Abstract
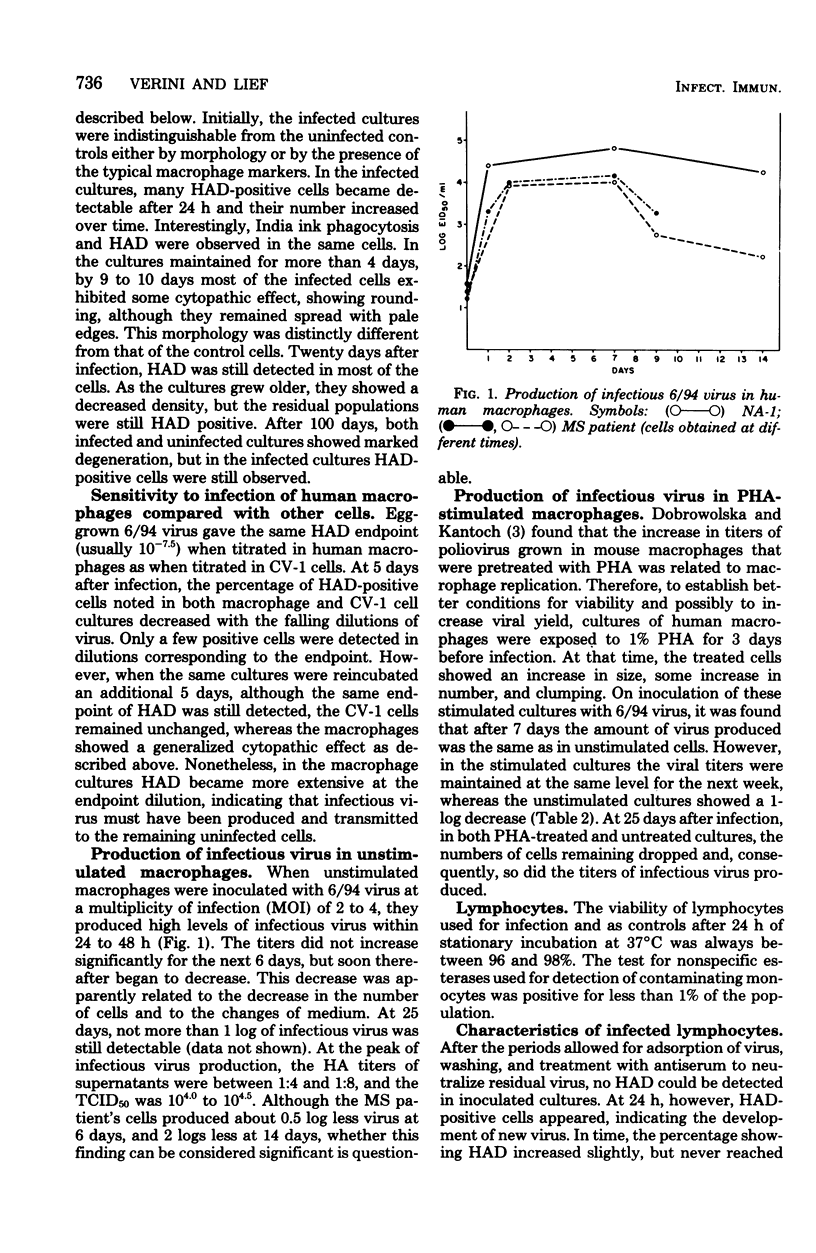
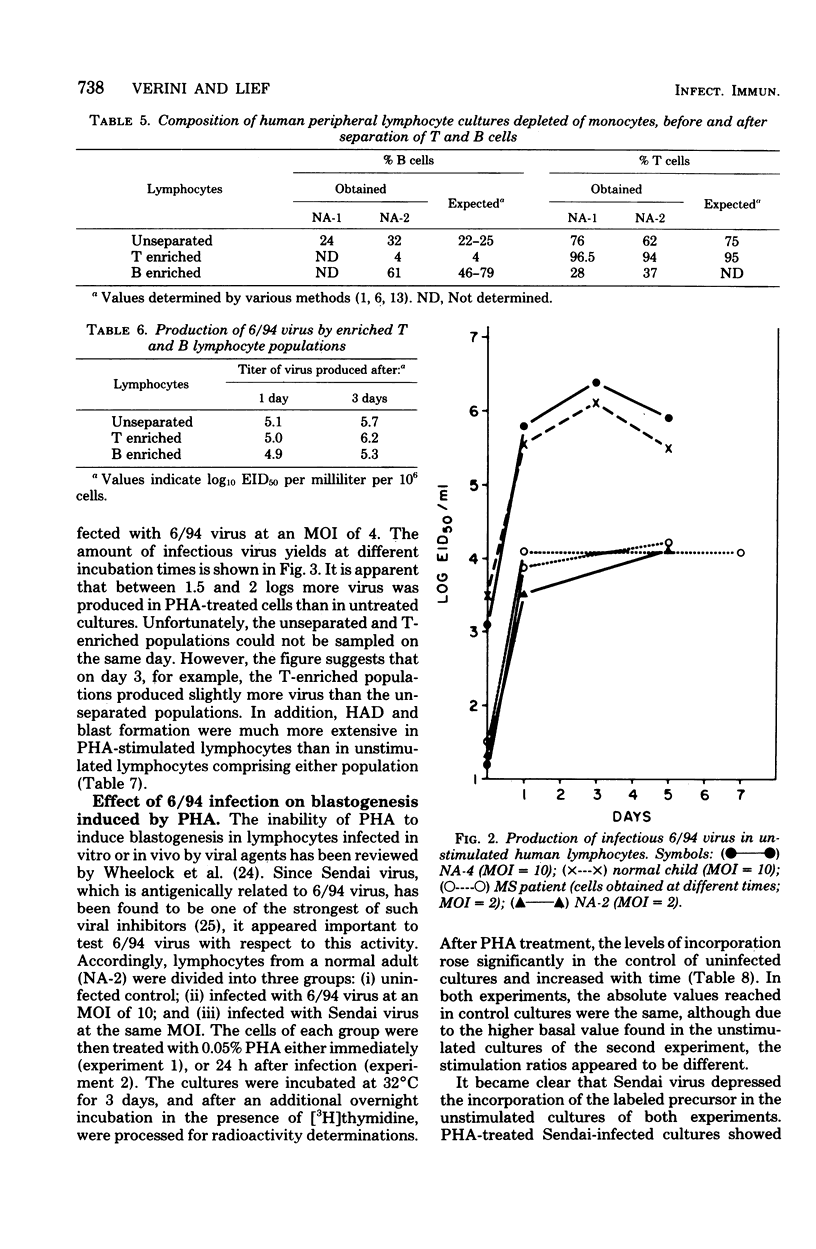
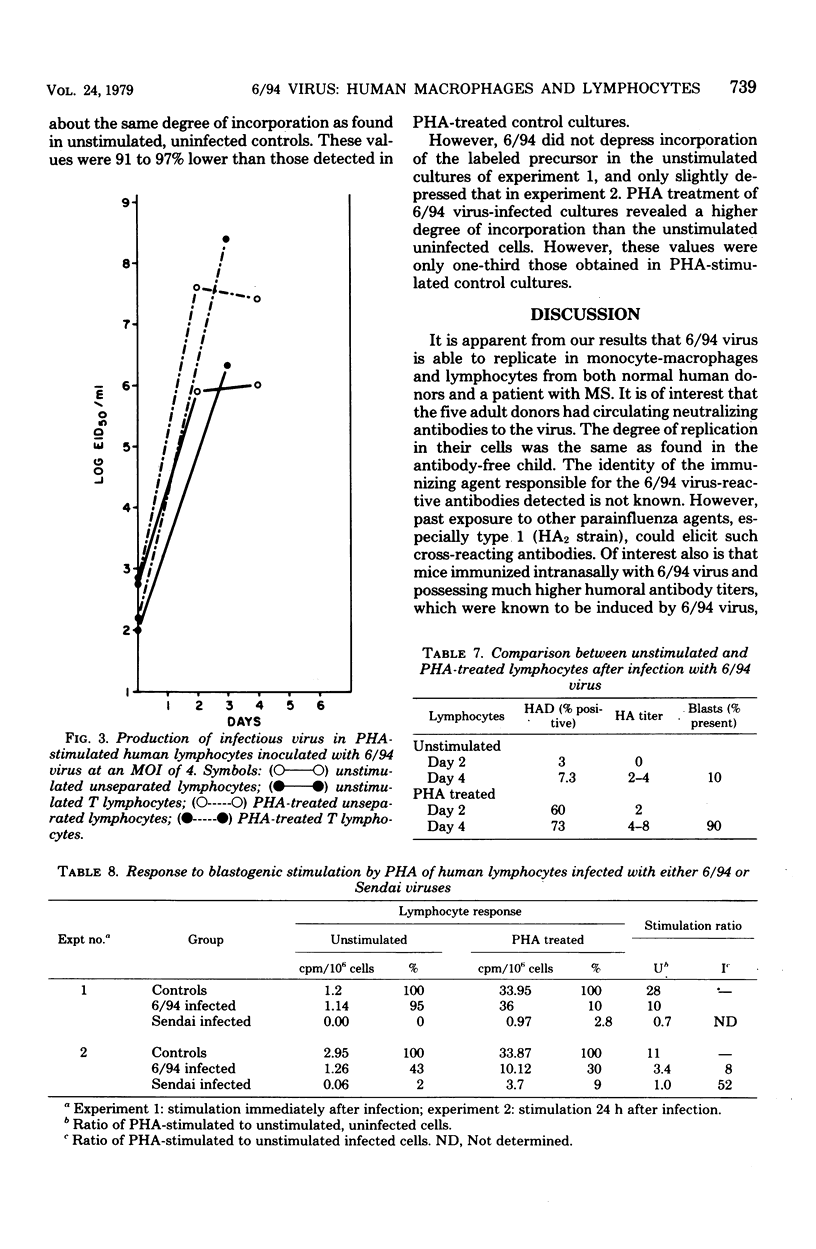
6/94 virus, a parainfluenza type 1 virus recovered by lysolecithin fusion of multiple sclerosis brain cell cultures with CV-1 cells, replicated in monocyte macrophages and lymphocytes from normal human donors and from a patient with multiple sclerosis. In macrophage cultures, hemadsorption-positive cells and high levels of infectious virus became apparent within 24 to 48 h after infection, persisted for 6 days, and then began to decrease. Phytohemagglutinin-stimulated macrophages yielded similar titers of virus, but the levels were maintained for a longer period of time. Macrophage-produced virus appeared to be infectious for other macrophages in the same culture. Both unstimulated and phytohemagglutinin-stimulated lymphocytes also supported virus replication. Significantly higher titers were produced in the stimulated cultures, T cell-enriched populations producing more virus than unseparated populations whether stimulated or unstimulated. The presence or absence of antibodies to the virus in the donors did not appear to influence the levels of virus obtained in any of the leukocyte cultures. However, an increase in blastic forms after 6/94 virus infection was noted in lymphocytes from donors with antibodies as revealed morphologically and by increased incorporations of tritiated thymidine. Furthermore, 6/94 virus-infected lymphocytes, unlike Sendai virus-infected lymphocytes, were able to respond well to mitogenic stimulation by phytohemagglutinin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobrove A. M., Strober S., Herzenberg L. A., DePamphilis J. D. Identification and quantitation of thymus-derived lymphocytes in human peripheral blood. J Immunol. 