Abstract
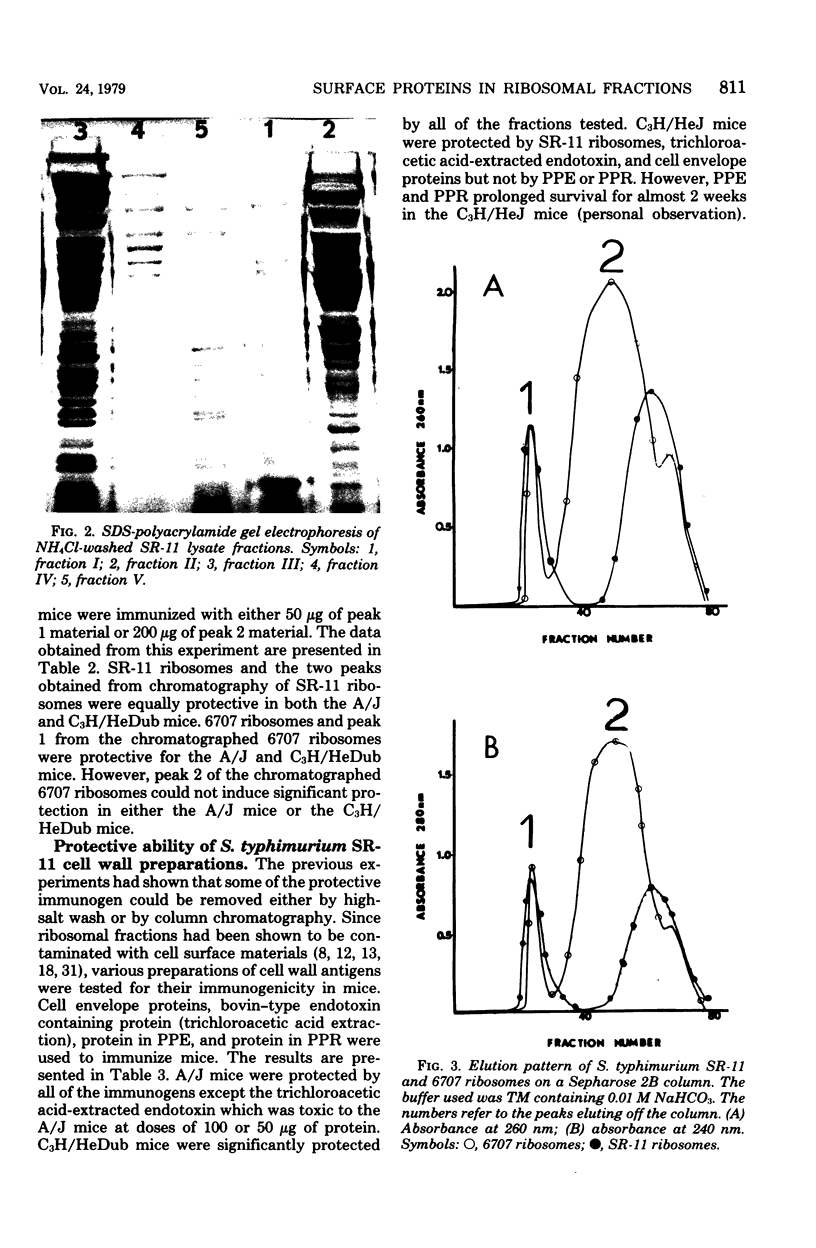
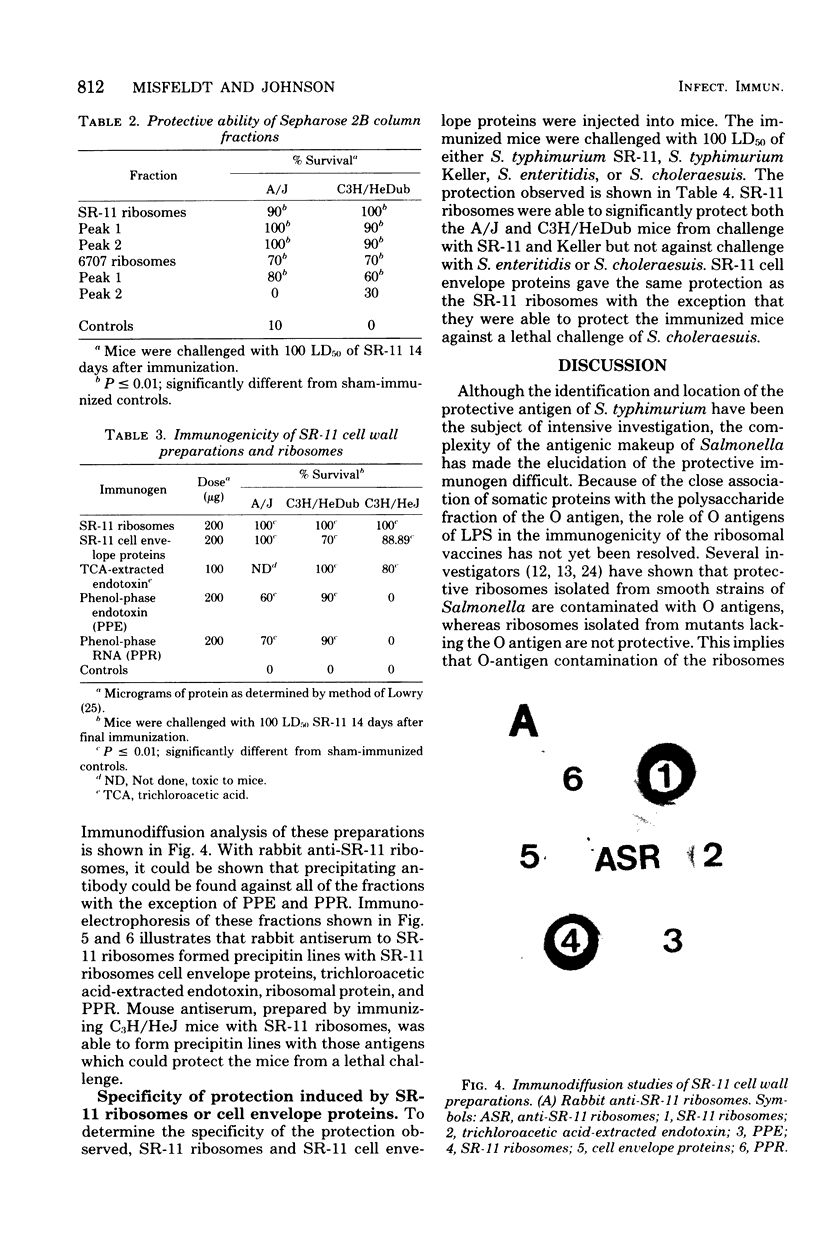
Cell surface antigen preparations from Salmonella typhimurium SR-11 prepared by either trichloroacetic acid extraction or boiling in sodium dodecyl sulfate were able to protect C3H/HeJ, C3H/HeDub, and A/J mice. Some of the proteins found in these preparations were shown to exist in the protective ribosomal fraction isolated from S. typhimurium SR-11. Passage of ribosomes isolated from S. typhimurium SR-11 and 6707 through a Sepharose 2B column removed the protective immunogen from 6707 ribosomes but did not completely remove it from SR-11 ribosomes. Immunity to salmonella infection in C3H/HeJ mice induced by ribosomal vaccines may be dependent on the presence of cell surface proteins in the ribosomal fraction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber C., Eylan E. Confirmation of the protective role of proteins from S. typhimurium in infection of mice with their natural pathogen. Zentralbl Bakteriol Orig A. 1975;230(4):461–465. [PubMed] [Google Scholar]
- Barber C., Eylan E., Heiber R. The protective role of proteins from Salmonella typhimurium in infection of mice with their natural pathogen. Rev Immunol (Paris) 1972 Apr-Jun;36(3):77–84. [PubMed] [Google Scholar]
- Barber C., Eylan E. Heterologous protections in experimental salmonellosis. Zentralbl Bakteriol Orig A. 1975;230(4):452–457. [PubMed] [Google Scholar]
- Barber C., Eylan E. The proteins from S. enteritidis; their protective role in mice, and the antibodies induced during infection with the homologous strain. Zentralbl Bakteriol Orig A. 1974 Mar;226(3):331–335. [PubMed] [Google Scholar]
- Barber C., Eylan E. The unfortunate role of precedent in bacteriology. I. The main antigens of salmonellae: the proteins. Zentralbl Bakteriol Orig A. 1976 Jan;234(1):53–59. [PubMed] [Google Scholar]
