009; the Swedish Crime Register, containing complete national data on all convictions from 1973 to 2011; the Swedish Suspicion Register, containing complete national data on all individuals strongly suspected of crime from 1998 to 2011; the Swedish Mortality Register, containing causes of death; and the Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA), containing annual information on socio-economic factors on all individuals from 16 years of age. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (no. 2008/409).
Sample
We created three cohorts for these analyses: 2006, 2001 and 1996. For the 2006 cohort, from the Swedish population residing in the country on 31 December 2006, we identified all individuals aged 15–44 years, excluding those previously registered for DA. Incident DA registration was then assessed in these individuals from January 2007 to December 2011. In a second step, using the Swedish Multi-Generation Register, we identified all MZ twin pairs, full sibling pairs, maternal and paternal half-sibling pairs, and first cousin pairs. We separated paternal and maternal half-siblings because, although they shared the same degree of genetic resemblance, maternal half-siblings were much more likely to live together while growing up than paternal half-siblings.
Cohorts 2001 and 1996 were replications of cohort 2006 but with different time windows; that is, instead of using 2006 as baseline, we used 31 December 2001 and 31 December 1996 respectively. Cohort 2001 had a follow-up period from January 2002 to December 2006 and cohort 1996 had a follow-up period from January 1997 to December 2001. Thus, to serve as replications of observed trends, these three cohorts were constructed to be as similar to one another as possible.
We used the broad age range of 15–44 years in our three cohorts because this reflected the age distribution [mean and standard deviation (S.D.)] of first registrations although it is noteworthy that this was declining substantially over the historical period included in our study: 34.2 (9.7) years in 1996, 30.2 (9.2) years in 2001 and 25.9 (8.0) years in 2006.
Outcome variable
DA was identified in the Swedish medical registries by ICD codes [ICD-8: Drug dependence (304); ICD-9: Drug psychoses (292) and Drug dependence (304); ICD-10: Mental and behavioral disorders due to psy-choactive substance use (F10–F19), except those due to alcohol (F10) or tobacco (F17)]; in the Suspicion register by codes 3070, 5010, 5011 and 5012, reflecting crimes related to DA; and in the Crime register by references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). Crimes involved only in selling or distributing drugs were not included. DA was identified in individuals (excluding those suffering from cancer) in the Prescribed Drug Register who had retrieved (on average) more than four defined daily doses for 12 months of either Hypnotics and Sedatives [Anatomical Therapeutic Chemical (ATC) Classification System: N05C and N05BA] or Opioids (ATC: N02A). Individuals were considered to have their first recorded event of DA in our analyses if they fulfilled any of the above criteria. The distributions of incident cases of DA among Small Areas for Market Statistics (SAMS) for our three cohorts were – 1996: mean 0.8% (S.D.: 0.9); percentiles: 10th, 0%; 25th, 0%; 50th, 0.6%; 75th, 1.1%; 90th, 1.8%; 2001: mean 1.1% (S.D.: 1.0); percentiles: 10th, 0%; 25th, 0.4%; 50th, 1.0%; 75th, 1.6%; 90th, 2.5%; and 2006: mean 1.3% (S.D.: 1.2); percentiles: 10th, 0%; 25th, 0.6%; 50th, 1.2%; 75th, 1.9%; 90th, 3.3%. We have previously shown that these sources of ascertainment for DA are highly intercorrelated, supporting their validity (Kendler et al. 2013).
Neighborhood- and individual-level information
Neighborhoods, as defined by Statistics Sweden, the Swedish government-owned statistics bureau, are known as SAMS. There are approximately 9200 SAMS throughout Sweden, with an average population of 1000. These SAMS units were initially created by the Swedish authorities for administrative and marketing purposes. They are often characterized by homogeneous types of buildings and are limited by ‘natural’ boundaries, such as highways, rivers or hills. In addition, they are relatively small, containing around 1000 residents on average, which is advantageous in neighborhood studies. Many previous neighborhood studies were based on larger geographic units, such as census tracts or even counties, which may not be a good measure of neighborhoods. Qualitative research has shown that small neighborhoods are consistent with how residents themselves define their neighborhoods (Huie, 2001).
