Abstract
Juvenile hormone (JH) plays important roles in regulation of many physiological processes including development, reproduction and metabolism in insects. However, the molecular mechanisms of JH signaling pathway are not completely understood. To elucidate the molecular mechanisms of JH regulation of Krüppel homolog 1 gene (Kr-h1) in Aedes aegypti, we employed JH-sensitive Aag-2 cells developed from the embryos of this insect. In Aag-2 cells, AaKr-h1 gene is induced by nanomolar concentration of JH III, its expression peaked at 1.5 hr after treatment with JH III. RNAi studies showed that JH induction of this gene requires the presence of Ae. aegypti methoprene-tolerant (AaMet). A conserved 13 nucleotide JH response element (JHRE, TGCCTCCACGTGC) containing canonical E box motif (underlined) identified in the promoter of AaKr-h1 is required for JH induction of this gene. Critical nucleotides in the JHRE required for JH action were identified by employing mutagenesis and reporter assays. Reporter assays also showed that basic helix loop helix (bHLH) domain of AaMet is required for JH induction of AaKr-h1. 5’ rapid amplification of cDNA ends method identified two isoforms of AaKr-h1, AaKr-h1α and AaKr-h1β, the expression of both isoforms is induced by JH III, but AaKr-h1α is the predominant isoform in both Aag-2 cells and Ae. aegypti larvae.
Keywords: Juvenile hormone, Methoprene-tolerant, Krüppel homolog 1, bHLH | JH response element
1. INTRODUCTION
Many physiological processes in insects including growth, development, metamorphosis and reproduction are under the control of juvenile hormone (JH). Due to their presence only in Arthropods, JH has attracted a lot of attention as selective targets for the design and development of environmentally friendly insecticides (Parthasarathy et al., 2012). There has been a tremendous progress in understanding JH action during the last few years (Jindra et al., 2013). However, the molecular mechanisms of JH action are not completely understood.
Methoprene-tolerant (Met) is a basic helix-loop-helix (bHLH)/Per-Arnt-Sim (PAS) domains containing protein that specifically binds to JH III (Charles et al., 2011; Miura et al., 2005). Steroid receptor co-activator (SRC/FISC/p160/Taiman) was also found to be required for Met function in activation of transcription of JH target genes (Li et al., 2011; Zhang et al., 2011). Krüppel homolog 1 gene (Kr-h1), a C2H2 zinc-finger type transcription factor, had been identified as an early JH-response gene in Drosophila melanogaster (Minakuchi et al., 2008), Tribolium castaneum (Minakuchi et al., 2009) and Bombyx mori (Kayukawa et al., 2012). It plays a primary role in the repression of metamorphosis (Konopova et al., 2011; Lozano and Belles, 2011; Minakuchi et al., 2009). A region in the promoter of Kr-h1 gene that interacts with JH/Met/SRC has been identified in T.castaneum (Kayukawa et al., 2013), B. mori (Kayukawa et al., 2012) and Ae. aegypti (Shin et al., 2012; Zou et al., 2013).
Although, JH signaling pathway is well conserved in insects, the action of methoprene appears to be different between mosquitoes and other insects. Methoprene is effective in blocking larval-pupal metamorphosis in lepidopteran and other holometabolous insects but not in mosquitoes. Mosquito larvae treated with methoprene any time during their larval stage undergo larval-pupal metamorphosis and die during the pupal stage mainly due to the persistence of larval tissues such as the midgut (Wu et al., 2006). However, the molecular mechanisms that regulate JH action during larval-pupal and pupal-adult metamorphosis in this insect are not well understood. In an attempt to understand mechanisms of JH action in this insect, we employed Aag-2, a JH sensitive mosquito cell line developed from Ae. aegypti embryos. We identified AaKr-h1 gene as a JH response gene in these cells and showed that its expression requires the presence of JH III and Met. Studies on the promoter region of this gene identified a 13 nucleotide canonical E box sequence containing JHRE that mediates JH action. These data provide important insights into the regulation of AaKr-h1 gene by JH in Ae. aegypti.
2. MATERIALS AND METHODS
2.1 Cell culture
Aag-2 cells were cultured in Schneider’s Drosophila medium (SDM; Sigma-Aldrich, St Louis, MO, USA) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) at 28 °C in 25-cm 2 cell culture flasks. The cells were sub-cultured every 4–5 days when they grew to 90% confluency.
2.2 RNA isolation, cDNA preparation and qRT-PCR
Total RNA was isolated using TRI reagent (Molecular Research Center Inc., Cincinnati, OH). To remove the contaminated DNA, the RNA samples were treated with RNase-free DNase I (Ambion Inc. Austin, TX, USA). Three micrograms of purified RNA was used to synthesize cDNA using the M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Based on the sequences available in the GenBank [AaMet (Wang et al., 2007), AY902310.1] and Vectorbase (AaKr-h1, AAEL002390), the gene-specific primers of AaMet and AaKr-h1 (Table 1S) were synthesized and used to quantify mRNA levels by quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) using MyiQ single color real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). The qRT-PCR was performed in 15 μl reaction volume containing 1 μl of cDNA, 1 μl primer mix (10 μM stock solution), 5.5 μl of H2O and 7.5 μl of Fast Start SYBR Green Master mix (Roche Applied Science, Indianapolis, IN, USA). PCR conditions of 95 °C for 3 min; followed by 45 cycles of 95 °C for 10 s, 55 °C for 15 s and 72 °C for 20 s were used. The expression of gene coding for ribosomal protein subunit S7RP (GenBank: AY380336.1) was used for normalization of mRNA levels. Each experiment was repeated using three independent biological samples. Statistical analyses were performed by student’s t-test or by univariate analysis of variance Post Hoc Tests using IBM SPSS Statistics 21 program.