1974 Feb;112(2):520–527. [PubMed] [Google Scholar]
- Denman A. M., Rager-Zisman B., Merigan T. C., Tyrrell D. A. Replication or inactivation of different viruses by human lymphocyte preparations. Infect Immun. 1974 Feb;9(2):373–376. doi: 10.1128/iai.9.2.373-376.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolska H., Kańtoch M. Badania nad reakywnościa makrofagów na zakazenie enterowirusami. II. Reakcja hodowli makrofagów na wprowadzenie wirusa. Med Dosw Mikrobiol. 1968;20(2):201–206. [PubMed] [Google Scholar]
- Eustatia J. M., Maase E., van Helden P., van der Veen J. Viral replication in mouse macrophages. Arch Gesamte Virusforsch. 1972;39(4):376–380. doi: 10.1007/BF01241017. [DOI] [PubMed] [Google Scholar]
- Frank A. L., Bissell J. A., Rowe D. S., Dunnick N. R., Mayner R. E., Hopps H. E., Parkman P. D., Meyer H. M., Jr Rhesus leukocyte-associated herpesvirus. I. Isolation and characterization of a new herpesvirus recovered from rhesus monkey leukocytes. J Infect Dis. 1973 Nov;128(5):618–629. doi: 10.1093/infdis/128.5.618. [DOI] [PubMed] [Google Scholar]
- GRESSER I., CHANY C. MULTIPLICATION OF POLIOVIRUS TYPE I IN PREPARATIONS OF HUMAN LEUKOCYTES AND ITS INHIBITION BY INTERFERON. J Immunol. 1964 Jun;92:889–895. [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Hartzman R. J., Segall M., Bach M. L., Bach F. H. Histocompatibility matching. VI. Miniaturization of the mixed leukocyte culture test: a preliminary report. Transplantation. 