Abstract
The process of Henle 407 embryonic intestinal epithelial cell infection by Shigella flexneri 2a M42-43 was studied in an in vitro model system. The role of the Henle cell was assessed. It was established that entry of S. flexneri into cells was suppressed by reagents which inhibit uptake of particles by phagocytic cells. The compounds tested included cytochalasin B, dibutyryl-cyclic adenosine monophosphate, choleragen (Vibrio cholera enterotoxin), iodoacetate, and dinitrophenol. Cytochalasin B inhibited infection at concentrations of 1.0 μg/ml or greater. Dibutyryl-cyclic adenosine monophosphate at concentrations of 1 mM and choleragen at 0.1 μg/ml caused significant suppression of infection. Iodoacetate or dinitrophenol, at 0.1 mM concentrations, inhibited internalization of virulent shigellae, and a combination of these compounds inhibited infection at 0.01 mM concentrations. Preincubation of Henle cell monolayers with the combination of iodoacetate and dinitrophenol (0.05 mM) also inhibited infection markedly. The data suggest that infection of epithelial cells by S. flexneri in vitro is accomplished by an endocytic process induced by virulent bacteria. The process appears to be similar to uptake of particles by phagocytic cells. Ultrastructural analysis by transmission electron microscopy provided corroborative evidence of phagocytosis of shigellae by Henle cells in that intracellular bacteria were often observed within membrane-limiting vacuoles resembling phagosomes.
Full text
PDF


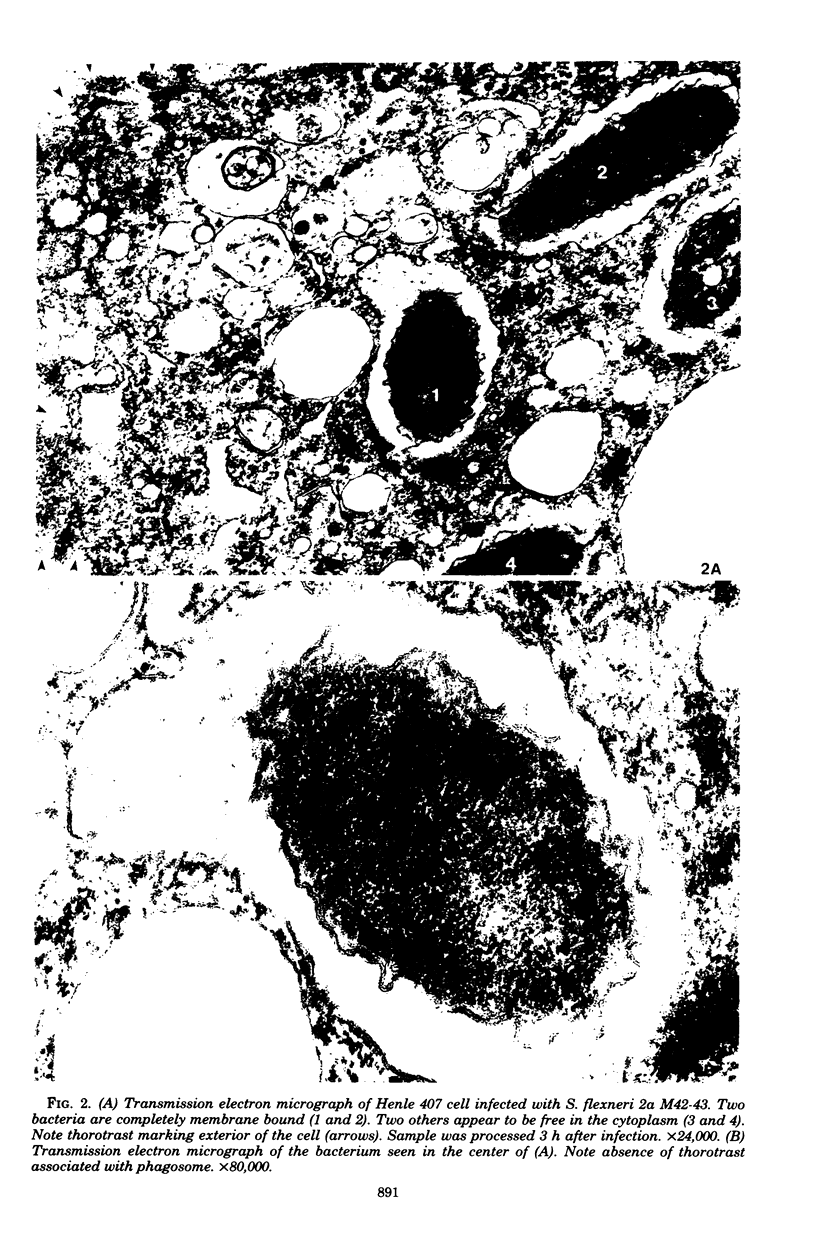
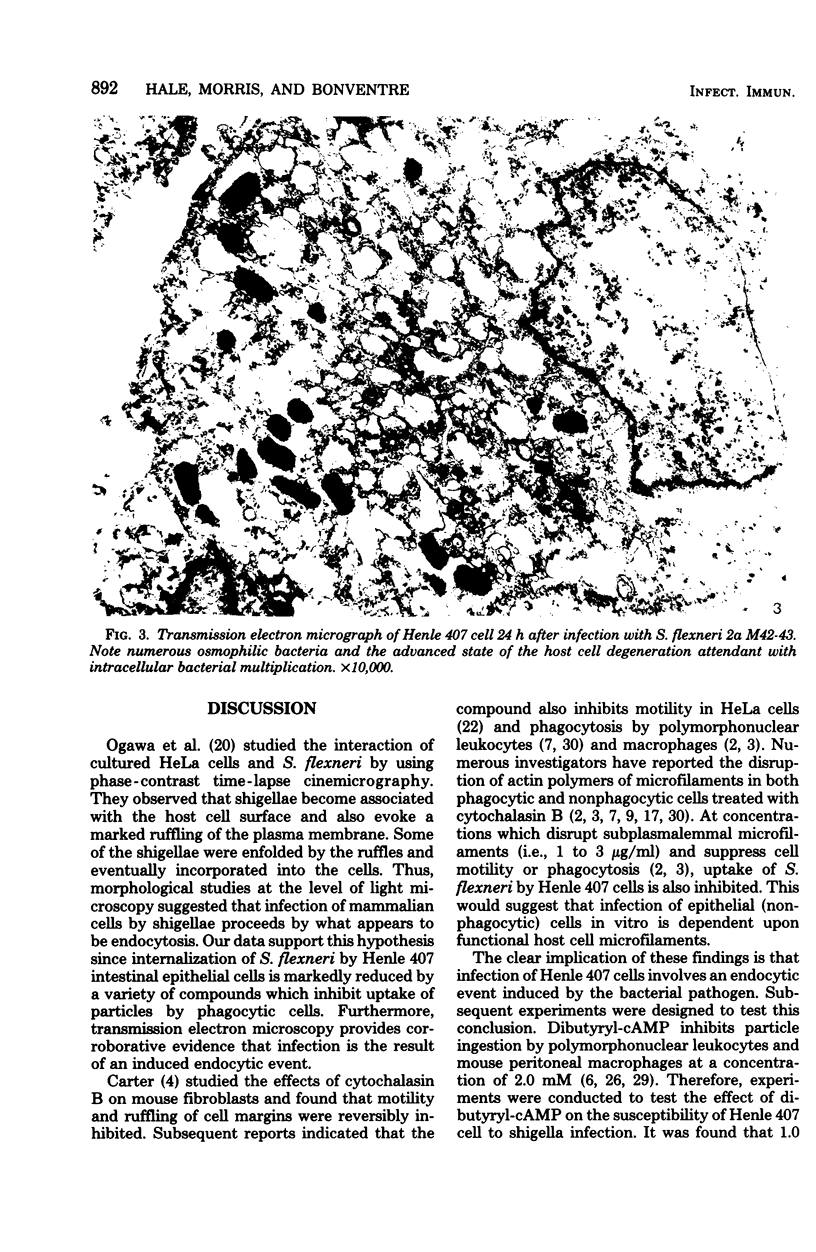


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Cox J. P., Karnovsky M. L. The depression of phagocytosis by exogenous cyclic nucleotides, prostaglandins, and theophylline. J Cell Biol. 1973 Nov;59(2 Pt 1):480–490. doi: 10.1083/jcb.59.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Fox R. I., Polyzonis M., Allison A. C., Haswell A. D. The inhibition of phagocytosis and facilitation of exocytosis in rabbit polymorphonuclear leukocytes by cytochalasin B. Lab Invest. 1973 Jan;28(1):16–22. [PubMed] [Google Scholar]
