Abstract
Because tobacco use has a large negative health and financial impact on society, it is critical to identify the factors that drive excessive use. These factors include the aversive withdrawal symptoms that manifest upon cessation of tobacco use, and may include increases in nociceptive processing. Corticotropin-releasing factor (CRF) signalling in the central amygdala (CeA) has been attributed an important role in: (1) central processing of pain, (2) excessive nicotine use that results in nicotine dependence, and (3) in mediating the aversive symptoms that manifest following cessation of tobacco exposure. Here, we describe three experiments in which the main hypothesis was that CRF/CRF1 receptor (CRF1R) signalling in the CeA mediates nicotine withdrawal-induced increases in nociceptive sensitivity in rats that are dependent on nicotine. In Experiment 1, nicotine-dependent rats withdrawn from chronic intermittent (14-h/day) nicotine vapor exhibited decreased hindpaw withdrawal latencies in response to a painful thermal stimulus in the Hargreaves test, and this effect was attenuated by systemic administration of the CRF1R antagonist, R121919. In Experiment 2, nicotine-dependent rats withdrawn from nicotine vapor exhibited robust increases in mRNA for CRF and CRF1Rs in CeA. In Experiment 3, intra-CeA administration of R121919 reduced thermal nociception only in nicotine-dependent rats. Collectively, these results suggest that nicotine dependence increases CRF/CRF1R signalling in the CeA that mediates withdrawal-induced increases in sensitivity to a painful stimulus. Future studies will build on these findings by exploring the hypothesis that nicotine withdrawal-induced reduction in pain thresholds drive excessive nicotine use via CRF/CRF1R signalling pathways.
Keywords: Nicotine Dependence, CRF1 Receptor, R121919, Hargreaves, Withdrawal
INTRODUCTION
Cigarette smoking leads to cancer, respiratory disease, and cardiovascular disease, and is responsible for 6 million deaths annually and more than 400,000 deaths per year in the U.S. (Cancer.gov; Who.int). Withdrawal from nicotine, the psychoactive ingredient in tobacco, produces both somatic and affective symptoms that contribute to relapse and continued nicotine use (Koob et al., 1993; Markou et al., 1998). This point is concerning in light of the recent explosion in the use of electronic cigarette (e-cigarette) products, which contain potentially harmful levels of nicotine, but whose negative health impacts have been grossly understudied (Henningfield & Zaatari, 2010; Trtchounian & Talbot, 2011). The cessation of nicotine use results in a number of aversive effects that include irritability, restlessness, increased anxiety, and enhanced nociceptive processing (Bruijnzeel, 2012; Hughes and Hatsukami, 1986; Yang et al, 1992). These aversive withdrawal symptoms, either separately or combined, may drive subsequent excessive nicotine use and relapse to nicotine use (Bruijnzeel, 2012; Piper et al, 2010). In the attempt to reduce abuse of tobacco products, it is critical to identify behaviors (e.g., nociceptive processing) and neural systems dysregulated by nicotine withdrawal that may drive subsequent use.