2.3 JH III dose-response and time-course experiments
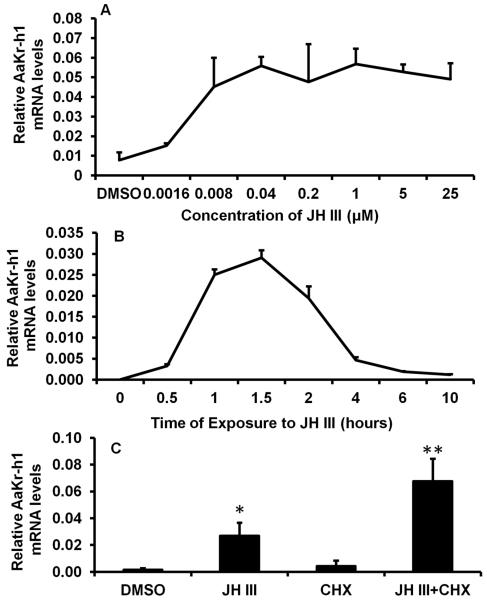
To determine dose-response of JH III induction of AaKr-h1 in Aag-2 cells, the cells were exposed to DMSO or 1.6 nM to 25 μM JH III for 2 hr. Total RNA was isolated and used in qRT-PCR to quantify AaKr-h1 mRNA levels as described above. To determine time-course of JH III induction, the cells were exposed to 1 μM of JH III for different time periods and total RNA was isolated and AaKr-h1 mRNA levels were quantified. The Aag-2 cells were also exposed to DMSO, 1 μM of JH III, 10 μg/ml cycloheximide or 1 μM of JH III and 10 μg/ml Cycloheximide for 2 hr, total RNA was isolated and AaKr-h1 mRNA levels were quantified.
2.4 Cloning of 5’ untranslated region of AaKr-h1
Gene-specific primers were designed based on the known sequence at the 5’ end of AaKr-h1 (Table 1S). The 5’ RACE was performed following the manufacturer’s Instruction manual (Takara, Mountain View, USA).
2.5 Synthesis of double-strand RNA (dsRNA)
Primers targeting regions of AaMet and AaFISC and containing T7 polymerase promoter sequence at their 5’ ends (Table 1S) were used to amplify fragments of these genes by PCR. dsRNAs were synthesized using the gene fragments as the templates using Ambion MEGAscript transcription kit (Ambion, Austin, TX). To transfect Aag-2 cells with the dsRNA, 4 × 105 cells were seeded into each well of 6-well plate and cultured at 28 °C in SDM plus 10% FBS. On the next day, the medium was removed and the cells were incubated for 6 hr in 8 μg dsRNA diluted with one ml of SDM without FBS. Then, one ml of SDM containing 20% FBS was added and the cells were cultured for three days. At the end of this incubation period, the medium was replaced with the fresh SDM with 10% FBS containing JH III (Sigma-Aldrich, St Louis, MO, USA). At 2 hr after addition of JH III, the cells were harvested and total RNA was isolated using TRI reagent and used in qRT-PCR analysis.
2.6 Construction and transfection of AaMet expression plasmids
To express AaMet in Aag-2 cells, a pair of primers (Table 1S) were designed in order to sub-clone the complete ORF of AaMet into pIEx-4 vector (Novagen, Madison, WI, USA). To investigate whether or not bHLH domain of AaMet is required for regulation of AaKr-h1, a forward primer (Table 1S) was synthesized to amplify AaMet gene coding for a protein without bHLH domain and this primer along with AaMet-R primer were used to amplify a fragment of AaMet. The PCR product was sub-cloned into pIEx-4. QuikChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA) was used to create E126A mutant of AaMet. The mutation was verified by sequencing performed by Functional Biosciences Inc (MGE Innovation Center, Madison, WI, USA). To transfect the cells with the plasmids, 6 × 105 cells were seeded into each well of 6-well plate and cultured at 28 °C in SDM containing 10% FBS. On the next day, the medium was removed and the cells were washed with SDM without FBS. For each well, 3 μg of plasmid and 10 μl of Cellfectin (Invitrogen, Carlsbad, CA, USA) were diluted with 100 μl of SDM without FBS. The diluted plasmid and Cellfectin were mixed gently and incubated for 15 min. Then the mixture was diluted with 0.8 ml of SDM and added to the washed cells. After 5 hr, the medium was replaced with fresh SDM containing 10% FBS and the cells were cultured for 72 hr. Then JH III diluted in the fresh SDM with 10% FBS was added to the cells for 2 hr. The cells were harvested and the total RNA was extracted.
2.7 Rescue experiment
To knockdown endogenous AaMet but not AaMet expressed from pIE-x-4 vector, two primers targeting 5’ UTR region of AaMet (Table 1S) were designed and used to amplify a fragment of AaMet that was used to prepare dsRNA. 2.5 × 105 cells were seeded into each well of 6-well plate and cultured overnight at 28 °C in SDM containing 10% FBS. The cells were then exposed to 8 μg of UTR-dsRNA for 72 hr. Then, pIEx-4 AaMet plasmid was transfected and the transfected cells were exposed to JH III for 2 hr.
2.8 Luciferase reporter assay
To identify JH-response region in the promoter of AaKr-h1 gene, the genomic DNA fragments containing E-boxes (CANNTG) were amplified using primers containing Kpn I and Xho I sites (Table 1S) and designed based on the sequence of AaKr-h1 gene. The PCR products were sub-cloned into pGL3-basic vector (Promega) containing a minimal promoter (TGGAAACATATACGCAGTAAACATATATAAAGCGCGGCGGGCGGCGCGCGGCGACAGTGTCGGCCGG). To perform luciferase reporter assay, 3 × 104 Aag-2 cells were seeded into each well of 96-well plate and cultured at 28 °C in SDM containing 10% FBS. For each well, 0.2 μg of plasmid and 1 μl of Cellfectin were used for transfection. At 24 hr after transfection, the cells were exposed to 1 μM JH III for 24 hr. The cells were washed with PBS, 50 μl of reporter lysis buffer was added to each well and the cells were assayed for the luciferase reporter activity using the luciferase reporter assay system from Promega (Madison, WI). The same experimental procedures were used to assay 36 nucleotide fragments containing K1 (GCCAAACGTCGCGTCCACCGCGTGACGTCACACC), K2 (CGACAAACGACAGCCGCGCGTGGATGAAATGTTG), K3 (GAAATGTTGTGCCGTGCCTCCACGTGCCCTAGAA) and K4 (AGAAATTGTCTTGCCTCGCACGCGTTGTTCAGTG) motifs fused to a minimal promoter and cloned into PGL3-basic vector. Six copies of 35 nucleotide fragment (GAAATGTTGTGCCGTGCCTCCACGTGCCCTAGAAA) containing K3 motif (underlined) were fused to the minimal promoter, cloned into pGL3 basic vector and tested in Aag-2 cells.
