Abstract
Objective
Prior research has found that within-person standard deviations across different neuropsychological domains are larger in various clinical groups than in healthy control groups, but little is known about the specificity of these measures to clinical conditions.
Method
Within-person standard deviations were computed across composite scores representing episodic memory, perceptual speed, inductive reasoning, and spatial visualization, and compared in older adults differing in the amount of subsequent cognitive change, and as a function of age in a large sample of adults ranging from 18 to 89 years of age.
Results
The standard deviations at an initial occasion were significantly greater in older adults who experienced the most negative longitudinal change, but relations of the standard deviations with age were only evident in adults under 65 years of age, and they were negative rather than positive.
Conclusions
These findings suggest that high values of within-person variability may have specificity in predicting late life cognitive decline.
Keywords: longitudinal change, within-person variability, across-domain diversity
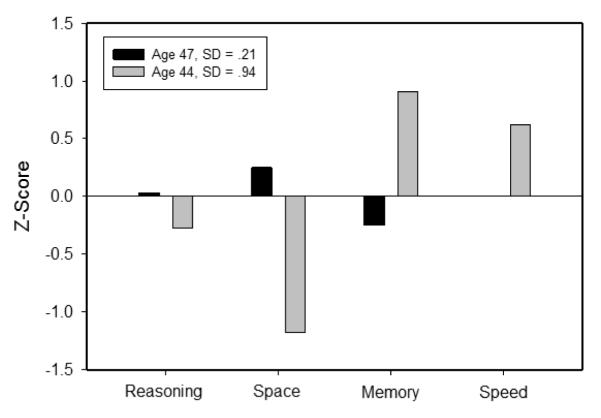
When multiple cognitive abilities are assessed in the same individuals it is possible to compare profiles based on the relative levels of different abilities. For example, Figure 1 portrays the mean levels (expressed in z-score units) of four cognitive abilities for two adults age 44 and 47 years of age. Despite having nearly identical overall means, the two individuals had markedly different ability profiles, with the 44-year-old having a more heterogeneous profile than the 47-year-old. A convenient way to represent differences in profiles is in terms of the standard deviation across the four abilities, and it can be seen that one of these individuals had a standard deviation of .21 whereas the other had a standard deviation of .94. The major questions in this report are whether this measure of within-person across-ability variability is higher at an initial occasion in individuals who subsequently experience large cognitive decline, and whether it is related to age in healthy adults.
Figure 1.
Illustration of ability profiles for two participants with nearly the same average level of cognitive performance. The z-score for speed in the 47-year-old was zero, and thus the value is not apparent in the figure.
Because they may reflect different phenomena, and could have different causes and consequences, it is important to distinguish different types of within-person variability. Each type is expressed relative to the individual’s own mean, but they differ in that the variability can be across trials within the same test, across sessions with the same test, or across different tests. The focus in this report is on the latter form of variability, namely, the heterogeneity of different cognitive abilities in the same individual, and not inconsistency of the individual’s performance across repetitions of trials within the same test, or inconsistency of the individual’s performance of the same test across separate sessions.
Interest in ability profiles has a long history in psychological assessment (e.g., Matarazzo et al., 1988; Matarazzo & Prifitera, 1989), but there has been a recent resurgence of interest in this topic for both theoretical and empirical reasons. One theoretical reason is that the multivariate information inherent in across-test variability has been postulated to be a more sensitive indicator of cognitive status than the measures from any single domain (Kliegel & Sliwinski, 2004). Another theoretical speculation is that greater across-domain variability may reflect poor sustained cognitive control, and/or allocation of resources across different types of cognitive tests (Morgan et al., 2012a). Still another proposal is that within-person variability might be an indirect measure of neural connectivity (Reichenberg et al. 2006). That is, if different brain regions are involved in different types of cognitive abilities (e.g., Colom et al., 2009; Jung & Haier, 2007), weaker structural or functional connectivity among regions could diminish the spread of influences across abilities and contribute to greater distinctiveness of ability profiles.
The major empirical reason for the interest in across-ability variability is that several reports have found greater variability in clinical groups, such as schizophrenics (Reichenberg et al. 2006), patients positive for HIV (Morgan et al., 2011, 2012b), patients with hepatitis C (Morgan et al., 2012a), patients with dementia (Reckess et al., 2013), athletes after a sports-related concussion (Rabinowitz & Arnett, 2013), and individuals with low functional status (Rapp et al., 2005). There are also reports of higher within-person variability for individuals who subsequently develop cognitive pathologies (Holtzer et al., 2008; Kliegel & Sliwinski, 2004). Taken together, these findings suggest that the distinctiveness or heterogeneity of cognitive ability profiles may convey important information about an individual’s cognitive status.
