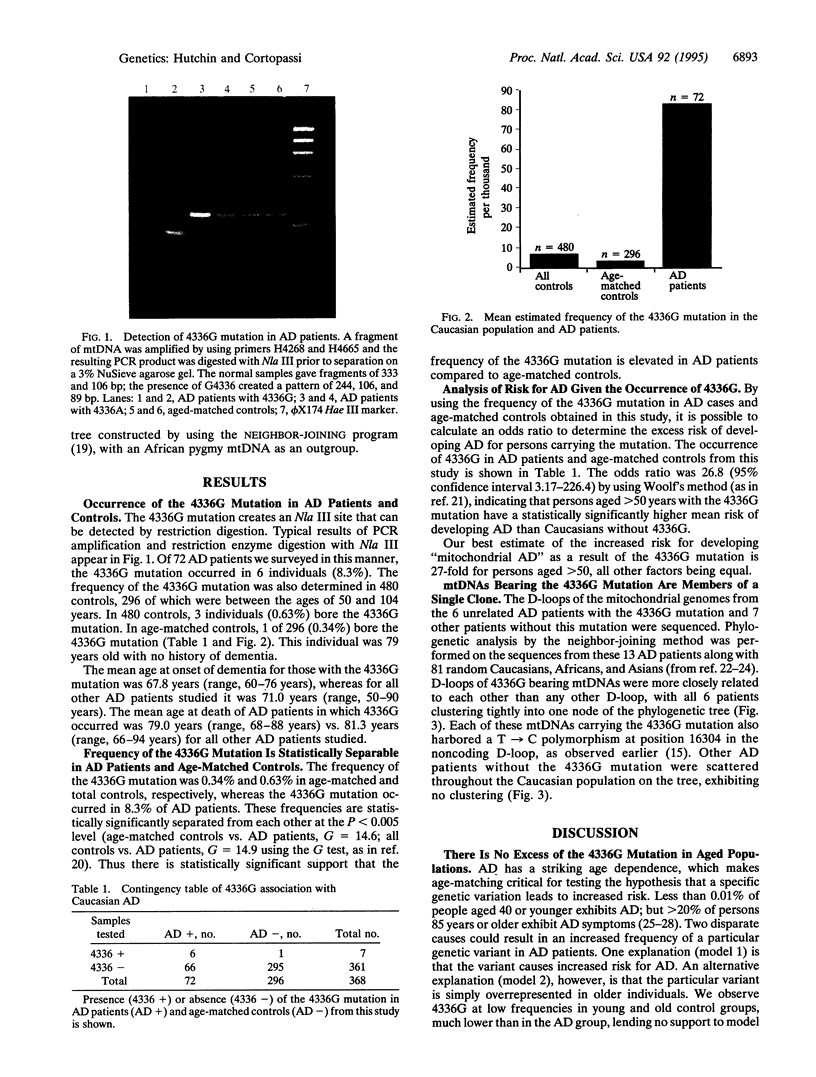
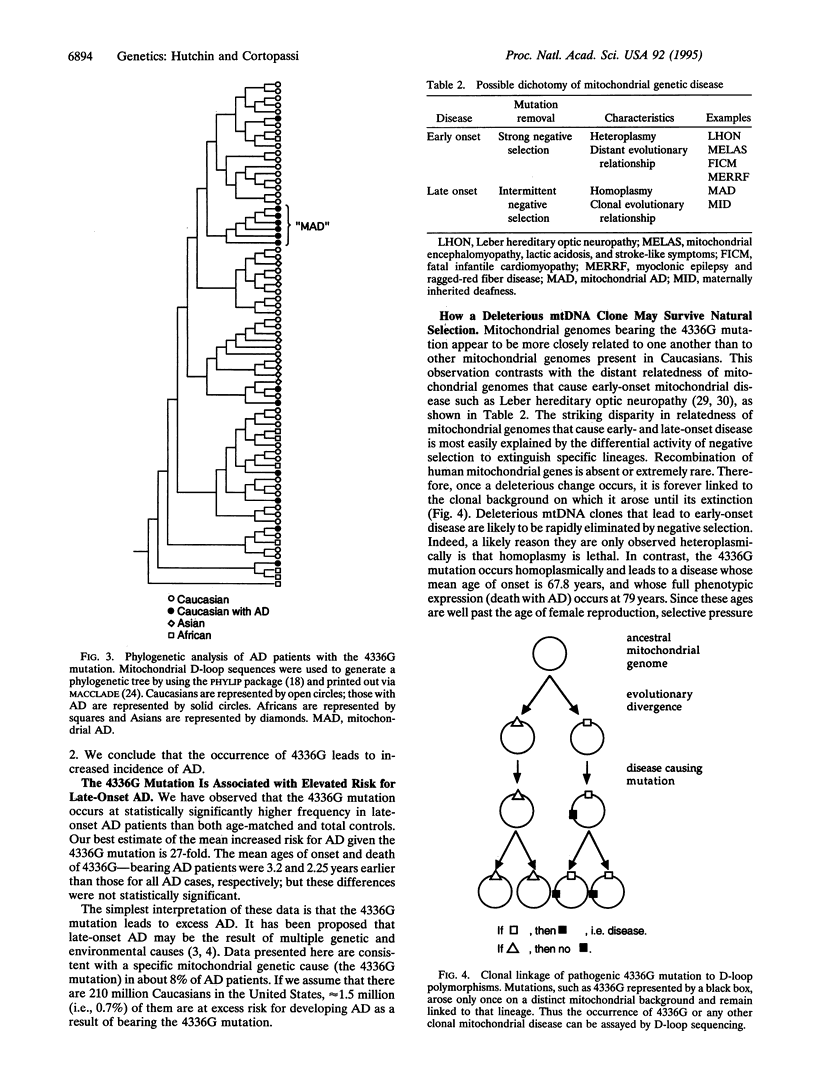
Abstract
Severe mitochondrial genetic mutations lead to early degeneration of specific human tissues; milder mitochondrial mutations may cause degeneration at a later point in life. A mutation at position 4336 was reported to occur at increased frequency in individuals with Alzheimer disease (AD) and Parkinson disease [Shoffner, J. M., Brown, M. D., Torroni, A., Lott, M. T., Cabell, M. F., Mirra, S. S., Beal, M. F., Yang, C.-C., Gearing, M., Salvo, R., Watts, R. L., Juncos, J. L., Hansen, L. A., Crain, B. J., Fayad, M., Reckord, C. L. & Wallace, D. C. (1993) Genomics 17, 171-184]. We have investigated the notion that this mutation leads to excess risk of AD by using a case-control study design of 72 AD autopsies and 296 race- and age-matched controls. The 4336G mutation occurred at higher frequency in AD autopsies than age-matched controls, a statistically significant difference. Evolutionary analysis of mtDNAs bearing the 4336G mutation indicated they were more closely related to each other than to other mtDNAs, consistent with the model of a single origin for this mutation. The tight evolutionary relatedness and homoplasmy of mtDNAs that confer elevated risk for a late-onset disease contrast strikingly with the distant relatedness and heteroplasmy of mitochondrial genomes that cause early-onset disease. The dichotomy can be explained by a lack of selection against mutations that confer a phenotype at advanced age during most of the evolution of humans. We estimate that approximately 1.5 million Caucasians in the United States bear the 4336G mutation and are at significantly increased risk of developing mitochondrial AD in their lifetime. A mechanism for 4336G-mediated cell death is proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachman D. L., Wolf P. A., Linn R., Knoefel J. E., Cobb J., Belanger A., D'Agostino R. B., White L. R. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992 Jan;42(1):115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Beard C. M., Kokmen E., Offord K., Kurland L. T. Is the prevalence of dementia changing? Neurology. 1991 Dec;41(12):1911–1914. doi: 10.1212/wnl.41.12.1911. [DOI] [PubMed] [Google Scholar]
- Borgaonkar D. S., Schmidt L. C., Martin S. E., Kanzer M. D., Edelsohn L., Growdon J., Farrer L. A. Linkage of late-onset Alzheimer's disease with apolipoprotein E type 4 on chromosome 19. Lancet. 1993 Sep 4;342(8871):625–625. doi: 10.1016/0140-6736(93)91458-x. [DOI] [PubMed] [Google Scholar]