- HENLE G., DEINHARDT F. The establishment of strains of human cells in tissue culture. J Immunol. 1957 Jul;79(1):54–59. [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Interactions of actin, myosin, and an actin-binding protein of rabbit pulmonary macrophages. III. Effects of cytochalasin B. J Cell Biol. 1976 Oct;71(1):295–303. doi: 10.1083/jcb.71.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Fishman P. H., Bennett V., Cuatrecasas P. Cholera toxin and cell growth: role of membrane gangliosides. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4224–4228. doi: 10.1073/pnas.71.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Morgan W. D., Pastan I. Regulation of cell motility by cyclic AMP. Nature. 1972 Jan 7;235(5332):54–56. doi: 10.1038/235054a0. [DOI] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke E., Carlberg K., Norrby R. Interactions between Toxoplasma gondii and its host cells: function of the penetration-enhancing factor of toxoplasma. Infect Immun. 1975 Apr;11(4):853–861. doi: 10.1128/iai.11.4.853-861.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech H. L., Lentz T. L. Microfilaments in epidermal cancer cells. J Cell Biol. 1974 Feb;60(2):473–482. doi: 10.1083/jcb.60.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A. Virulence of Shigella isolated from healthy carriers. Jpn J Med Sci Biol. 1967 Jun;20(3):213–223. doi: 10.7883/yoken1952.20.213. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Nakamura A., Nakaya R. Cinemicrographic study of tissue cell cultures infected with Shigella flexneri. Jpn J Med Sci Biol. 1968 Aug;21(4):259–273. doi: 10.7883/yoken1952.21.259. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Sanger J. W., Holtzer H. Cytochalasin B: effects on cell morphology, cell adhesion, and mucopolysaccharide synthesis (cultured cells-contractile microfilaments-glycoproteins-embryonic cells-sorting-out). Proc Natl Acad Sci U S A. 1972 Jan;69(1):253–257. doi: 10.1073/pnas.69.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Takeuchi A., Formal S. B., Sprinz H. Exerimental acute colitis in the Rhesus monkey following peroral infection with Shigella flexneri. An electron microscope study. Am J Pathol. 1968 Mar;52(3):503–529. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]
- Welscher H. D., Cruchaud A. The relationship between phagocytosis, release of lysosomal enzymes and 3', 5' cyclic adenosine monophosphate in mouse macrophages. Adv Exp Med Biol. 1976;66:705–710. doi: 10.1007/978-1-4613-4355-4_109. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]