Results of two studies are described in the current report. The first study examined the predictive power of across-domain variability at the initial occasion on subsequent cognitive change in older adults. The second study examined properties of within-person variability among healthy adults across a wide age range in both cross-sectional and longitudinal comparisons.
Several issues need to be considered when examining group differences in within-person variability. First, although it is desirable that the variability reflect a number of different domains of cognitive functioning, it may be misleading to include tests of acquired knowledge in the computations because tests of knowledge reflect the products of processing carried out earlier in life rather than efficiency or effectiveness of processing at the time of assessment. Furthermore, the existence of different age trends in product and process measures may introduce artifactual decreases followed by increases in relations of age to within-person variability as ability levels first converge and then diverge with increased age. Vocabulary or other measures of knowledge have sometimes been used as a reference to compare with other cognitive measures because they have been postulated to reflect early-life intelligence (Christensen et al., 1999; Rabbitt, 1993), but their inclusion in the computation of across-ability variability should either be avoided, or implemented with caution.
Second, because variability will likely be lowest near the floors and ceilings of measurement, there may be an inverted-U function relating variability to mean performance. It is therefore important to control for the mean when analyzing group differences in variability if the groups differ in average level of performance.
And third, although variability could be computed across scores on individual tests, the resulting variability measure will include measurement error and influences of characteristics of specific tests in addition to those of relevant abilities. However, influences of the relevant ability can be emphasized, and reliability increased, by conducting analyses on composite scores instead of scores of individual tests.
Study 1
The purpose of the first study was to investigate relations of across-domain variability on a first occasion with cognitive decline from the first (T1) to a second (T2) occasion. The key question was whether within-person variability was greater in individuals who could be considered to be vulnerable because they subsequently exhibited the most negative cognitive change.
Many different criteria could be used to classify individuals as vulnerable or normal. The criteria used here were based on the magnitude of longitudinal change from a first to a second measurement occasion. Individuals in the bottom 10% of the distribution of changes were considered to be in the vulnerable group. In order to compare individuals exhibiting the most negative change with those exhibiting more typical change, the normal group was defined as individuals in the middle 50% of the distribution of changes. However, it should be noted that similar results were obtained when the normal group was defined as the 90% of the individuals not in the bottom 10% of the change distribution.
Method
Sample
The sample consisted of a subset of participants from the Virginia Cognitive Aging Project (VCAP; Salthouse, in press; Salthouse et al., 2008) who were 65 years of age and older and who had completed at least two longitudinal occasions. Participants in VCAP were recruited from newspaper advertisements, flyers, and referrals from other participants. Approximately 79% of the VCAP participants were white, 11% African-American, and the remainder distributed across other racial categories or reporting more than one race. Information on the selectivity of the longitudinal participants relative to the initial sample is provided in other reports (Salthouse, 2010; in press-a).
Two groups of participants were formed based on the T2 residual of the average composite score across four ability domains after controlling the T1 average composite score, age, and years of education. The groups consisted of “at risk” individuals in the bottom 10% of the distribution of T2 residuals, and individuals in the middle 50% of the distribution, who were considered normals. Characteristics of the individuals in the two groups are reported in Table 1.
Table 1.