1971 Mar;11(3):268–273. doi: 10.1097/00007890-197103000-00005. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHMIAS A. J., KIBRICK S., ROSAN R. C. VIRAL LEUKOCYTE INTERRELATIONSHIPS. I. MULTIPLICATION OF A DNA VIRUS--HERPES SIMPLEX--IN HUMAN LEUKOCYTE CULTURES. J Immunol. 1964 Jul;93:69–74. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOCH M., WARWICK A., BANG F. B. The cellular nature of genetic susceptibility to a virus. J Exp Med. 1963 May 1;117:781–798. doi: 10.1084/jem.117.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedar E., Ortiz de Landazuri M., Bonavida B. Cellular immunoadsorbents: a simplified technique for separation of lymphoid cell populations. J Immunol. 1974 Mar;112(3):1231–1243. [PubMed] [Google Scholar]
- Lewandowski L. J., Lief F. S., Verini M. A., Pienkowski M. M., ter Meulen V., Koprowski H. Analysis of a viral agent isolated from multiple sclerosis brain tissue: characterization as a parainfluenzavirus type 1. J Virol. 1974 May;13(5):1037–1045. doi: 10.1128/jvi.13.5.1037-1045.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Enders J. F. Vaccinia virus replication and cytopathic effect in cultures in phytohemagglutinin-treated human peripheral blood leukocytes. J Virol. 1968 Aug;2(8):787–792. doi: 10.1128/jvi.2.8.787-792.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata I., Kimura Y., Ito Y., Tanaka T. Temperature-sensitive phenomenon of viral maturation observed in BHK cells persistently infected with HVJ. Virology. 