Under normal physiological conditions, corticotropin releasing factor (CRF) contributes to emotional regulation via both hypothalamic and amygdalar circuits (Heilig et al., 1994). Dysregulation of these brain CRF systems is heavily implicated in multiple psychiatric disorders including anxiety, depression, and alcohol and drug use disorders (Arborelius et al, 1999; Bale and Vale, 2004; Koob, 2010; Risbrough, 2006). For example, alcohol withdrawal in alcohol-dependent animals is defined by mechanical allodynia (i.e., pain in response to a stimulus that does not normally produce pain) and increased anxiety-like behavior that are mediated by CRF1R signalling (Edwards et al., 2012; Funk et al., 2006, 2007; Richardson et al., 2007; Valdez et al., 2002, 2003), which is particularly interesting because antagonism of CRF1Rs reduces excessive alcohol drinking by alcohol-dependent rats (Funk et al., 2007; Roberto et al., 2010), an effect that is mediated by the central nucleus of the amygdala (CeA; Funk et al., 2006). Animals that are dependent on nicotine exhibit increases in nociceptive processing, anxiety-like behavior and nicotine self-administration (Cohen et al., 2013; George et al., 2007; Gilpin et al., 2013; Grabus et al., 2005; Yang et al., 1992). Antagonism of CRF1Rs attenuates nicotine withdrawal-induced mechanical allodynia, as well as heightened anxiety-like behavior and nicotine self-administration under long-access (23-h/day) conditions (Cohen et al., 2013; George et al., 2007). Antagonism of CRF1Rs or reduction of CRF1R expression attenuates hyperalgesia associated with inflammatory and neuropathic pain in mice (Hummel et al., 2010). Furthermore, CRF1Rs specifically in the CeA have been implicated in mediating brain reward deficits associated with nicotine withdrawal (Marcinkiewcz et al., 2009; Bruijnzeel et al., 2012). In the present investigation, we examined thermal hyperalgesia in rats withdrawn from chronic intermittent nicotine vapor, as well as the role of CRF1Rs in CeA in mediating this hyperalgesia.
The CeA, a limbic region critical for converting emotionally relevant sensory information into appropriate behavioral and physiological responses, has been identified as a potential “on-off” switch for central transmission of pain information (Rouwette et al., 2012). In humans, oral administration of a CRF1R antagonist dampens the amygdalar activation produced by pain expectation (Hubbard et al., 2011). In rats, administration of exogenous CRF into CeA promotes nociception via effects at CRF1Rs (Ji & Neugebauer, 2008). The CeA is recruited during the transition to dependence on drugs of abuse, including nicotine, and CRF/CRF1R signaling in CeA undergoes neuroadaptations that are particularly important for producing the aversive aspects of drug withdrawal and driving future drug use (Koob, 2008). Furthermore, heightened pain processing has been cited as a potential motivating factor for alcohol and drug abuse in humans (e.g.,Brennan et al, 2005; Riley and King, 2009; Egli et al., 2012).
A recently published study showed that CRF1Rs in the CeA mediate mechanical allodynia during withdrawal from long-access nicotine self-administration (Cohen et al., in press). In the present investigation, we aimed to extend those findings by investigating the role of CRF1Rs in the CeA in thermal hyperalgesia during withdrawal from a nicotine vapor exposure protocol that produces nicotine dependence (George et al., 2010) and escalates nicotine self-administration in rats (Gilpin et al., 2013). We hypothesized that nicotine dependence would increase thermal nociception and CRF and CRF1R gene expression in CeA, and also that antagonism of CRF1Rs, both systemically and in the CeA, would reverse withdrawal-induced hyperalgesia.
METHODS
Animals
Specific-pathogen free adult male Wistar rats (Charles River, Kingston, NY) weighing 200-250 g at the time of arrival were pair-housed and fed a standard rat diet (Purina Rat Chow, Ralston Purina, St. Louis, MO) with water available ad libitum except during experimental procedures. Rats were exposed to a reverse 12-h light/12-h dark cycle (lights off at 8 AM). All behavioral testing was conducted in the dark cycle. Animal procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center (LSUHSC IACUC 2908) and were in accordance with the National Institute of Health guidelines.
Drugs and Chemicals
The CRF-1 antagonist, R121919, was a generous gift from Neurocrine Biosciences Inc (San Diego, CA). R121919 was dissolved in hydroxypropyl-β-cyclodextrin with a final concentration of 20%. Vehicle-treated animals received 20% hydroxypropyl-β-cyclodextrin. Mecamylamine hydrochloride (Sigma, St. Louis, MO) was dissolved in saline.