Sample characteristics for “at risk” and normal individuals age 65 and older with longitudinal data
| Mean T2 Residual | “At Risk” Bottom 10% |
Normal Middle 50% |
t | d |
|---|---|---|---|---|
| N | 58 | 294 | ||
| Age | 74.3 (5.8) | 72.8 (5.6) | 1.87 | 0.20 |
| Proportion Female | .48 | .59 | −1.44 | −0.15 |
| Self-Rated Health | 2.6 (0.8) | 2.3 (0.9) | 2.33 | 0.25 |
| Years of Education | 16.0 (2.7) | 16.4 (3.0) | −0.89 | −0.09 |
| T1 MMSE | 27.8 (2.3) | 28.4 (1.6) | −1.86 | −0.22 |
| T2 MMSE | 27.0 (3.2) | 28.1 (1.8) | −4.01* | −0.43 |
| Scaled Scores | ||||
| Vocabulary | 13.1 (2.7) | 13.2 (2.7) | −0.39 | −0.04 |
| Digit Symbol | 11.0 (2.6) | 11.7 (2.6) | −2.13 | −0.23 |
| Logical Memory | 12.6 (3.4) | 12.5 (2.7) | 0.39 | 0.04 |
| Word Recall | 12.2 (4.2) | 12.3 (3.1) | −0.16 | −0.02 |
| T1-T2 Interval (years) | 3.8 (1.5) | 2.5 (0.9) | 8.53* | 0.91 |
| T1 Mean Cognition | −.55 (.61) | −.44 (.56) | −1.20 | −0.13 |
| T2-T1 Mean Difference | −.58 (.30) | .02 (.09) | −27.51* | −2.94 |
| T2-T1 Memory Difference | −.61 (.42) | −.03 (.37) | −8.26* | −0.95 |
| T2-T1 Speed Difference | −.42 (.44) | −.00 (.32) | −8.85* | −0.91 |
| T2-T1 Reasoning Difference | −.40 (.33) | −.05 (.34) | −4.04* | −0.52 |
| T2-T1 Space Difference | −.31 (.29) | .01 (.30) | −5.73* | −0.66 |
| T1 Within-Person SD | .59 (.29) | .48 (.22) | 2.85* | 0.31 |
Note: p<.01. Values in parentheses are standard deviations. MMSE is the Mini Mental State Exam (Folstein et al., 1975), and self-rated health is on a scale from 1 for excellent to 5 for poor.
Neurocognitive Tests
At each occasion the participants performed 12 neuropsychological tests selected to reflect four different abilities. Reasoning ability was represented by Matrix Reasoning, Shipley Abstraction, and Letter Sets; Spatial Visualization ability was represented by Spatial Relations, Paper Folding, and Form Boards; Episodic Memory ability was represented by Word Recall, Paired Associates and Logical Memory; and Perceptual Speed ability by Digit Symbol, Pattern Comparison, and Letter Comparison. The tasks have been described in more detail in other articles (e.g., Salthouse, in press-b; Salthouse & Ferrer-Caja, 2003; Salthouse, Pink & Tucker-Drob, 2008; Salthouse, Siedlecki & Krueger, 2006). These other articles also report coefficient alpha estimates of the reliabilities, which ranged from .71 to .91, and results of confirmatory factor analyses supporting the construct validity of the variables.
The scores on each of the 12 tests were converted to z-scores and then averaged across the tests representing a given domain to allow analyses to be conducted on composite scores representing each ability domain. The measure of across-domain within-person variability was the standard deviation of the composite scores for the four abilities.
Results and Discussion
Characteristics of the two groups are reported in Table 1. Note that, as expected based on how the groups were selected, the “at risk” group had much more negative change from the first to the second occasion than the “normal” group in the mean cognitive score, and in the composite scores in each cognitive domain. The only other significant differences between the “at risk” and the “normal” groups were with the length of the interval between T1 and T2 and the measure of within-person variability. Additional analyses revealed that the group differences in the within-person variability measure were still significant (p<.01) when the T1-T2 interval was included as a covariate in the analysis.
Two additional analyses were conducted to examine the robustness of the results. One analysis investigated the possibility that the results in Table 1 might have been an artifact of contrasting an extreme group with a typical group. The analysis was therefore repeated with the two groups consisting of individuals in the top 10% (instead of the bottom 10%) of change and individuals in the middle 50% of change. The average within-person standard deviations in the two groups were .45 and .48, respectively, and the difference was not significant (i.e., t = −.95, p>.34, d = −.10). These results are therefore inconsistent with the possibility that the within-person standard deviations are greater in any group selected on the basis of extreme values of change, and instead indicate that the higher variability appears to be specific to the group with the most negative change.
A second analysis reversed the direction of the relation and compared the magnitude of cognitive change in groups defined on the basis of within-person variability at the initial occasion. The normal group in this analysis was defined as individuals with T1 standard deviations in the middle 50% of the distribution, and the vulnerable group was defined as individuals in the top 10% of the distribution of T1 standard deviations. As expected from the results in Table 1, the group with the greatest within-person variability at T1 had significantly more negative average cognitive change than the normal group (i.e., −.16 vs. −.06, t = 2.59, p<.01, d = −.28). These results therefore indicate that the relation between within-person variability at baseline and subsequent cognitive change is evident regardless whether the groups are defined on the basis of initial variability, or in terms of the magnitude of change.