2.9 Construction of the luciferase reporter plasmid with JHRE mutations
The reporter plasmid with one copy of JHRE was mutated using QuikChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA). The mutations were verified by sequencing.
3. RESULTS
3.1 AaKr-h1 is a JH inducible gene in Aag-2 cell
Previous studies showed that Aag-2 cells respond to both JH and 20-hydroxyecdysone (20E) and induce expression of JH and 20E response genes respectively (Zhang et al., 2011). To determine JH III dose-response induction of AaKr-h1, Aag-2 cells were cultured in the medium containing different concentrations of JH III (1.6 × 10−9 M to 25 × 10−6 M) for 2 hr. qRT-PCR analysis showed that the AaKr-h1 mRNA levels increased in cells exposed to 1.6 × 10−9 M (Fig.1A). The AaKr-h1 mRNA levels gradually increased in cells exposed to increasing concentrations of JH III and reached the maximum levels in cells exposed to 1 μM of JH III and stayed at this level in the cells exposed to 5 and 25 μM of JH III (Fig. 1A). Since the primers used in qRT-PCR target common region of AaKr-h1, the mRNA levels measured represent both the isoforms.
Fig. 1.
JH dose-response and time-course in induction of AaKr-h1 in Aag-2 cells. Aag-2 cells were exposed to different concentrations of JH III for 2 hr (A) or to 1 μM of JH III for different time periods (B) or to 1 μM of JH III and 10 μg/ml Cycloheximide (CHX) for 2 hr (C). Total RNA was isolated and used in qRT-PCR to quantify AaKr-h1 mRNA levels. AaS7RP mRNA levels measured in the same samples at the same time were used for normalization. Mean + S.E. (n=3) are shown. The data were analyzed using one-way ANOVA. **, significantly different at P<0.01; *, significantly different at P<0.05.
To determine the time-course of AaKr-h1 gene response to JH III, Aag-2 cells were exposed to 1 μM JH III for different time periods from 0.5 hr to 10 hr. The mRNA levels of AaKr-h1 increased beginning at 0.5 hr after JH treatment, reached the maximum levels at 1.5 hr, then the mRNA levels gradually decreased until 10 hr of hormone exposure (Fig. 1B). In addition, the JH induction of this gene is not blocked by the presence of a protein synthesis inhibitor, cycloheximide (CHX) (Fig. 1C). The mRNA levels of AaKr-h1 were higher in the cells exposed to both JH III and cycloheximide when compared to their levels in the cells exposed to JH III alone. This might be due to stabilization of Kr-h1 mRNA by cycloheximide. Alternatively, a gene induced by the continuous presence of JH codes for a protein that suppresses the expression of Kr-h1.
3.2 JH regulates AaKr-h1 gene through Met
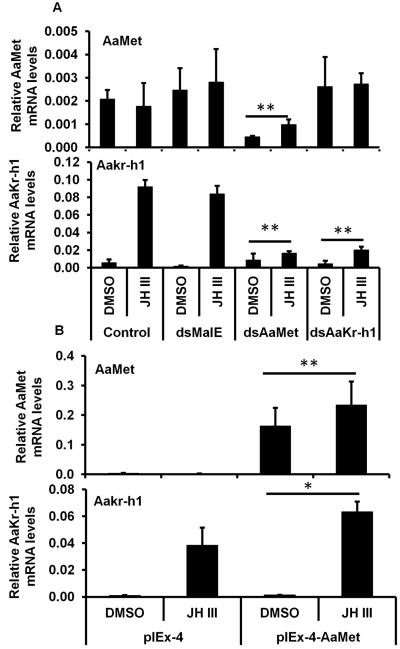
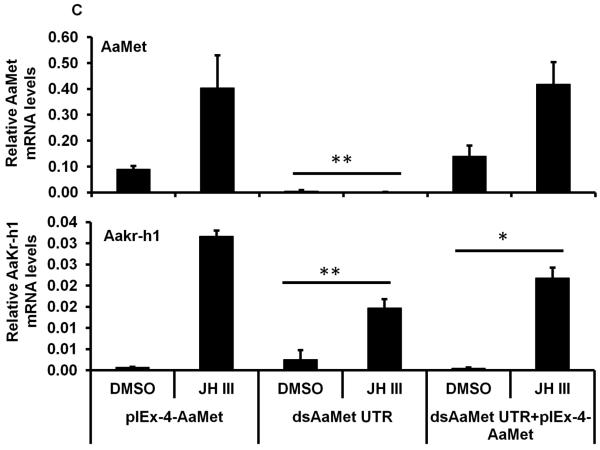
To determine whether the induction of AaKr-h1 gene by JH is mediated by Met, RNAi and over-expression of AaMet were performed in Aag-2 cells. Aag-2 cells exposed to AaMet dsRNA and treated with JH III or DMSO showed 60-70% decrease in AaMet mRNA levels (Fig. 2A). Control cells that were not exposed to dsRNA or the cells exposed to malE or AaKr-h1 dsRNA and treated with JH III or DMSO showed no significant changes in AaMet mRNA levels. The mRNA levels of AaKr-h1 increased in control cells or those exposed to malE dsRNA and treated with JH III (Fig. 2A). However, when compared to the control or malE dsRNA treated cells, the increase in the mRNA levels of AaKr-h1 was significantly lower in Aag-2 cells exposed to AaMet or AaKr-h1 dsRNA and treated with JH III (Fig.2A). Transfection of Aag-2 cells with AaMet expression plasmid increased AaMet mRNA levels (Fig. 2B). Exposure of these cells to JH III induced AaKr-h1 mRNA levels by two-fold higher than in untransfected Aag-2 cells exposed to JH III (Fig.2B). To confirm the requirement of AaMet in JH induction of AaKr-h1, dsRNA targeting 5’ UTR region (that is not present in pIEx-4AaMet expression plasmid) of AaMet was used to silence endogenous AaMet in Aag-2 cells. The results showed that transfection of pIEx-4 AaMet expression plasmid resulted in an increase in both AaMet and Aakr-h1 mRNA levels in cells exposed to dsRNA targeting 5’ UTR region of AaMet (Fig. 2C). The induction of AaKr-h1 mRNA in cells exposed to AaMet dsRNA is likely due to the presence of some AaMet in these cells.