- Chedid L., Parant M., Damais C., Parant F., Juy D., Galelli A. Failure of endotoxin to increase nonspecific resistance to infection of lipopolysaccharide low-responder mice. Infect Immun. 1976 Mar;13(3):722–727. doi: 10.1128/iai.13.3.722-727.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauve R. M., Hevin B. Immunostimulation with bacterial phospholipid extracts. Proc Natl Acad Sci U S A. 1974 Feb;71(2):573–577. doi: 10.1073/pnas.71.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero F., Romano C. The protection of mice against experimental infection by means of immunization with enriched membrane fraction of Salmonella typhimurium. Experientia. 1975 Feb 15;31(2):238–238. doi: 10.1007/BF01990726. [DOI] [PubMed] [Google Scholar]
- Gürtler L. G., Rank W. R. Genetic differences between endotoxin-sensitive and resistant C3H mice. Intraperitoneal cell response to lipopolysaccharide and prostaglandins. Z Immunitatsforsch Immunobiol. 1976 Aug;151(1):420–429. [PubMed] [Google Scholar]
- Hoops P., Prather N. E., Berry J., Ravel J. M. Evidence for an extrinsic immunogen in effective ribosomal vaccines from Salmonella typhimurium. Infect Immun. 1976 Apr;13(4):1184–1192. doi: 10.1128/iai.13.4.1184-1192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
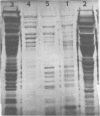
- Houchens D. P., Wright G. L., Jr Immunity to Salmonella typhimurium infection: characterization of antigens in active protection by polyacrylamide gel electrophoresis. Infect Immun. 1973 Mar;7(3):507–511. doi: 10.1128/iai.7.3.507-511.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis. Infect Immun. 1972 Jun;5(6):947–952. doi: 10.1128/iai.5.6.947-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin J. H., Berry L. J. The use of strain LT2-Ml in identifying the protective antigens in a Salmonella typhimurium-derived ribosomal vaccine. J Reticuloendothel Soc. 1978 Feb;23(2):135–143. [PubMed] [Google Scholar]