We created a neighborhood SD index for each of the SAMS based on registry data for all residents in the neighborhood aged 25–64 years (i.e. the working-age population) that are thought to have a stronger impact on the neighborhood than others. The SD composite contained the following derived at baseline: the proportion of residents with low education (≤ 9 years), the proportion of residents with low household income (less than half the median income), the proportion of unemployed residents, and the proportion of individuals on financial assistance (Winkleby et al. 2007). This measure has been validated in prior studies predicting risk and mortality for coronary heart disease (Winkleby et al. 2007; Chaikiat et al. 2012) and access to health-care resources (Kawakami et al. 2011). In the model the composite was kept as a continuous variable, with the SD score ranging between −3 and 11, with higher values indicating greater levels of neighborhood deprivation. The distributions of SD among SAMS for our three cohorts were respectively: 1996: mean 0.00 (S.D. = 1.42), percentiles: 10th, −1.57; 25th, −0.95; 50th, −0.17; 75th, 0.66; 90th, 1.59; 2001: mean 0.00 (S.D. = 1.41), percentiles: 10th, −1.44; 25th, −0.91; 50th, −0.22; 75th, 0.58; 90th, 2.33; and 2006: mean −0.03 (S.D. = 1.40), percentiles: 10th, −1.38; 25th, −0.91; 50th, −0.29; 75th, 0.44; 90th, 1.47.
Our individual-level information was defined at baseline for each cohort and included age (a continuous variable); gender (male and female); annual household income (size-weighted, divided into quartiles: highest, mid-high, low-mid, and lowest); financial assistance (dichotomized into yes/no); employment status (dichotomized into yes (sometime during the baseline year)/no and country of birth [categorized into four groups: Sweden, other Nordic countries, other Western countries (Western Europe, the USA, Canada, Oceania) and others]. In Sweden, financial assistance is intended to act as a last-resort safety net for people who have temporary financial problems. The Municipality Social Services assess each application individually and individuals must totally lack financial resources to be entitled. Before receiving such support, individuals must first apply for general benefits and compensation for which they may be eligible such as sickness benefit, parental allowance, housing benefit and maintenance support.
Using a multilevel logistic regression model, adjusted for individual covariates, showed that the proportions of variance in incident DA in our cohorts accounted for by SAMS were 3.4, 3.9 and 3.6% for our 1996, 2001 and 2006 cohorts respectively.
Statistical methods
We investigated the association between SD and individual DA risk. First, we used a generalized estimating equation (GEE) approach with individuals nested within their neighborhoods at baseline. This technique accounts for the hierarchical structure of the information with individuals nested within neighborhoods and estimates regression coefficients with a population average interpretation (Hu et al. 1998). Second, we sought to compare the results from the GEE method with the results from our co-relative design. Using conditional logistic regression (CLR), we performed five analyses on all MZ twin pairs, full sibling pairs, maternal half-sibling pairs, paternal half-sibling pairs and first cousin pairs that were discordant for DA and levels of SD. Discordance for DA was defined as one member of a relative pair having at least one lifetime DA registration and the co-relative never having a registration. SD discordance was defined as not residing in the same SAMS or in different SAMS with the same SD level.
In all models, we also controlled for individual income, age and gender. The co-relative design allowed us to contrast the rates of DA in relatives living in different neighborhoods with dissimilar levels of SD. The CLR provides a subject-specific regression coefficient for SD that is adjusted for the familial cluster, and therefore accounts for an array of unknown shared genetic and environmental factors. All analyses were repeated for each gender. The statistical analyses were performed using SAS version 9.2 (SAS Institute, 2008).
Results
Tables 1–3 provide the descriptive statistics for our 1996, 2001 and 2005 cohorts respectively. The total numbers of individuals contained in these samples were similar, at ~3.5 million each. Over the 5-year follow-up periods, increasing proportions of incident DA cases were seen, with rates ranging from 1.3% to 2.2% in males and from 0.5% to 0.7% in females. These tables also provide the total number of relative pairs contained in each cohort, their correlation for SD and the number of pairs who met criteria for inclusion in our analyses, being discordant for both DA and level of SD experienced. In all cohorts, cousin pairs were most common, followed by full siblings, paternal half-siblings and maternal half-siblings. As expected, for all classes of relatives, we had far fewer female–female than male–male pairs for analysis.
Table 1.
Sample size by gender, prevalence of drug abuse (DA), sample size by relative pairs and their correlation for social deprivation (SD) in all individuals included in the analysis of the 1996 cohort
| n | Prevalence of DA (%), 1997–2001 | ||
|---|---|---|---|
| Women | 1694037 | 0.5 | |
| Men | 1769952 | 1.3 | |
|
| |||
| Pair correlation for SD | n pairs (total) | n pairs included in the CLR | |
|
| |||
| Cousins | |||
| Women | 0.107 | 1117951 | 22 608 |
| Men | 0.118 | 1249095 | 58 540 |
| Paternal half-siblings | |||
| Women | 0.111 | 63146 | 2446 |
| Men | 0.128 | 66666 | 6428 |
| Maternal half-siblings | |||
| Women | 0.216 | 50439 | 1826 |
| Men | 0.225 | 53677 | 4540 |
| Full siblings | |||
| Women | 0.392 | 437421 | 6104 |
| Men | 0.426 | 487085 | 13 188 |
CLR, Conditional logistic regression.