Fig. 2.
AaMet is required for JH III induction of AaKr-h1 gene.
A. Aag-2 cells were incubated with AaMet or AaKr-h1 dsRNA for 72 hr. Then the cells were exposed to 1 μM of JH III for 2 hr. Total RNAs were isolated and used in qRT-PCR to quantify AaMet and AaKr-h1 mRNA levels. AaS7RP mRNA levels were used for normalization. Mean + S.E. (n=3) are shown.
B. Aag-2 cells were transfected with empty pIEx-4 expression plasmid and this plasmid containing complete ORF of AaMet. Three days after transfection, the cells were exposed to 1 μM of JH III for 2 hr. Total RNAs were isolated and used in qRT-PCR to quantify AaMet and AaKr-h1 mRNA levels. AaS7RP mRNA levels were used for normalization. Mean + S.E. (n=3) are shown.
C. Aag-2 cells were incubated with AaMet or AaKr-h1 dsRNA targeting 5’ UTR for 72 hr. Then the cells were transfected with pIEx-4 AaMet expression plasmid. Three days after transfection, the cells were exposed to 1 μM of JH III for 2 hr. Total RNAs were isolated and used in qRT-PCR to quantify AaMet and AaKr-h1 mRNA levels. AaS7RP mRNA levels were used for normalization. Mean + S.E. (n=3) are shown.
The data were analyzed using Univariate analysis of variance Post Hoc Tests. **, significantly different at P<0.01; *, significantly different at P<0.05.
3.3 bHLH domain of Met is required for induction of AaKr-h1
Since the overexpressed AaMet could promote JH III induction of AaKr-h1 gene, we asked whether or not DNA binding by AaMet is required for JH III induction of this gene. AaMet expression plasmids containing the complete ORF or the ORF without bHLH domain were transfected into Aag-2 cells. JH III induction of AaKr-h1 decreased significantly in cells transfected with AaMet lacking bHLH domain when compared to its induction in cells transfected with complete AaMet (Fig. 3A). These data suggest that DNA binding by bHLH domain is required for JH induction of AaKr-h1 gene. Mutation to a single glutamic acid residue that is conserved in all bHLH proteins identified so far abolishes DNA binding of these proteins (Fisher and Goding, 1992). To confirm that the DNA binding by bHLH domain is required for JH induction of AaKr-h1 gene, the conserved glutamic acid, E126 in the bHLH domain of AaMet was mutated to alanine. JH III induction of AaKr-h1 decreased significantly in cells transfected with pIEx-4AaMetE126A when compared to the induction levels in cells transfected with plasmid expressing wild-type AaMet (Fig. 3B). These data suggest that DNA binding by Met is required for JH induction of AaKr-h1 gene.
Fig. 3.
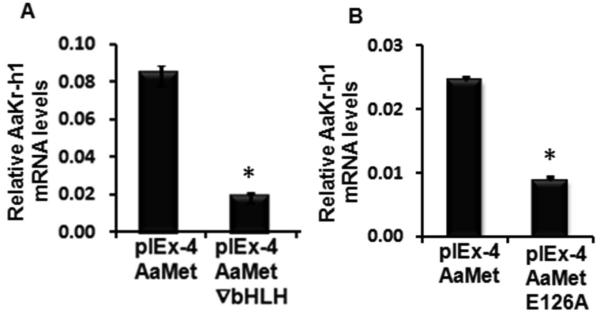
AaMet bHLH domain and its DNA binding are required for JH III induction of AaKr-h1.
A. Aag-2 cells were transfected with pIEx-4AaMet or the same expression plasmid containing AaMet lacking bHLH domain. Three days after transfection, the cells were exposed to 1 μM of JH III for 3 hr. Total RNAs were isolated and used in qRT-PCR to quantify AaKr-h1 mRNA levels. AaS7RP mRNA levels were used for normalization. Mean + S.E. (n=3) are shown. The data were analyzed using Student t-test. *, significantly different at P<0.01.
B. AaMet containing E126A mutation that abolishes DNA binding ability was tested as described in 3A.
3.4 Identification of AaKr-h1 isoforms, promoter and JH response element
To identify a region in the promoter of AaKr-h1 that supports JH induction, a series of fragments (1539, 3075, 728 and 853) present upstream to the AaKr-h1 open reading frame (ORF) and included conserved E-box motif (CANNTG) were screened using the luciferase reporter assay (Fig. 1S A). As shown in the supplementary data section (Fig. 1S B-D), none of these fragments supported JH III induction of the luciferase gene suggesting that these fragments may not represent JH-responsive AaKr-h1 gene promoter.
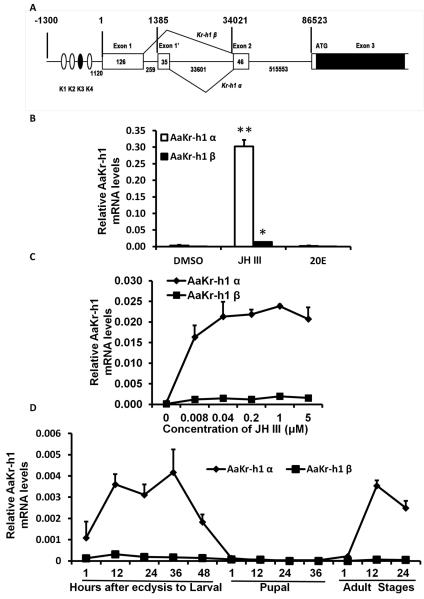
To identify 5’ end of AaKr-h1 mRNAs, we performed 5’RACE and identified two isoforms of AaKr-h1 expressed in Aag-2 cells. These two isoforms were named as α and β based on their sequence differences in the 5’ end of mRNA. The Kr-h1α and Kr-h1β include two alternative exons of 35 and 126 nucleotides respectively (Fig. 4A). Shin et al. (2012) identified Kr-h1β and included as the supplementary data in their publication. Kr-h1α-specific sequence identified in our RACE experiments is shown in Figure 2S. qRT-PCR analysis showed that Kr-h1α is the predominant isoform expressed in Aag-2 cells and the Kr-h1β isoform is expressed at lower levels (Fig 4B). However, both AaKr-h1 isoforms are induced by JH III but not 20E (Fig.4B). JH III dose-response analysis showed that both isoforms are induced beginning at 0.008 μM JH III (Fig.4C). Studies on developmental expression of both AaKr-h1 isoforms during last instar larval, pupal and adult stages showed that Kr-h1α mRNA levels are higher than the Kr-h1β mRNA levels during all stages tested (Fig. 4D). The Kr-h1α mRNA started to increase soon after molt to final instar and reached the maximum levels by 12 hr after ecdysis and remained high until 36 hr after ecdysis. The Kr-h1α mRNA levels then decreased and reached the minimum levels soon after ecdysis into the pupal stage (Fig. 4D). The Kr-h1α mRNA levels remained low during the pupal stage and started to increase again after adult ecdysis and reached the maximum by 12 hr after ecdysis into the adult stage. The Kr-h1β mRNA levels remained low during all stages tested (Fig. 4D).