1972 Aug;49(2):453–461. doi: 10.1016/0042-6822(72)90497-7. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Selection of temperature-sensitive mutants during persistent infection: role in maintenance of persistent Newcastle disease virus infections of L cells. J Virol. 1973 Sep;12(3):481–491. doi: 10.1128/jvi.12.3.481-491.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J. A. GROWTH OF VIRULENT AND ATTENUATED ECTROMELIA VIRUS IN CULTURED MACROPHAGES FROM NORMAL AND ECTROMELIAIMMUNE MICE. J Immunol. 1964 Jun;92:837–842. [PubMed] [Google Scholar]
- Ueda S., Nozima T. Delayed hypersensitivity in vaccinia-infected mice. II. Resistance of peritoneal macrophages against vaccinia infection. Acta Virol. 1973 Jan;17(1):41–49. [PubMed] [Google Scholar]
- Verini M. A., Lief F. S. Interaction between 6/94 virus, a parainfluenza type 1 strain, and mouse macrophages. Infect Immun. 1979 Jun;24(3):720–728. doi: 10.1128/iai.24.3.720-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verini M. A., Lief F. S. Interaction between 6/94 virus, a parainfluenza type 1 strain, and unstimulated mouse lymphocytes. Infect Immun. 1979 Jun;24(3):729–733. doi: 10.1128/iai.24.3.729-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Viral inhibition of the phytohemagglutinin response of human lymphocytes and application to viral hepatitis. Proc Soc Exp Biol Med. 1969 Feb;130(2):652–661. doi: 10.3181/00379727-130-33628. [DOI] [PubMed] [Google Scholar]
- Zisman B., Denman A. M. Inactivation of myxoviruses by lymphoid cells. J Gen Virol. 1973 Aug;20(2):211–223. doi: 10.1099/0022-1317-20-2-211. [DOI] [PubMed] [Google Scholar]