Table 3.
Sample size by gender, prevalence of drug abuse (DA), sample size by relative pairs and their correlation for social deprivation (SD) in all individuals included in the analysis of the 2006 cohort
| n | Prevalence of DA (%), 2007–2011 | ||
|---|---|---|---|
| Women | 1727783 | 0.7 | |
| Men | 1770317 | 2.2 | |
|
| |||
| Pair correlation for SD | n pairs (total) | n pairs included in the CLR | |
|
| |||
| Cousins | |||
| Women | 0.106 | 1591384 | 43876 |
| Men | 0.107 | 1734343 | 122332 |
| Paternal half-siblings | |||
| Women | 0.138 | 72802 | 3610 |
| Men | 0.136 | 73008 | 8482 |
| Maternal half-siblings | |||
| Women | 0.228 | 60191 | 2738 |
| Men | 0.208 | 60473 | 6000 |
| Full siblings | |||
| Women | 0.485 | 414713 | 9930 |
| Men | 0.493 | 453212 | 30284 |
CLR, Conditional logistic regression.
Table 4 depicts for each cohort both the raw odds ratios (ORs) and the ORs adjusted for individual SES with multiple indices (and 95% confidence intervals, CIs) between SD assessed at baseline and subsequent risk for first registration for DA in the entire population and in relative pairs ordered by their degree of genetic relationship. These adjusted ORs are consistently substantially smaller than the raw ORs, suggesting that a substantial proportion of the raw SD–DA association results from factors related to individual SES. Nonetheless, after controlling for individual-level factors, a highly significant SD–DA relationship remains. Furthermore, the raw ORs for close relative pairs are substantially lower than those seen within the entire population in all three cohorts, suggesting considerable familial confounding for the raw SD–DA association. However, as shown in Figs 1–3, where the adjusted ORs are depicted graphically, this effect disappears almost entirely when controlling for individual-level SES.
Table 4.
Raw odds ratios (ORs) and ORs adjusted for individual socio-economic status (SES)for the association between neighborhood social deprivation (SD) and risk for subsequent registration for drug abuse (DA) in three nationwide Swedish cohorts
| Relative | 1996 Cohort
|
2001 Cohort
|
2006 Cohort
|
|||
|---|---|---|---|---|---|---|
| Raw OR (95% CI) | aOR (95% CI) | Raw OR (95% CI) | aOR (95% CI) | Raw OR (95% CI) | aOR (95% CIs) | |
| Entire population | 1.24 (1.23–1.26) | 1.09 (1.08–1.09) | 1.19 (1.18–1.21) | 1.06 (1.05–1.07) | 1.18 (1.16–1.19) | 1.08 (1.06–1.09) |
| Cousins | 1.22 (1.22–1.23) | 1.11 (1.10–1.12) | 1.18 (1.17–1.19) | 1.10 (1.09–1.10) | 1.17 (1.16–1.17) | 1.12 (1.11–1.12) |
| Paternal half-siblings | 1.22 (1.19–1.24) | 1.11 (1.08–1.13) | 1.15 (1.13–1.17) | 1.08 (1.05–1.10) | 1.15 (1.13–1.17) | 1.11 (1.08–1.13) |
| Maternal half-siblings | 1.16 (1.13–1.19) | 1.08 (1.05–1.11) | 1.14 (1.12–1.17) | 1.07 (1.05–1.10) | 1.12 (1.10–1.14) | 1.09 (1.06–1.11) |
| Full siblings | 1.17(1.16–1.19) | 1.11 (1.09–1.12) | 1.11 (1.09–1.12) | 1.07 (1.06–1.08) | 1.08 (1.06–1.09) | 1.06 (1.05–1.08) |
| MZ twins | 1.53 (1.17–1.99) | 3.42 (1.23 –9.55) | 1.18 (0.89 –1.58) | 1.28 (0.91–1.81) | 1.15 (0.93–1.44) | 1.23 (0.95–1.59) |
CI, Confidence interval; MZ, monozygotic; aOR, adjusted odds ratio; models adjusted for individual income, financial assistance, employment status, country of birth, age and gender.
Fig. 1.