Fig. 4.
AaKr-h1 encodes two isoforms and both the isoforms are induced by JH III.
A. The structure and the splicing pattern of the two isoforms are shown. Exons in the untranslated regions are shown as open boxes and the exons in the translated region are sown as filled boxes. The numbers in the boxes or below line show nucleotides present in each region and the numbers on the top show nucleotide location of different regions. E box and E box-like motifs are shown as closed and open circles respectively.
B. JH III induces production of mRNAs of both isoforms of AaKr-h1. Aag-2 cells were exposed to 1 μM JH III or 10 μM 20E for 3 hr. Total RNAs were isolated and used in qRT-PCR to quantify AaKr-h1α and AaKr-h1β mRNA levels. Mean + S.E. (n=3) are shown. The data were analyzed using Univariate analysis of variance Post Hoc Tests. **, significantly different at P<0.01; *, significantly different at P<0.05.
C. JH III dose-response in induction AaKr-h1 isoforms. Experimental procedures are the same as in Figure 4B except that the cells were exposed to various doses of JH III for 3 hr. Mean + S.E. (n=3) are shown.
D. AaKr-h1 α is the predominant isoform in larvae, pupae and adults. Total RNAs were isolated from staged Ae aegypti larvae, pupae and adults and used in qRT-PCR to quantify AaKr-h1α and AaKr-h1β mRNA levels. Mean + S.E. (n=3) are shown.
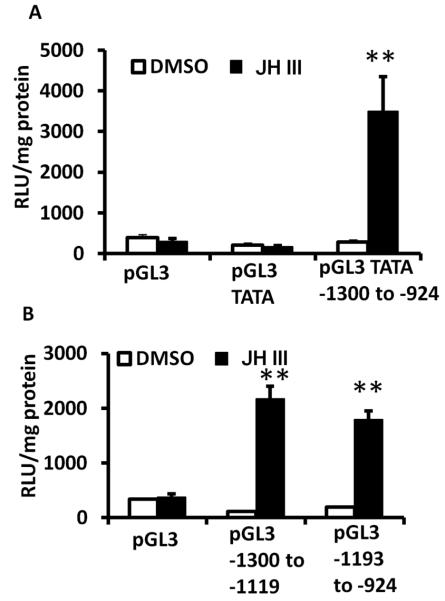
A 374 nucleotide fragment containing conserved E-box motif (CANNTG) and present in the promoter (−1300 to −924) of AaKr-h1 was tested in the luciferase assay. As shown in Figure 5A, this fragment supported JH III induction of the luciferase reporter in Aag-2 cells. To identify regions of 374 nucleotide fragment that support JH induction, two overlapping regions of this fragment were PCR amplified and tested in reporter assays. Both these fragments supported JH III induction of the luciferase reporter gene suggesting that the functional JHRE could be located in the overlapping region (Fig. 5B).
Fig. 5.
374 nucleotide fragment (−1300 to −924) of AaKr-h1 promoter supports JH III induction of the luciferase reporter.
A. Aag-2 cells were transfected with pGL3 basic vector or pGL3 containing minimal promoter or pGL3 containing minimal promoter and 374 nucleotide (nt) AaKr-h1 promoter region. Twenty four hours after transfection, the cells were exposed to JH III for 24 hr. The cells were harvested and the luciferase activity was quantified. Mean + S.E. (n=3) are shown. The data were analyzed using Univariate analysis of variance Post Hoc Tests. **, significantly different at P<0.01.
B. Two sub-fragments of 374 nucleotide AaKr-h1 promoter, −1300 to −1119 and −1193 to −924 were tested as described in Figure 5A.
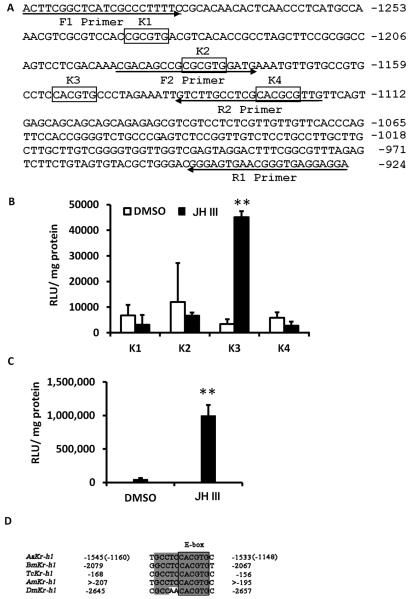
Shin et al., (Shin et al., 2012) recently identified four motifs, three E box-like (K1, K2 and K4) and one E box (K3) in the promoter region of AaKr-h1 (Fig 6A). Interestingly, all four motifs are located in the 374 nucleotide AaKr-h1 promoter fragment that supported JH induction of AaKr-h1 in Aag-2 cells. In addition, only K3 that contains E box is in the overlapping region of both the fragments of 374 nucleotide promoter region suggesting that K3 may be functional JHRE in Aag-2 cells. To test this hypothesis, 36 nucleotide fragments containing underlined K1, K2, K3 and K4 motifs were fused to a minimal promoter and cloned into PGL3 basic vector. Transfection of these reporter vectors into Aag-2 cells followed by their exposure to JH III showed that only K3 motif containing E box supported JH III induction of the luciferase reporter (Fig 6B). To further validate that K3 is a functional JHRE, six copies of 35 nucleotide fragment containing K3 motif were fused to the minimal promoter, cloned into pGL3 basic vector and tested in Aag-2 cells. As shown in Figure 6C, JH III induced reporter gene under the control of 6XJHRE. Sequence comparison showed that the E-box motif present in AaKr-h1 promoter is identical to the JHRE identified in the Kr-h1 promoter regions of D. melanogaster, T.castaneum and B. mori (Kayukawa et al., 2012; Kayukawa et al., 2013) and Apis mellifera (Fig 6D).