- Lugowski C., Romanowska E. Biological properties of lipid A from Shigella sonnei. Eur J Biochem. 1974 Oct 1;48(1):81–87. doi: 10.1111/j.1432-1033.1974.tb03745.x. [DOI] [PubMed] [Google Scholar]
- Mates A., Yosipovici H. Localization of the protective antigen in Salmonella typhimurium. Microbios. 1976;16(64):81–90. [PubMed] [Google Scholar]
- Medina S., Vas S. I., Robson H. G. Effect of nonspecific stimulation on the defense mechanisms of inbred mice. J Immunol. 1975 Jun;114(6):1720–1725. [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Protective ability of Salmonella ribosomal protein and RNA in inbred mice. Infect Immun. 1978 Jul;21(1):286–291. doi: 10.1128/iai.21.1.286-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Role of endotoxin contamination in ribiosomal vaccines prepared from Salmonella typhimurium. Infect Immun. 1977 Jul;17(1):98–104. doi: 10.1128/iai.17.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Variability of protection in inbred mice induced by a ribosomal vaccine prepared from Salmonella typhimurium. Infect Immun. 1976 Sep;14(3):652–659. doi: 10.1128/iai.14.3.652-659.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Romanowska E. Sepharose gel filtration method of purification of lipopolysaccharides. Anal Biochem. 1970 Feb;33(2):383–389. doi: 10.1016/0003-2697(70)90309-x. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Wu M. C., Heath E. C. Isolation and characterization of lipopolysaccharide protein from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2572–2576. doi: 10.1073/pnas.70.9.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]