The results of this study extend earlier studies in the finding that vulnerable individuals, in this case defined as those with the most negative change from T1 to T2, had higher across-domain variability at the initial (T1) occasion than normal individuals. An intriguing implication of this finding is that across-domain within-person variability may be a sensitive indicator of impending cognitive decline.
Study 2
The purpose of Study 2 was to examine whether there were also relations of within-person across-ability variability to age in healthy adults. A discovery that within-person variability was greater with increased age in healthy adults might imply that heterogeneity of abilities is a sensitive measure of an individual’s overall cognitive status, and would lead to questions as to the specificity of within-person variability as an indicator of subsequent decline.
In addition to determining whether increased variability might be specific to late-life decline in vulnerable individuals, the relation of age to within-person variability across cognitive abilities may be relevant to the nature of age-related influences on cognitive functioning. For example, Lindenberger and Baltes (1997) suggested that the degree of within-person variability would be expected to be smaller at older ages if increased age is associated with a greater contribution of general influences relative to ability-specific influences on cognitive functioning.
However, an opposite pattern might be expected if large proportions of the age-related influences on cognitive functioning are specific rather than general. For example, differential ability change could occur if there are greater age-related changes in the brain regions responsible for certain abilities than in others, or if selective engagement in certain activities results in greater preservation of some abilities compared to others. Regardless of the reason, if age-related influences vary across abilities, the relative levels of abilities might become more distinct with increased age and result in larger across-ability standard deviations, and more pronounced, or heterogeneous, ability profiles.
Another prediction from the differential-change interpretation is that the age-related differences should be less negative for measures of the individuals’ best ability compared to his or her worst ability. That is, regardless of which specific abilities are the best or worst for particular individuals, if age-related influences are primarily ability-specific, the age trends on the individual’s best ability might be expected to be less negative than those on his or her worst ability.
Only a few studies have examined within-person variability across different cognitive domains in healthy adults, and the results have been inconsistent. For example, in adults over 64 years of age, Lindenberger and Baltes (1997) found that increased age was associated with smaller within-person variability, but Christensen et al. (1999) and Hilburn et al. (2009) both found larger within-person standard deviations at older ages. Adults from 20 to 92 years of age were examined by Schretlen et al. (2003), who reported a correlation of .22 with age and the difference between the individual’s highest and lowest scores. Longitudinal changes in within-person dispersion were investigated by Christensen et al (1999) who found no relation of age to change in within-person variability in a sample of adults 70 years of age and older.
Method
Sample
Characteristics of the VCAP participants in the cross-sectional sample and the subset of these individuals in the longitudinal sample are reported in Table 2.
Table 2.
Sample characteristics for age comparisons
| Cross-Sectional | Longitudinal | |
|---|---|---|
| N | 4775 | 2250 |
| Age | 50.8 (18.0) | 53.4 (16.4) |
| Proportion Female | .65 | .67 |
| Self-Rated Health | 2.2 (0.9) | 2.2 (0.9) |
| Years of Education | 15.6 (2.7) | 15.7 (2.7) |
| T1 MMSE | 28.4 (1.9) | 28.5 (1.7) |
| Scaled Scores | ||
| Vocabulary | 12.4 (3.2) | 12.5 (3.0) |
| Digit Symbol | 11.2 (2.9) | 11.4 (2.8) |
| Logical Memory | 11.6 (3.0) | 11.9 (2.9) |
| Word Recall | 12.0 (3.3) | 12.2 (3.3) |
| T1-T2 Interval (years) | NA | 3.0 (1.6) |
Neurocognitive tests
The tests were the same as those described in Study 1, and composite scores for each ability and measures of within-person across-ability variability were computed in the same manner as described earlier.