Fig. 6.
A response element containing canonical E box supports JH III induction of AaKr-h1 gene.
A. AaKr-h1 promoter sequence showing one E box and three E box-like motifs identified by Shin et al., (2012). Primers used to amplify two sub-fragments of 374 nucleotide promoter regions are also shown. The numbers on the right show location of the promoter.
B. Aag-2 cells were transfected with pGL3 basic vector containing minimal promoter and K1, K2, K3 or K4 motifs. Twenty four hours after transfections, the cells were exposed to JH III for 24 hr. The cells were harvested and the luciferase activity was quantified. Mean + S.E. (n=3) are shown. The data were analyzed using Univariate analysis of variance Post Hoc Tests. **, significantly different at P<0.01.
C. Same as Figure 6B except pGL3 containing a minimal promoter and 6XJHRE was used.
D. E box motif and surrounding sequences are conserved in Aedes, Drosophila, Bombyx, Tribolium and Apis. Thirteen nucleotide sequence (6 nucleotides upstream to the E box, six nucleotides E box and one nucleotide downstream to E box) conserved in Drosophila, Bombyx, Tribolium and Apis are shown.
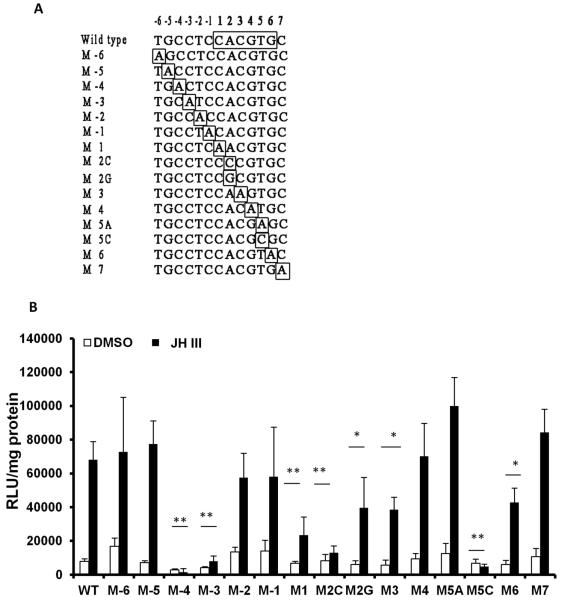
The E box motif and six nucleotides upstream to the E box and one nucleotide downstream to the E box to a total of 13 nucleotides are well conserved in Kr-h1 promoters of Ae. aegypti, D. melanogaster, A. mellifera, T.castaneum and B. mori (Fig. 6D) suggesting that not only E box but also residues surrounding the E box may play important roles in JH induction of this gene. To test this hypothesis, mutant versions of 13 nucleotide motifs were created by changing one nucleotide at a time to adenosine (Fig.7A). The adenosine residue in the E box was changed to guanine [M2G, this mutation changes E box of K3 motif (CACGTG) to E box-like of K1 and K2 motifs, (CGCGTG). The adenosine residue in the E box was changed to cytosine (M2C). To change E box of K3 motif (CACGTG) to E box-like motif of K4 (CACGCG), the T in E box was changes to C (M5C). Motifs containing mutated nucleotides at positions -4 and -3 preceding the E box as well as first nucleotide and second nucleotide (changed to C) of E box showed significantly lower induction of the luciferase reporter by JH III suggesting that these four nucleotides play important roles in JH III induction of Kr-h1 gene in Aag-2 cells (Fig. 7B). When compared to the JH III induced the luciferase levels in wild-type K3 motif containing reporter plasmid transfected cells, the levels of JH III induced luciferase in cells transfected with reporter plasmids containing mutations to second residue (changed to G), the third or the sixth residues of E box were significantly lower suggesting that these residues also contribute to JH III induction of Kr-h1 gene in Aag-2 cells (Fig. 7B). Interestingly, mutating a single adenosine residue in the E box in K3 (CACGTG) to Guanine to mimic E-box-like motifs K1 and K2 (CGCGTG) or a single thymine residue in the E box in K3 (CACGTG) to mimic E-box-like motif in K4 (CACGCG) reduced JH III induction of the luciferase reporter confirming that K3 is the functional JHRE in Aag-2 cells. In addition, the nucleotide immediately following E box is highly conserved in all the insects studied; however, mutating this cytosine to adenosine had no effect on the activity of the promoter (Fig. 7B).
Fig. 7.
Both E box and nucleotides surrounding E box are important for JH III induction of the luciferase reporter.
A. Mutations performed to 13 nucleotide JHRE including six upstream and one downstream to the E box are shown. E box nucleotides and nucleotides mutated are identified by open boxes.
B. Evaluation of JHRE mutants in the luciferase reporter assay. Aag-2 cells were transfected with pGL3 basic vector containing a minimal promoter and wild-type or mutated 13 bp JHRE. Twenty four hours after transfection, the cells were exposed to JH III for 24 hr. The cells were harvested and the luciferase activity was quantified. Mean + S.E. (n=3) are shown. The data were analyzed using Univariate analysis of variance Post Hoc Tests. **, significantly different at P<0.01; *, significantly different at P<0.05.
