TO THE EDITOR: B-cell depletion is an effective treatment for a number of autoimmune diseases in which B cells were not previously considered to be important, such as multiple sclerosis.1 In renal transplantation, acute cellular rejection has been viewed as a T-cell–dependent process, but B cells are required for alloantibody production and may also play other roles, including alloantigen presentation to T cells.2
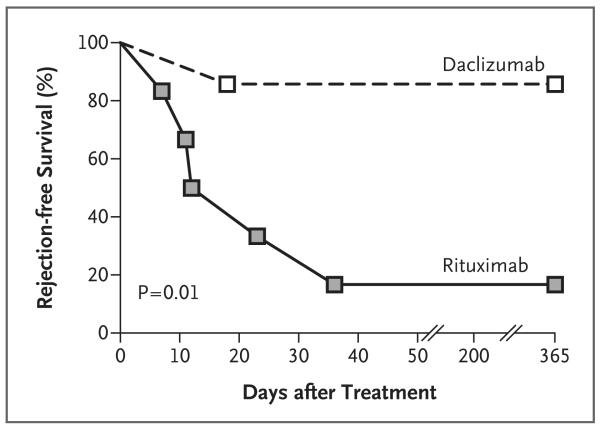
Published data on the use of B-cell depletion at induction in nonsensitized patients undergoing organ transplantation are lacking. We conducted an open-label, randomized, controlled trial comparing rituximab, a B-cell–depleting, chimeric, anti-CD20 monoclonal antibody, with an anti-CD25 monoclonal antibody (daclizumab) as induction therapy in patients undergoing renal transplantation. We planned to recruit 120 patients, but the study was suspended after recruitment of the first 13 patients, owing to an excess incidence of acute cellular rejection in the ritux imab group. Five of six patients (83%) who received rituximab had an episode of biopsy-confirmed acute rejection in the first 3 months after transplantation, as compared with one of seven patients (14%) in the daclizumab group (P = 0.01) (Table 1 and Fig. 1). All the episodes of rejection responded to intravenous methylprednisolone, and allograft function was similar in the two groups at 12 months (Table 1, and Fig. 1A in the Supplementary Appendix). After rituximab treatment, peripheral B cells were undetectable in all patients (Fig. 1B in the Supplementary Appendix). Serum cytokines, including tumor necrosis factor α, interleukin-6, and interleukin-10, were increased after transplantation, as compared with baseline values, in some of the patients who were treated with rituximab (Fig. 2, 3, and 4 in the Supplementary Appendix).
Table 1. Immunosuppression, Acute Rejection, and Allograft Function.*.
| Variable | Daclizumab Group (N = 7) |
Rituximab Group (N = 6) |
|---|---|---|
| Immunosuppression | ||
| Induction | Daclizumab, 1 mg/kg of body weight (day 0, day 7) | Rituximab, 10 mg/kg (day 0, day 7) and methylprednisolone, 10 mg/kg (day 0 and 7 before rituximab) |
| Maintenance (corticosteroid-free) | Tacrolimus (8–15 ng/ml) and mycophenolate mofetil (1 g twice a day) | Tacrolimus (8–15 ng/ml) and mycophenolate mofetil (1 g twice a day) |
| Tacrolimus level in months 1–3 — ng/ml | 10.6±1.0 | 12.2±2.3 |
| Mean no. of HLA mismatches | 3.1 | 2.8 |
| HLA-A | 1.1 | 1.2 |
| HLA-B | 1.0 | 1.0 |
| HLA-DR | 1.0 | 0.7 |
| First or second transplantation — no. (%) | ||
| First | 6 (86) | 6 (100) |
| Second | 1 (14) | 0 |
| Delayed graft function — no. (%) | 2 (29) | 1 (17) |
| Acute rejection at 3 mo — no. (%) | 1 (14) | 5 (83) |
| Banff grade for severity of acute rejection† | Patient 1, IB | Patient 1, IB; Patient 2, IB; Patient 3, IB and IA; Patient 4, IIB; Patient 5, IB |
| Peritubular capillaries on C4d immunoper oxidase staining of biopsy specimen — % | Patient 1, 0 | Patient 1, <50; Patients 2–5, 0 |
| Interval between transplantation and biopsy-confirmed rejection — days‡ | Patient 1, 18 | Patient 1, 12; Patient 2, 36; Patient 3, 11 and 35, respectively; Patient 4, 7; Patient 5, 23 |
| Antibody-mediated rejection — no. (%) | 0 | 0 |
| Corticosteroid-resistant rejection — no. (%) | 0 | 0 |
| Development of donor-specific antibody — no. (%) | 1 (14)§ | 0 |
| Glomerular filtration rate as measure of graft function — ml/min/1.73 m2 | ||
| At 3 mo | 57.3±8.0 | 45.5±9.7 |
| At 12 mo | 48.9±10.6 | 44.4±8.1 |
Plus–minus values are means ±SD.