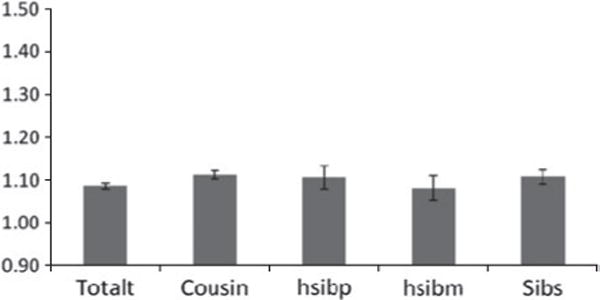
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between neighborhood social deprivation (SD) and risk for subsequent drug abuse (DA) registration in both sexes from our 1996 cohort. A population-wide sample of individuals aged 15–44 years without prior for DA was selected as of December 1996 and the follow-up period defined as January 1997 to December 2001. The ORs are presented for the entire population (totalt) and then within relative pairs of first cousins (Cousin), paternal half-siblings (hsibp), maternal half-siblings (hsibm) and full-siblings (Sibs). For relative pairs, we only examined those that were doubly discordant (for both level of SD and DA outcome) as these were the only pairs that were statistically informative.
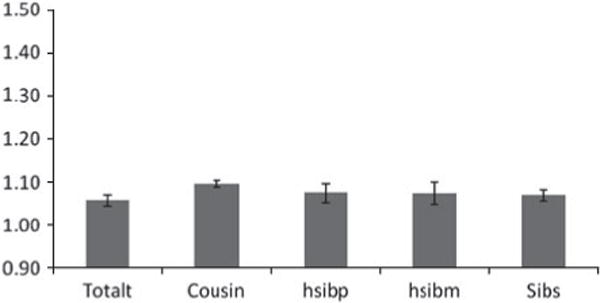
Fig. 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between neighborhood social deprivation (SD) and risk for subsequent drug abuse (DA) registration in both sexes from our 2006 cohort. A population-wide sample of individuals aged 15–44 years without prior for DA was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. The ORs are presented for the entire population (totalt) and then within relative pairs of first cousins (Cousin), paternal half-siblings (hsibp), maternal half-siblings (hsibm) and full-siblings (Sibs). For relative pairs, we only examined those that were doubly discordant (for both level of SD and DA outcome) as these were the only pairs that were statistically informative.
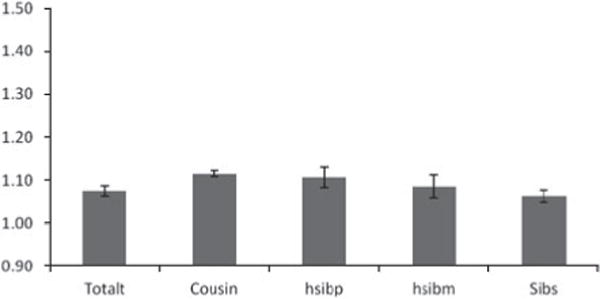
In each cohort, the OR within cousin, half-sibling and full sibling pairs was very similar to that seen in the entire population. Only in full siblings in the 2006 cohort do we observe an OR marginally smaller than that seen in the general population. The number of MZ pairs discordant for both SD and DA was small so these ORs were known imprecisely. In the 1996 cohort, the OR in these pairs was implausibly high but with very wide CIs: OR 3.42, 95% CI 1.23–9.55. In the 2001 and 2006 cohorts, the ORs in the MZ twins were also higher than those observed in the entire population (OR 1.28, 95% CI 0.91–1.81 and OR 1.23, 95% CI 0.95–1.59 respectively) but the population estimates were well within the wide CIs.
We examined whether the SD–DA association was moderated by age. As shown in Figs A1–A6 (see online Supplementary material) for the 2006 cohort (similar results were obtained with the other cohorts), the association between SD and DA was stronger in the older age groups. In age groups 25–29, 30–34, 35–39 and 40–49 years, the ORs were similar in the general population and in the cousin, half-sibling and sibling pairs. In the two younger age groups (15–19 and especially 20–24 years), a decline was seen in ORs in the more closely related relative pairs.
We were concerned about reverse causation in which early symptoms of DA that preceded official detection might cause downward social drift that could mimic a causal effect of SD on DA. We attempted to evaluate this empirical hypothesis in three ways. First, we repeated all of our analyses in all three cohorts restricting them to individuals who had resided in the same location for a minimum of a 5-year period prior to baseline (see online Supplementary Figs A7–A9). We found no evidence that the overall SD–DA association declined in the population screened for geographical stability. Second, we reanalyzed our data including a 2-year lag in DA registration so that anyone with a DA registration within 2 years of baseline was censored from each cohort. The aim of these analyses was to attempt to remove cases who had undetected DA at baseline. For the 2006 cohort, 47.7% (n=24846) of all DAs were registered within 2 years after baseline. Excluding them from the analysis gave an OR in the total population for SD of 1.07 (95% CI 1.06–1.08) compared to 1.08 (95% CI 1.06–1.09) when all DA cases were included. Excluding them from the sibling comparison (full siblings) gave an OR for SD of 1.06 (95% CI 1.04–1.08), identical to that seen when all DA cases were included. Similar results were obtained for the 1996 and 2001 cohorts. Third, we examined only individuals who did not move during the follow-up period. For the 2006 cohort, 56% of the individuals lived in the same area at the end of the follow-up. The ORs for the total population adjusted for all individual covariates and for full siblings pairs were both increased compared with those seen in the entire cohort (OR 1.10, 95% CI 1.10–1.11 and OR 1.18, 95% CI 1.13–1.23 respectively). Similar results were obtained for the 1996 and 2001 cohorts.