- Breteler M. M., Claus J. J., van Duijn C. M., Launer L. J., Hofman A. Epidemiology of Alzheimer's disease. Epidemiol Rev. 1992;14:59–82. doi: 10.1093/oxfordjournals.epirev.a036092. [DOI] [PubMed] [Google Scholar]
- Cleeter M. W., Cooper J. M., Schapira A. H. Irreversible inhibition of mitochondrial complex I by 1-methyl-4-phenylpyridinium: evidence for free radical involvement. J Neurochem. 1992 Feb;58(2):786–789. doi: 10.1111/j.1471-4159.1992.tb09789.x. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Di Rienzo A., Wilson A. C. Branching pattern in the evolutionary tree for human mitochondrial DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1597–1601. doi: 10.1073/pnas.88.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A., Funkenstein H. H., Albert M. S., Scherr P. A., Cook N. R., Chown M. J., Hebert L. E., Hennekens C. H., Taylor J. O. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989 Nov 10;262(18):2551–2556. [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa E., Takeshige K., Oishi T., Murai Y., Minakami S. 1-Methyl-4-phenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1049–1055. doi: 10.1016/0006-291x(90)90498-c. [DOI] [PubMed] [Google Scholar]
- Howell N., Bindoff L. A., McCullough D. A., Kubacka I., Poulton J., Mackey D., Taylor L., Turnbull D. M. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991 Nov;49(5):939–950. [PMC free article] [PubMed] [Google Scholar]
- Hutchin T., Haworth I., Higashi K., Fischel-Ghodsian N., Stoneking M., Saha N., Arnos C., Cortopassi G. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res. 1993 Sep 11;21(18):4174–4179. doi: 10.1093/nar/21.18.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M., Houlden H., Windelspecht M., Fidani L., Lombardi C., Diaz P., Rossor M., Crook R., Hardy J., Duff K. A locus for familial early-onset Alzheimer's disease on the long arm of chromosome 14, proximal to the alpha 1-antichymotrypsin gene. Nat Genet. 1992 Dec;2(4):340–342. doi: 10.1038/ng1292-340. [DOI] [PubMed] [Google Scholar]
- Pfeffer R. I., Afifi A. A., Chance J. M. Prevalence of Alzheimer's disease in a retirement community. Am J Epidemiol. 1987 Mar;125(3):420–436. doi: 10.1093/oxfordjournals.aje.a114548. [DOI] [PubMed] [Google Scholar]
- Prezant T. R., Agapian J. V., Bohlman M. C., Bu X., Oztas S., Qiu W. Q., Arnos K. S., Cortopassi G. A., Jaber L., Rotter J. I. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993 Jul;4(3):289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Rao V. S., van Duijn C. M., Connor-Lacke L., Cupples L. A., Growdon J. H., Farrer L. A. Multiple etiologies for Alzheimer disease are revealed by segregation analysis. Am J Hum Genet. 