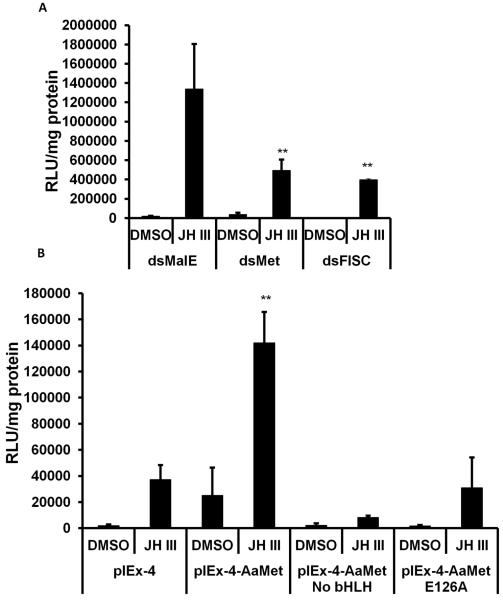
3.5 JH induces AaKr-h1 gene through conserved JHRE and Met
As shown in Figure 2, JH III induction of Kr-h1 in Aag-2 cells requires the presence of AaMet protein. To determine whether JH III induction of Kr-h1 through JHRE identified in the experiments reported here also require Met, RNAi was employed to knockdown the expression of AaMet in Aag-2 cells. Knockdown in expression of Met or SRC/FISC reduced JH III induction of 6XJHRE suggesting that the products of both these genes are required for JH induction of the reporter gene regulated by 6XJHRE (Fig. 8A). In addition, Aag-2 cells transfected with AaMet lacking bHLH domain or E126A mutant of AaMet showed significantly lower induction of 6XJHRE regulated luciferase gene by JH III when compared to the cells transfected with wild-type AaMet (Fig. 8B). These data confirm that JH III induces Kr-h1 in Aag-2 cells through JHRE identified in these studies.
Fig. 8.
AaMet and AaFISC are required for JH III induction of the luciferase through JHRE.
A. Aag-2 cells were exposed to malE, AaMet or AaFISC dsRNA for 72 hr. Then the cells were transfected with pGL3 basic vector containing minimal promoter and 6XJHRE. The cells were exposed to JH III for 24 hr. The cells were harvested and the luciferase activity was quantified. Mean + S.E. (n=3) are shown. The data were analyzed using Univariate analysis of variance Post Hoc Tests. **, significantly different at P<0.01.
B. Same as in Figure 8A except pIEx-4, pIEx-4AaMet, AaMet lacking bHLH domain (pIEx-4-AaMet▽bHLH) or E126A mutant of AaMet were tested.
4. DISCUSSION
The first major contribution of this study is the confirmation that Kr-h1 is a JH- response gene in Aag-2 cells of Ae. aegypti. The Kr-h1 gene was originally identified in D. melanogaster as a homolog of segmentation gene Krüppel (Schuh et al., 1986). In this insect, the Kr-h1 encodes three isoforms that show stage- and tissue-specific expression and are also regulated by 20E (Pecasse et al., 2000). Later, JH regulation of this gene in D. melanogaster has been reported (Minakuchi et al., 2008). The Kr-h1 homologs identified in T. castaneum (Minakuchi et al., 2009) and B. mori (Kayukawa et al., 2012) are also JH-responsive and shown to play essential roles in the repression of metamorphosis (Kayukawa et al., 2012). Shin et al., (2012) reported that the Kr-h1 homolog in the mosquito, Ae. aegypti is regulated by JH III. The data included in this paper also showed that Kr-h1 is a JH-response gene in Aag-2 cells.
The second major contribution of these studies is the finding that the Kr-h1 gene in mosquito, Ae. aegypti is regulated by JH III through Met and a 13 nucleotide JHRE containing canonical E box motif. The Kr-h1 in D. melanogaster, B. mori (Kayukawa et al., 2012) and T. castaneum (Kayukawa et al., 2013) are shown to be regulated by JH through Met and SRC and JHRE containing canonical E box motif. In Ae. aegypti Kr-h1 has been identified as a JH-response gene by microarray analysis (Zhu et al., 2010). Shin et al (2012) reported that similar to Met and Cycle, FISC is also required for expression of Kr-h1. However, FISC was not found in the protein complex bound to the E-box-like motif analyzed by EMSA using FISC antibodies suggesting that FISC may function as a co-activator rather than a heterodimeric partner binding to both Met and DNA. The possibility that the Met in complex with different partners may bind to different JHRE containing E box or E box-like motifs is interesting and warrants further investigation. In the current study, RNAi assays showed that Met and SRC/FISC are the major proteins that are required for JH regulation of Kr-h1 gene through JHRE containing E box in Aag-2 cells.
Recently Zou et al., (2013) employed RNAi, pattern search and EMSA analysis and identified a nine nucleotide consensus Met-binding motif (CACGYGRWG; Y=C or T, R= A or G and W= T or A) that is present in JH III inducible genes in Ae. aegypti. The consensus sequence of this motif matches with all previously reported JHRE that were shown to bind Met. The sequence (CCACACGCGAAG) in the promoter midgut-specific early trypsin and the sequence (CACGCGGTG) in the promoter of krüppel homolog 1 of Ae. aegypti match well with this consensus sequence. The JHRE sequence (GGCTCCACGTG) identified in the Kr-h1 promoter of T. castaneum (Kayukawa et al., 2013) and B. mori (Kayukawa et al., 2012) also match with this consensus motif. However, this consensus sequence does not show similarity with the consensus sequence identified in the promoters of JH inducible genes of D. melanogaster and A. mellifera (Li et al., 2007). Interestingly, the protein complex identified by affinity purification using this consensus motif does not include Met and SRC. This raises an interesting possibility of JH acting on the promoters of some genes through proteins other than Met and SRC. The consensus sequence identified by Zou et al., 2013 includes nine nucleotides beginning with the E box. Data presented here showed that not only E box but also nucleotides upstream to the E box (TGCCTCCACGTG) especially CC at -3 and -4 position upstream to the E box are essential for JH III activation of Kr-h1 gene in Aag-2 cells. It will be interesting to perform pattern searches on the JH inducible genes to determine whether or not these nucleotides upstream to the E box are conserved in the promoters of these genes.