Discussion
In accordance with prior results (Faris & Dunham 1939; Warner et al. 1995; Muntaner et al. 1998; Compton et al. 2007), we found, in Swedish national data after controlling for individual income, financial assistance, employment status and country of birth, a substantial relationship between the experience of neighborhood-level SD and risk for DA. However, this relationship is open to two major causal interpretations. First, the traits that increase risk for DA could also predispose individuals to poor occupational functioning, which would in turn increase the probability of residence in low SES areas with high SD. In this selection model, living in a neighborhood with high SD does not directly impact on risk for DA. Instead, the association arises because of a set of personal attributes that both increase risk for DA and increase selection into high SD environments.
The second possible interpretation of the SD–DA relationship is a causal one. The social environment itself could impact directly on risk for DA through a range of mechanisms including greater drug and crime exposure, an increased sense of helplessness, poorer social support with reduced ties to employed individuals, reduced economic opportunities and more deviant behavior in available peers (Crum et al. 1996; Williams & Latkin, 2007). The potential importance of this SD–DA pathway is supported by meta-analytic results documenting the clustering of DA outcomes in geographical areas (Karriker-Jaffe, 2011).
Discriminating between a selection and a causal model for the association between SD and risk for DA is both difficult and important. In this report, we used a combined prospective and familial design to address this issue. As the risk for DA is strongly familial, with contributions from both genetic and shared environmental factors (Cadoret et al. 1986, 1996; Tsuang et al. 1996; Merikangas et al. 1998; van den Bree et al. 1998; Kendler et al. 2000, 2012; Lynskey et al. 2002), relatives of affected individuals will share a substantial range of risk factors for DA. The magnitude of sharing will be greater the closer the familial relationship. If the SD–DA correlation arises from selection, then the association between SD and DA should be lower in pairs of relatives discordant for SD exposure than in the general population because the unexposed relative (the one living in the low SD environment) will share some of the familial risk factors for DA and therefore will have an increased risk for DA. Because of this sharing, these relative pairs should differ less in risk for DA than unrelated individuals who vary to a similar degree in their exposure to SD.
Consistent with prior studies of this topic (Dohrenwend et al. 1992; Sareen et al. 2011), five of our findings provided evidence favoring the causal explanation for the SD–DA association. First, in our three cohorts, when we examined the prospective association between SD and DA in the entire population and in large samples of first cousins, half-siblings and full siblings, we found no evidence for a decline in the association with increasing familial relationships in the 1996 and 2001 cohorts and a very modest decline restricted to full sibling pairs in the 2006 cohort. In each cohort, controlling for a range of measures of individual SES, the member of the sibling pair living in the area with the higher levels of SD had an appreciably greater risk for subsequent DA than the co-sibling who experienced lower levels of neighborhood deprivation.
Second, among the small number of informative MZ twin pairs in each cohort, we consistently observed a higher prospective risk for DA in the MZ twins exposed to higher levels of SD. The CIs on these observations were wide but the trend was consistent across all three cohorts. Third, selecting a sample screened for geographical stability for a minimum of 5 years produced a pattern of SD–DA associations very similar to that for unscreened cohorts. Fourth, censoring all subjects who were registered for DA in the first 2 years of ascertainment, and so might have had an earlier onset of DA that could have led to downward social drift, produced almost no change in SD–DA association in the entire population and in full sibling pairs. Fifth, if we eliminated from our cohorts all subjects who moved during the follow-up, the magnitude of the SD–DA association increased slightly in the general population and even more so in the sibling pairs discordant for SD exposure.