1994 Nov;55(5):991–1000. [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Saunders A. M., Strittmatter W. J., Schmechel D., George-Hyslop P. H., Pericak-Vance M. A., Joo S. H., Rosi B. L., Gusella J. F., Crapper-MacLachlan D. R., Alberts M. J. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993 Aug;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Orr H. T., Anderson L., Nemens E., White J. A., Bonnycastle L., Weber J. L., Alonso M. E. Genetic linkage evidence for a familial Alzheimer's disease locus on chromosome 14. Science. 1992 Oct 23;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Brown M. D., Torroni A., Lott M. T., Cabell M. F., Mirra S. S., Beal M. F., Yang C. C., Gearing M., Salvo R. Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics. 1993 Jul;17(1):171–184. doi: 10.1006/geno.1993.1299. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Myers R. H., Haines J. L., Farrer L. A., Tanzi R. E., Abe K., James M. F., Conneally P. M., Polinsky R. J., Gusella J. F. Familial Alzheimer's disease: progress and problems. Neurobiol Aging. 1989 Sep-Oct;10(5):417–425. doi: 10.1016/0197-4580(89)90082-1. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P., Haines J., Rogaev E., Mortilla M., Vaula G., Pericak-Vance M., Foncin J. F., Montesi M., Bruni A., Sorbi S. Genetic evidence for a novel familial Alzheimer's disease locus on chromosome 14. Nat Genet. 1992 Dec;2(4):330–334. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi R., Takeshige K., Minakami S. NADH- and NADPH-dependent lipid peroxidation in bovine heart submitochondrial particles. Dependence on the rate of electron flow in the respiratory chain and an antioxidant role of ubiquinol. Biochem J. 1980 Dec 15;192(3):853–860. doi: 10.1042/bj1920853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem J. 1979 Apr 15;180(1):129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Broeckhoven C., Backhovens H., Cruts M., De Winter G., Bruyland M., Cras P., Martin J. J. Mapping of a gene predisposing to early-onset Alzheimer's disease to chromosome 14q24.3. Nat Genet. 1992 Dec;2(4):335–339. doi: 10.1038/ng1292-335. [DOI] [PubMed] [Google Scholar]
- Vigilant L., Stoneking M., Harpending H., Hawkes K., Wilson A. C. African populations and the evolution of human mitochondrial DNA. Science. 1991 Sep 27;253(5027):1503–1507. doi: 10.1126/science.1840702. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wolvetang E. J., Johnson K. L., Krauer K., Ralph S. J., Linnane A. W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994 Feb 14;339(1-2):40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]