The data included here clearly showed that Met is required for JH III induction of Kr-h1 gene or the luciferase gene regulated by 6XJHRE containing canonical E box motifs. Two-hybrid assays performed in L57 cells showed that PAS-A and PAS-B domains of AaMet are essential for the Met-FISC interaction (Li et al., 2011). The data presented here showed that bHLH domain of AaMet is required for JH III induction of Kr-h1 gene or the luciferase gene regulated by 6XJHRE in Aag-2 cells. Electrophoretic mobility shift assay (EMSA) has been one of the most commonly used methods to assess interaction between DNA binding proteins and DNA response elements. However, despite numerous attempts using Met and FISC proteins expressed in vitro using TNT kit (Promega) or Met produced in baculovirus expression system or using nuclear proteins isolated from Met/FISC overexpressed Aag-2 cells, we were unable to obtain consistent and repeatable results in EMSA assays. The main problem has been specific binding of other bHLH proteins present in the rabbit lysate and nuclear extracts of insect cells to JHRE containing canonical E box motif. This may be due to the fact that bHLH proteins are capable of binding to E box motif; rabbit and insect cells could express many bHLH proteins (Bitra et al., 2009). A combination of reporter assays, RNAi, overexpression, truncations and mutagenesis of DNA binding protein could serve as an excellent alternative to EMSA to address questions on interaction between transcription factors and the DNA response elements. Taken together, the data included in this paper showed that binding of Met to 13 nucleotide JHRE identified is required for JH III induction of Kr-h1 gene,
Three isoforms of Kr-h1, one regulated by 20E and two regulated by JH have been identified in D. melanogaster (Pecasse et al., 2000; Minakuchi et al., 2008). In B. mori, two isoforms were found, one isoform is predominant in the larval epidermis (Kayukawa et al., 2012) and both are induced by JH. In Aag-2 cells and Ae. aegypti larvae, pupae and adults, we detected two isoforms of Kr-h1 and both the isoforms are JH-responsive although Kr-h1α isoform is expressed at much higher levels than the β isoform. Further studies are required to determine specific function of each Kr-h1 isoform. It is interesting that neither of the two Kr-h1 isoforms identified in Ae. aegypti are 20E responsive (Fig. 4B). Further investigations are required to determine whether there are additional isoforms of Ae. aegypti that are 20E sensitive or Kr-h1 gene in this insect is not sensitive to 20E.
Supplementary Material
Highlights.
AaKr-h1 is a JH-response gene in Aag-2 cells
JH induction of Aakr-h1 requires the presence of AaMet
A conserved 13 nucleotide JHRE identified in the promoter of Aakr-h1 is required for JH induction of this gene.
bHLH domain of AaMet is required for JH induction of Aakr-h1.
The expression of both AaKr-h1α and AaKr-h1β is induced by JH III.
5. ACKNOWLEDGEMENTS
This is contribution number 14-08-05 from the Kentucky Agricultural Experimental Station. This work was supported by National Institutes of Health (GM070559-11).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bitra K, Tan A, Dowling A, Palli SR. Functional characterization of PAS and HES family bHLH transcription factors during the metamorphosis of the red flour beetle, Tribolium castaneum. Gene. 2009;448:74–87. doi: 10.1016/j.gene.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles JP, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M. Ligand- binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. U.S.A. 2011;108:21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher F, Goding CR. Single amino acid substitutions alter helix-loop-helix protein specificity for bases flanking the core CANNTG motif. The EMBO J. 1992;11:4103–4109. doi: 10.1002/j.1460-2075.1992.tb05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindra M, Palli SR, Riddiford LM. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. 58. [DOI] [PubMed] [Google Scholar]
- Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, Kamimura M, Mita K, Imanishi S, Kiuchi M, Ishikawa Y, Shinoda T. Transcriptional regulation of juvenile hormone-mediated induction of Kruppel homolog 1, a repressor of insect metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 2012;109:11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayukawa T, Tateishi K, Shinoda T. Establishment of a versatile cell line for juvenile hormone signaling analysis in Tribolium castaneum. Sci. Rep. 2013;3 doi: 10.1038/srep01570. doi:10.1038/srep01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopova B, Smykal V, Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE. 2011;6:e28728. doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Mead EA, Zhu JS. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc.Natl. Acad. Sci. U.S.A. 2011;108:638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Z, Robinson GE, Palli SR. Identification and characterization of a juvenile hormone response element and its binding proteins. J. Biol. Chem. 2007;282:37605–37617. doi: 10.1074/jbc.M704595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano J, Belles X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 2011;1 doi: 10.1038/srep00163. doi:10.1038/srep00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi C, Namiki T, Shinoda T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 2009;325:341–350. doi: 10.1016/j.ydbio.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Minakuchi C, Zhou X, Riddiford LM. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008;125:91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene -tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. Febs J. 2005;272:1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Farkas R, Palli SR. Recent Progress in Juvenile hormone analogs (JHA) research Adv. Insect Physiol. 2012;43:353–436. [Google Scholar]
- Pecasse F, Beck Y, Ruiz C, Richards G. Kruppel-homolog, a stage-specific modulator of the prepupal ecdysone response, is essential for Drosophila metamorphosis. Dev. Biol. 2000;221:53–67. doi: 10.1006/dbio.2000.9687. [DOI] [PubMed] [Google Scholar]
- Schuh R, Aicher W, Gaul U, Cote S, Preiss A, Maier D, Seifert E, Nauber U, Schroder C, Kemler R, et al. A conserved family of nuclear proteins containing structural elements of the finger protein encoded by Kruppel, a Drosophila segmentation gene. Cell. 1986;47:1025–1032. doi: 10.1016/0092-8674(86)90817-2. [DOI] [PubMed] [Google Scholar]
- Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc.Natl. Acad. Sci. U.S.A. 2012;109:16576–16581. doi: 10.1073/pnas.1214209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Baumann A, Wilson TG. Drosophila melanogaster Methoprene-tolerant (Met) gene homologs from three mosquito species: Members of PAS transcriptional factor family. J. Insect Physiol. 2007;53:246–253. doi: 10.1016/j.jinsphys.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: Juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mech. Dev. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J. Biol. Chem. 2011;286:8437–8447. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JS, Busche JM, Zhang X. Identification of juvenile hormone target genes in the adult female mosquitoes. Insect Biochem. Mol. Biol. 2010;40:23–29. doi: 10.1016/j.ibmb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Zou Z, Saha TT, Roy S, Shin SW, Backman TW, Girke T, White KP, Raikhel AS. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc.Natl. Acad. Sci. U.S.A. 2013;110:E2173–2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.