All of these findings suggest that the SD–DA association we have observed in the Swedish population results, to a large extent, from the direct effect of experienced deprivation on risk for DA. However, two of our findings indicate caution in this conclusion. First, in the 2006 cohort we observe a small decline in the magnitude of the SD–DA association in full sibling pairs. Second, when we examine the SD–DA associations by age, we consistently see, across all three cohorts, a reduced association in closer relatives (full siblings and sometimes maternal half-siblings) in the youngest age groups. These results are open to two discrepant interpretations. Favoring the stress hypothesis, it might be that younger sibling pairs have had fewer years of living outside of their home of origin and thus more limited exposure to divergent levels of deprivation. Favoring the drift hypothesis, they may have had more limited opportunity than older pairs for downward drift to impact on the DA-prone member of the pair.
These two results suggest that selection may play some role in the association between SD and DA. However, it is noteworthy that, in all of our analyses, we controlled for a wide range of measures of personal SES. As shown in Table 4, on aggregate, these measures substantially attenuated the raw SD–DA association seen in the general population and in all relative pairs. Furthermore, the raw SD–DA association was considerably stronger in the general population than in close relative pairs, a pattern consistent with substantial familial confounding. However, controlling carefully for individual SES eliminated this pattern as the ORs in relative pairs closely resembled those seen in the population. Taken together, these results argue against the hypothesis that increased exposure to SD due to drug problems prior to official DA registration explains a large proportion of the observed SD–DA findings.
One important limitation of causal inference from the co-relative design is the role of non-familial con-founders. Our results, with their apparent evidence for an SD→DA causal relationship, could arise entirely from risk factors that predispose to both living in high SD environments and having DA as long as all those risk factors were uncorrelated in relatives. Although we cannot rule out this possibility, we suggest that it is implausible given the strong evidence for familial aggregation of the vast majority of human behavioral traits (Turkheimer, 2001).
We are not the first to use co-relative designs to clarify the causal origin of the association between psychiatric illness and social class. For example, Silverton & Mednick (1984) examined whether social class of the home of origin or downward drift best explained the SES–schizophrenia association. They found that the SES was lower in the schizophrenic subjects than in their unaffected siblings, and they interpreted this as evidence for drift.
The problem of causal inference is problematic and some epidemiologists argue that, in the absence of experimental data, causal attribution is impossible. Our conclusion is more sanguine. Our analyses, which combined prospective and family-based elements, provide reasonable support for the hypothesis that neighborhood factors contribute meaningfully to the etiology of DA. Although not definitive, these findings have sufficient empirical merit to warrant consideration when developing etiologic models for DA and when planning for primary prevention.
Strengths and limitations
These results should be interpreted in the context of both methodological strengths and limitations of our study. The strengths include the application of a longitudinal co-relative design to nationwide and nearly complete samples, ascertainment of DA from multiple sources, the control in all of our analyses for personal income, and the use of a lar ge number of small and precise geographical units.
Five potential methodological limitations are noteworthy. First, these results are limited to Sweden and may not apply to other countries, where the nature and distribution of SD might differ. Second, our findings do not provide any insight into the mechanisms whereby deprivation increases risk for DA. This is an important research topic that we hope to address in the future using the data available to us. Third, the co-relative design is based on the comparison of population-average models, which use standard logistic regression, and those from discordant relative pairs, which use conditional regression methods (Hu et al. 1998). Discrepancies in the estimates between these two approaches can occur and are largely dependent on the correlation between relatives. Hence, an estimate from a conditional regression can be approximated to a population-averaged coefficient based on the variance of the random effects (Hu et al. 1998). To explore this issue, we estimated the variance of the random effects among full siblings to be 0.56. This means that, for example, in our 2006 cohort, the population average OR for full siblings would decrease from 1.09 to 1.08. For the other relative pairs in this and other cohorts, the correlation is even smaller and is very unlikely to significantly bias our findings. Third, Frisell et al. (2012) have noted that a potential problem with the interpretation of within-relative-pair coefficients is that only pairs that differ in the exposure variable (in our case SD) will contribute to the estimation of the OR and these pairs may be unrepresentative of the entire population with respect to confounders. However, this critique is of limited relevance to us because our analytic methods differ from those critiqued by Frisell et al. (2012). Furthermore, omitted confoun-ders produce biased estimates in any regression model. However, the bias in conditional logistic regression (which provides us with the key finding that, in matched relative pairs, the one experiencing higher SD goes on to have higher rates of incident DA) is likely to be small and is usually much smaller than in standard logistic regression when, as in this study, large amounts of matched data are available (Greenland et al. 2000). Finally, it is plausible that the ascertainment of DA from health and criminal records varies across communities in Sweden. It is possible that this variation has introduced noise or bias in our findings.
Supplementary Material
Figure A1 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 15–19 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A2 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 20–24 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A3 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 25–29 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A4 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 30–34 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A5 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 35–39 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A6 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 40–49 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A7 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in both sexes from our 1996 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 1996 and the follow-up period defined as January 1997 to December 2001. However, in these analyses, we removed individuals who had moved residence one or more times in the five years preceding December 1996. For abbreviations, see figure legends in paper.