Results and Discussion
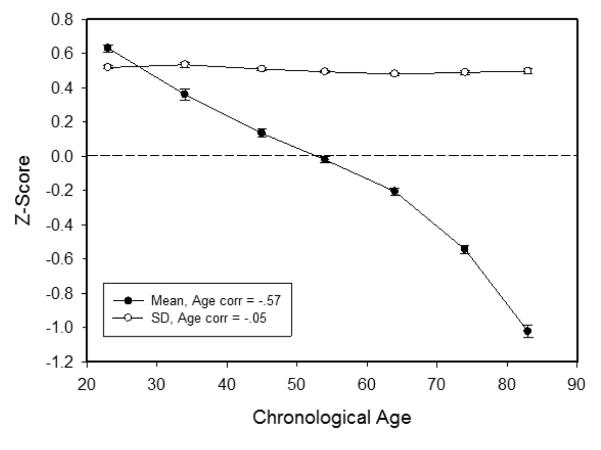
The means and within-person standard deviations across the four composite ability scores in the cross-sectional sample are plotted as a function of age decade in Figure 2. It can be seen that there was a moderate negative correlation of age (i.e., r = −.57) with the mean of the four composite scores, but a very small age relation (i.e., r = .05) with the within-person standard deviations across the four composite scores.
Figure 2.
Means (and standard errors) of the across-ability mean and across-ability standard deviation in each age decade.
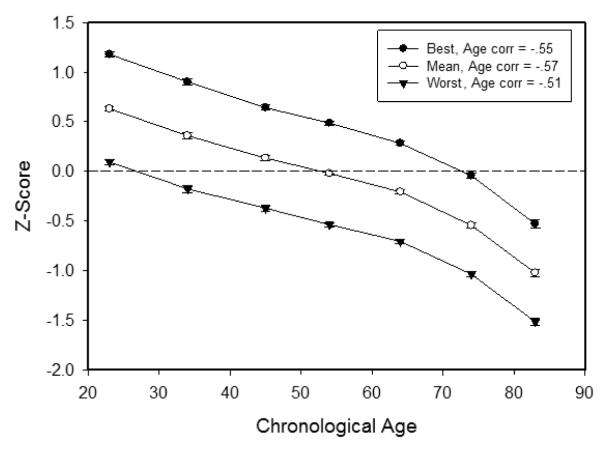
The prediction of less negative age trends for an individual’s best ability compared to his or her worst ability was examined by rank-ordering each individual’s z-scores for the composite scores in the four abilities, and then determining the age relations for the different ranks. These data are portrayed in Figure 3, which contains the means by decade of each individual’s best (rank 1), average, and worst (rank 4) ability by decade in the cross-sectional data. Note that the average levels of performance were highest for the best ability and lowest for the worst ability, and that the age trends for the best, average, and worst abilities were nearly parallel.
Figure 3.
Means (and standard errors) of the scores for each individual’s best, average, and worst ability by age decade.
The data summarized in Figure 3 were subjected to hierarchical regression analyses to determine the proportion of variance associated with age in the individuals’ best and worst values after control of the variance in other measures. The initial percentage of variance associated with age in the best ability was 31%, and after control of the average value it was 0.1%, and after control of the value corresponding to the worst ability it was 3.8%. Corresponding values for the worst ability were 26%, 0%, and 1.2%, respectively, without any control, after controlling the average value, and after controlling the value for the best ability. These results indicate that not only are the age trends in the three measures nearly parallel, but that there is considerable overlap of the age-related variance in the three measures, with very small independent age relations.
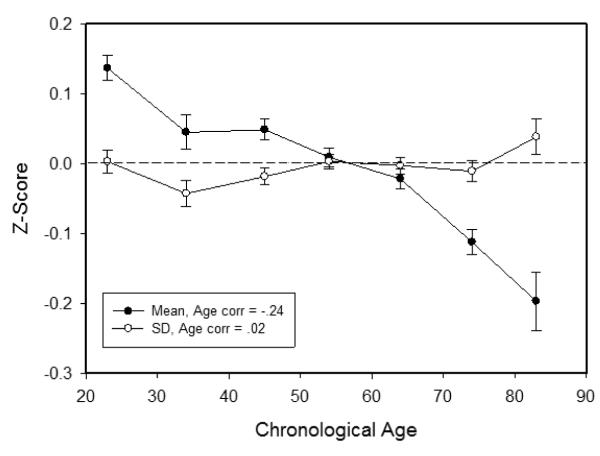
Longitudinal changes in within-individual variability were also examined, and Figure 4 portrays across-ability means and within-person standard deviations of the longitudinal differences from T1 to T2. The results were similar to Figure 3 in that there was a moderate negative age relation (i.e., r = −.24) on the longitudinal differences in the means, indicating more negative change at older ages, but little age relation (i.e., r = .02) on the longitudinal difference in the within-individual variability measure.
Figure 4.
Means (and standard errors) of the T2-T1 differences in across-ability mean and across-ability standard deviation in each age decade.