Banff grades for acute rejection range from I to III; I is defined as acute rejection with substantial interstitial infiltration with moderate (IA) or severe (IB) tubulitis, and II with mild-to-moderate (IIA) or severe (IIB) intimal arteritis.
All biopsies were performed to investigate a rise in the creatinine level.
A weak class I antibody developed in a patient who had no biopsy-confirmed acute rejection.
Figure 1. Increased Rate of Acute Rejection in Rituximab-Treated Patients.
Kaplan–Meier curves are shown for rejection-free survival at 1 year in patients who received rituximab as induction therapy, as compared with those who received daclizumab. The P value for the difference is based on a log-rank test.
Our findings are surprising; patients who received rituximab had a rate of acute rejection that was not only higher than the rate in the control group (83% vs. 14%) but also was higher than that previously observed among patients who have not received induction therapy (35%).3 One possible explanation may be that proinflammatory cytokine release associated with B-cell depletion might prime antigen-presenting cells. A short-lived cytokine-release syndrome often occurs after administration of the first dose of rituximab4; in our study, some patients who were treated with rituximab had elevated levels of proinflammatory cytokines. However, we cannot exclude the possibility that the increased levels of cytokines were the result rather than the cause of acute rejection.
Although B cells may enhance immune responses, some B cells have immunoregulatory properties. In animal models, depletion of such B cells before disease induction can worsen auto-immunity,5 and rituximab therapy can exacerbate ulcerative colitis and psoriasis. Similarly, depletion of immunoregulatory B cells may have contributed to the increased rejection in the rituximab-treated patients.
Recipients of renal transplants in whom rituximab is administered for desensitization do not appear to be at an increased risk for acute cellular rejection. Such patients usually receive rituximab well before transplantation, and rituximab treatment is often accompanied by plasma exchange and corticosteroid therapy; thus, any associated cytokine storm would probably resolve by the time of transplantation.
B-cell depletion has emerged as a powerful treatment strategy in autoimmunity; however, our results show that this strategy should be undertaken with caution when the precise role of B cells in a disease is unclear. (EudraCT Number, 2005-001496-35.)
Supplementary Material
Acknowledgments
Supported by grants from Roche Pharmaceuticals and the National Institute for Health Research Cambridge Biomedical Research Centre.
Drs. Clatworthy and Watson report receiving grants from Roche to attend scientific meetings and lecture fees from Wyeth and Astellas; Dr. Bradley, receiving grant support from Roche; and Dr. Smith, receiving consulting fees from and serving on advisory boards for Roche and GlaxoSmithKline and receiving grant support from GlaxoSmithKline.
Footnotes
No other potential conflict of interest relevant to this letter was reported.
References
- 1.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 2.Zarkhin V, Li L, Sarwal M. “To B or not to B?” B-cells and graft rejection. Transplantation. 2008;85:1705–14. doi: 10.1097/TP.0b013e318177793e. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Kirkman R, Light S, et al. Interleukin-2–receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med. 1998;338:161–5. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Vieira CA, Book BK, Sidner RA, Fineberg NS, Pescovitz MD. Rituximab, anti-CD20, induces in vivo cytokine release but does not impair ex vivo T-cell responses. Am J Transplant. 2004;4:1357–60. doi: 10.1111/j.1600-6143.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–30. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.