Figure A8 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in both sexes women from our 2001 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as January 2002 to December 2006. However, in these analyses, we removed individuals who had moved residence one or more times in the five years preceding December 2001. For abbreviations, see figure legends in paper.
Figure A9 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in women from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. However, in these analyses, we removed individuals who had moved residence one or more times in the five years preceding December 2006. For abbreviations, see figure legends in paper.
Fig. 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between neighborhood social deprivation (SD) and risk for subsequent drug abuse (DA) registration in both sexes from our 2001 cohort. A population-wide sample of individuals aged 15–44 years without prior for DA was selected as of December 2001 and the follow-up period defined as January 2002 to December 2006. The ORs are presented for the entire population (totalt) and then within relative pairs of first cousins (Cousin), paternal half-siblings (hsibp), maternal half-siblings (hsibm) and full-siblings (Sibs). For relative pairs, we only examined those that were doubly discordant (for both level of SD and DA outcome) as these were the only pairs that were statistically informative.
Table 2.
Sample size by gender, prevalence of drug abuse (DA), sample size by relative pairs and their correlation for social deprivation (SD) in all individuals included in the analysis of the 2001 cohort
| n | Prevalence of DA (%), 2002–2006 | ||
|---|---|---|---|
| Women | 1675686 | 0.7 | |
| Men | 1734772 | 1.9 | |
|
| |||
| Pair correlation for SD | n pairs (total) | n pairs included in the CLR | |
|
| |||
| Cousins | |||
| Women | 0.116 | 1393852 | 38568 |
| Men | 0.119 | 1544334 | 92126 |
| Paternal half-siblings | |||
| Women | 0.141 | 67136 | 3470 |
| Men | 0.137 | 69255 | 7762 |
| Maternal half-siblings | |||
| Women | 0.237 | 54551 | 2686 |
| Men | 0.221 | 56628 | 5530 |
| Full siblings | |||
| Women | 0.459 | 413419 | 8058 |
| Men | 0.476 | 457905 | 16152 |
CLR, Conditional logistic regression.
Acknowledgments
This study was funded by grant RO1 DA030005 from the National Institute of Drug Abuse, the Swedish Research Council (2012–2378), the ALF project grant, Lund, Sweden, the Swedish Council for Information on Alcohol and Other Drugs (CAN) and The Swedish Research Council for Health, Working Life and Welfare (reg. no: 2013-1836). C. Gardner provided helpful statistical advice.
Footnotes
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713003048.
Declaration of Interest
None.
References
- Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Archives of General Psychiatry. 1998;55:982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Troughton E, O’Gorman TW, Heywood E. An adoption study of genetic and environmental factors in drug abuse. Archives of General Psychiatry. 1986;43:1131–1136. doi: 10.1001/archpsyc.1986.01800120017004. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. An adoption study of drug abuse/dependency in females. Comprehensive Psychiatry. 1996;37:88–94. doi: 10.1016/s0010-440x(96)90567-2. [DOI] [PubMed] [Google Scholar]
- Chaikiat A, Li X, Bennet L, Sundquist K. Neighborhood deprivation and inequities in coronary heart disease among patients with diabetes mellitus: a multilevel study of 334,000 patients. Health and Place. 2012;18:877–882. doi: 10.1016/j.healthplace.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Crum RM, Lillie-Blanton M, Anthony JC. Neighborhood environment and opportunity to use cocaine and other drugs in late childhood and early adolescence. Drug and Alcohol Dependence. 1996;43:155–161. doi: 10.1016/s0376-8716(96)01298-7. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, Skodol AE, Stueve A. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science. 1992;255:946–952. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- Faris RE, Dunham HW. Mental Disorders in Urban Areas: An Ecological Study of Schizophrenia and Other Psychoses. University of Chicago Press; Chicago, IL: 1939. [Google Scholar]
- Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Morrison SL. Schizophrenia and social class. British Journal of Psychiatry. 1963;109:785–802. doi: 10.1192/bjp.109.463.785. [DOI] [PubMed] [Google Scholar]
- Greenland S, Schwartzbaum JA, Finkle WD. Problems due to small samples and sparse data in conditional logistic regression analysis. American Journal of Epidemiology. 2000;151:531–539. doi: 10.1093/oxfordjournals.aje.a010240. [DOI] [PubMed] [Google Scholar]
- Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. American Journal of Epidemiology. 1998;147:694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- Huie SAB. The concept of neighborhood in health and mortality research. Social Spectrum. 2001;21:341–358. [Google Scholar]