Because measures of variability may be related to the mean, regression analyses were conducted examining the relation of age to the within-person standard deviations after controlling linear and quadratic relations of the mean of the composite scores. Identical analyses were conducted in the complete sample, and also separately in adults between 18 and 64 years of age and in adults between 65 and 89 years of age because the age range in the latter group was similar to earlier studies reporting different patterns of age relations.
The left panel of Table 3 reports the standardized coefficients from regression analyses predicting within-person standard deviations at the first occasion, and the right panel contains results with the longitudinal T2-T1 differences. Several points should be noted about these results. First, although a number of the relations were significant, all of the analytical models accounted for very small proportions of variance (i.e., R2 values). Second, significant relations of age were evident in the cross-sectional sample, but they were negative and primarily evident among individuals between 18 and 64 years of age because the relation in the older group was not significant, and positive rather than negative. And third, there were no significant relations of age to the measure of longitudinal difference in within-person variability.
Table 3.
Standardized coefficients predicting across-domain variability from regression analyses
| Cross-Sectional | Longitudinal | |||||
|---|---|---|---|---|---|---|
| All | 18-64 | 65-89 | All | 18-64 | 65-89 | |
| MeanT11 | ||||||
| Linear | −.05 | −.04 | .13 | .00 | .02 | −.01 |
| Quadratic | −.08* | −.09* | .04 | .03 | .00 | .07 |
| Age | −.08* | −.09* | .08 | .02 | .02 | .00 |
| R2 | .01 | .01 | .01 | .00 | .00 | .01 |
General Discussion
The results of Study 1 indicate that, compared to individuals with typical longitudinal change, vulnerable individuals, defined as those with the most negative longitudinal change, had a more heterogeneous ability profile, with greater within-person variability at the first occasion. Furthermore, additional analyses revealed that the relations were still evident when the analyses were reversed, and mean change was examined in groups differing in magnitude of within-person variability at the initial occasion. These findings extend results of earlier studies with various patient groups in demonstrating that across-ability within-person variability at an initial occasion is predictive of the individual’s future status, as reflected in subsequent cognitive change.
Although the discovery of a relation between the heterogeneity of ability profiles at an initial occasion and amount of cognitive decline is interesting, it is important to examine relations of diversity to age in healthy adults to determine if relations involving within-person variability are specific to individuals about to experience substantial decline. The only relations of age on within-person across-ability variability in Study 2 were negative, and restricted to the period between about 18 and 64 years of age. The absence of age relations in healthy adults, together with the discovery of significant differences in individuals with the most negative change, suggests that higher levels of within-person variability may be specific to impending pathological decline, and not a component of normal aging. If the pattern of little relation in normal adults but significant relations in individuals who might be considered at risk is confirmed in subsequent studies, measures of within-person variability could be valuable as indicators of potentially pathological change, distinct from normal aging.
The lack of positive age relations on the measures of within-person variability is inconsistent with differential ability-specific age-related influences because that should have resulted in an age-related increase in across-ability standard deviations. As indicated in Table 3, the only relations with age were negative, and they were restricted to the period under about 65 years of age. Furthermore, the age trends for the best and worst abilities for each individual were very similar, instead of diverging as one would expect if there is relative preservation of one’s best abilities.
One of the reasons for expecting greater across-domain variability with increased age is that a number of studies have reported weaker functional connectivity across different brain regions at older ages (e.g., Andrews-Hanna et al., 2007; Dennis et al., 2008; Park et al., 2010; Wang et al., 2010). This reduction in connectivity among the brain regions involved in different cognitive abilities might therefore be expected to weaken the diffusion of homogenizing influences across abilities, in a manner analogous to how species might exhibit greater differentiation as they become isolated in different ecological niches. Because this expectation was not supported in these data, it would be informative to investigate the validity of the assumption that connectivity is related to within-person variability by comparing across-ability variability in the same individuals for whom measures of functional or structural connectivity are available.
In summary, the results of these studies indicate that measures of across-domain variability could be valuable indicators of impending cognitive decline. The finding in the first study that individuals who subsequently experienced the most negative cognitive decline had higher within-individual standard deviations than individuals within the middle of the distribution for decline suggests that the measure has sensitivity, and the finding in the second study of little or no relation of within-person variability with age in healthy individuals suggests that the measure may be specific to late-life pathological aging.