- Karriker-Jaffe KJ. Areas of disadvantage: a systematic review of effects of area-level socioeconomic status on substance use outcomes. Drug and Alcohol Review. 2011;30:84–95. doi: 10.1111/j.1465-3362.2010.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N, Winkleby M, Skog L, Szulkin R, Sundquist K. Differences in neighborhood accessibility to health-related resources: a nationwide comparison between deprived and affluent neighborhoods in Sweden. Health and Place. 2011;17:132–139. doi: 10.1016/j.healthplace.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Archives of General Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Sundquist K, Sundquist J. Environmental influences on familial resemblance for drug abuse in first-cousin pairs: a Swedish national study. Psychological Medicine. 2013 doi: 10.1017/S0033291713000846. Published online: April 23, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Sundquist K, Ohlsson H, Palmer K, Maes H, Winkleby MA, Sundquist J. Genetic and familial-environmental influences on risk for drug abuse: a national Swedish adoption study. Archives of General Psychiatry. 2012;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychological Medicine. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Muntaner C, Eaton WW, Diala C, Kessler RC, Sorlie PD. Social class, assets, organizational control and the prevalence of common groups of psychiatric disorders. Social Science and Medicine. 1998;47:2043–2053. doi: 10.1016/s0277-9536(98)00309-8. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten TR, Weissman MM, Prusoff B, Pauls D, Anton SF, Merikangas K. Psychiatric disorders in relatives of probands with opiate addiction. Archives of General Psychiatry. 1991;48:33–42. doi: 10.1001/archpsyc.1991.01810250035004. [DOI] [PubMed] [Google Scholar]
- Rutter M. Identifying the environmental causes of disease: how should we decide what to believe and when to take action? Academy of Medical Sciences; London: 2007a. ( http://acmedsci.ac.uk/policy/policy/identifying-the-environmental-causes-of-disease/) [Google Scholar]
- Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiments. Perspectives on Psychological Science. 2007b;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Sareen J, Afifi TO, McMillan KA, Asmundson GJ. Relationship between household income and mental disorders: findings from a population-based longitudinal study. Archives of General Psychiatry. 2011;68:419–427. doi: 10.1001/archgenpsychiatry.2011.15. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS OnlineDoc Version 9.2. SAS Institute Inc; Cary, NC: 2008. [Google Scholar]
- Silverton L, Mednick S. Class drift and schizophrenia. Acta Psychiatrica Scandinavica. 1984;70:304–309. doi: 10.1111/j.1600-0447.1984.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. American Journal of Medical Genetics. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Turkheimer E. Three laws of behavior genetics and what they mean. Current Directions in Psychological Science. 2001;9:160–164. [Google Scholar]
- Turner RJ, Wagenfeld MO. Occupational mobility and schizophrenia: an assessment of the social causation and social selection hypotheses. American Sociological Review. 1967;32:104–113. [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug and Alcohol Dependence. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlatesofdrug use and dependence in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:219–229. doi: 10.1001/archpsyc.1995.03950150051010. [DOI] [PubMed] [Google Scholar]
- Williams CT, Latkin CA. Neighborhood socioeconomic status, personal network attributes, and use of heroin and cocaine. American Journal of Preventive Medicine. 2007;32:S203–S210. doi: 10.1016/j.amepre.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby M, Sundquist K, Cubbin C. Inequities in CHD incidence and case fatality by neighborhood deprivation. American Journal of Preventive Medicine. 2007;32:97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A1 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 15–19 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A2 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 20–24 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A3 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 25–29 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A4 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 30–34 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A5 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 35–39 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A6 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in individuals aged 40–49 from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. For abbreviations, see figure legends in paper.
Figure A7 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in both sexes from our 1996 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 1996 and the follow-up period defined as January 1997 to December 2001. However, in these analyses, we removed individuals who had moved residence one or more times in the five years preceding December 1996. For abbreviations, see figure legends in paper.
Figure A8 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in both sexes women from our 2001 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as January 2002 to December 2006. However, in these analyses, we removed individuals who had moved residence one or more times in the five years preceding December 2001. For abbreviations, see figure legends in paper.
Figure A9 – Odds Ratios (and 95% Confidence Intervals) for the Association Between Neighborhood Social Deprivation and Risk for Subsequent Drug Abuse (DA) Registration in women from our 2006 cohort. A population wide sample of individuals aged 15–44 years without prior for Drug Abuse was selected as of December 2001 and the follow-up period defined as from January 2007 to December 2011. However, in these analyses, we removed individuals who had moved residence one or more times in the five years preceding December 2006. For abbreviations, see figure legends in paper.