Acknowledgments
This research was supported by Award Number R37AG024270 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
There are no conflicts of interest.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P. Dispersion in cognitive ability as a function of age: A longitudinal study of an elderly community sample. Aging, Neuropsychology and Cognition. 1999;6:214–229. [Google Scholar]
- Colom R, Haier RJ, Head K, Alvarez-Linera J, Quiroga MA, Shih PC, Jung RE. Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the PFIT model. Intelligence. 2009;37:124–135. [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory and Cognition. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, Hunter MA. Intraindividual variability across cognitive domains: Investigation of dispersion levels and performance profiles in older adults. Journal of Clinical and Experimental Neuropsychology. 2009;31:412–424. doi: 10.1080/13803390802232659. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. Journal of American Medical Association. 2008;300:823–830. doi: 10.1001/jama.300.7.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of young and older adults. Journal of Gerontology: Psychological Sciences. 2002;57B:P101–P115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30:135–187. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Sliwinski M. MMSE cross-domain variability predicts cognitive decline in centenarians. Gerontology. 2004;50:39–43. doi: 10.1159/000074388. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: Cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997;12:410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Matarazzo JD, Prifitera A. Subtest scatter and premorbid intelligence: Lessons from the WAIS-R standardization sample. Psychological Assessment. 1989;1:186–191. [Google Scholar]
- Matarazzo JD, Daniel MH, Prifitera A, Herman DO. Inter-subtest scatter in the WAIS-R standardization sample. Journal of Clinical Psychology. 1988;44:940–950. [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I, HIV Neurobehavioral Research Program (HNRP) Group Intraindividual variability in HIV infection: Evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25:645–654. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Grant I, HIV Neurobehavioral Research Program (HNRP) Group Archives of Clinical Neuropsychology. 2012;27:293–303. [Google Scholar]
- Morgan EE, Woods SP, Rooney A, Perry W, Grant I, Letendre SL, HIV Neurobehavioral Research Program (HNRP) Group Intraindividual variability across neurocognitive domains in chronic Hepatitis C infection: Elevated dispersion is associated with serostatus and unemployment risk. Clinical Neuropsychologist. 2012;26:654–674. doi: 10.1080/13854046.2012.680912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Frontiers in Human Neuroscience. 2010;3 doi: 10.3389/neuro.09.075.2009. Article #75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz AR, Arnett PA. Intraindividual cognitive variability before and after sports-related concussion. Neuropsychology. 2013;27:481–490. doi: 10.1037/a0033023. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Sano M, Silverman JM, Haroutunian V. Cross-domain variability of cognitive performance in very old nursing home residents and community dwellers: Relationship to functional status. Gerontology. 2005;51:206–212. doi: 10.1159/000083995. [DOI] [PubMed] [Google Scholar]
- Reckess GZ, Varvaris M, Gordon B, Schretlen DJ. Within-person distributions of neuropsychological test scores as a function of dementia severity. Neuropsychology. doi: 10.1037/neu0000017. (in press) [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, Knobler HY, Lubin G, Nahon D, Harvey PD, Davidson M. Premorbid intra-individual variability in intellectual performance and risk for schizophrenia: A population-based study. Schizophrenia Research. 2006;85:49–57. doi: 10.1016/j.schres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of age on practice effects in longitudinal neurocognitive change. Neuropsychology. 2010;24:563–572. doi: 10.1037/a0019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selectivity of attrition in longitudinal studies of cognitive functioning. Journal of Gerontology: Psychological Sciences. doi: 10.1093/geronb/gbt046. (in press-a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Correlates of cognitive change. Journal of Experimental Psychology: General. doi: 10.1037/a0034847. (in press-b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging. 2003;18:91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Pink JE, Tucker-Drob EM. Contextual analysis of fluid intelligence. Intelligence. 2008;36:464–486. doi: 10.1016/j.intell.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Siedlecki KL, Krueger LE. An individual differences analysis of memory control. Journal of Memory and Language. 2006;55:102–125. doi: 10.1016/j.jml.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Munro CA, Anthony JC, Pearlson GD. Examining the range of normal intraindividual variability in neuropsychological test performance. Journal of the International Neuropsychological Society. 2003;9:864–870. doi: 10.1017/S1355617703960061. [DOI] [PubMed] [Google Scholar]
- Wang L, LaViolette P, O’Keefe K, Putcha D, Bakkour A, van Dijk KRA, Pihlajamaki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]