Nicotine Vapor Inhalation
To induce nicotine dependence, animals were housed in nicotine vapor inhalation chambers (La Jolla Alcohol Research, Inc.; La Jolla, CA) and exposed daily to intermittent (14-h ON/10-h OFF) nicotine vapor. Nicotine vapor was produced by bubbling air at a flow rate of 10 l/min/cage through a gas-washing bottle containing a solution of pure nicotine (free base, Sigma, St. Louis, MO). Nicotine vapor was produced by vaporization that is maximized by the bubbling of air with a constant airflow. The highly concentrated nicotine vapor was then passed through a drop-catch bottle and further diluted by the addition of 60 l/min of clean air in a 2000 mL Erlenmeyer vacuum flask at room temperature. The final nicotine–air mixture was homogeneously distributed between chambers at a flow rate of 15 l/min. Nicotine-air concentrations, which can be tightly regulated by this procedure (Gilpin et al., 2013), were adjusted by varying the flow rate at which nicotine was bubbled. Air controls were treated in a similar manner except that air entering the cages did not contain nicotine.
Hargreaves Test
We utilized the Hargreaves method to test nociceptive processing in nicotine-dependent rats. Animals were placed in the examination room 10 min prior to testing to allow acclimation to the light and testing environment. After 5 min, animals were placed in Plexiglas enclosures with glass floors (IITC Life Sciences, Inc., Woodland Hills, CA) suspended 30 cm from the table top and allowed to habituate for 5 min prior to testing. The hind paws were individually stimulated from below using a halogen heat source from an IITC model 309 Hargreaves apparatus (IITC Life Sciences, Inc., Woodland Hills, CA). The intensity of the beam (75 A.I.) was selected to produce an average baseline threshold of approximately 8 seconds. A 20- second cut-off was employed to prevent tissue damage in non-responsive subjects. The latency to produce a nocifensive paw withdrawal response was used to measure thermal hypersensitivity. Each hind paw was targeted twice in alternating order, producing 4 scores of nociception that were averaged and analyzed as described below.
Stereotaxic Surgeries
Surgical implantation of cannulae was conducted using aseptic procedures. Rats were anesthetized via inhalation of isoflurane (IsoFlo, Abbott Laboratories, North Chicago, IL) before and during surgery. The incision area of the scalp was shaved, the rat was placed in a Kopf stereotaxic instrument, and a sagittal incision (approximately 2 cm long) was made in the midline exposing the surface of the skull. Two holes were drilled through the skull targeted above the left and right central amygdaloid nuclei according to the appropriate stereotaxic coordinates and a guide cannula was implanted. The stereotaxic coordinates were determined according to Paxinos and Watson (1998). The coordinates relative to bregma were (AP-2.6, ML±4.2, DV-7.0) from skull surface. Microinjection cannulae components (Plastics One Inc., Roanoke, VA) included guide cannulae (26 gauge), internal injection cannulae (33 gauge), and dummy cannulae (33 gauge). The injection cannula extended 1.0 mm beyond the tip of the guide cannula when inserted. A dummy cannula, cut to the same length as the guide cannulae, was maintained in the guide cannula at all times except during infusions. Four stainless steel screws were inserted into the skull at positions around the cannula implant site. Cranioplastic cement was applied over the open surface of the skull covering both the screws and the guide cannula. The incision was closed around the implants and the dummy cannula was inserted. Immediately after surgery, antibiotic ointment was applied to the surgical wound area. The rats were monitored during five days of recovery to determine that the animal had resumed normal activity such as mobility, and consumption of liquid diet and water.
Experiment 1
Rats were exposed to chronic intermittent (14-h/day) nicotine vapor (n=8) or ambient air (n=8) for 6 weeks prior to the start of Hargreaves testing. Physical dependence on nicotine was confirmed by precipitating withdrawal with mecamylamine (1.5 mg/3 ml/kg, s.c). Animals were then tested for thermal nociception during nicotine withdrawal both 6-h and 72-h following termination of nicotine vapor exposure. Rats were then re-exposed to nicotine vapor or control air for 4 days (14-h/day) and once again tested for thermal nociception both 6-h and 72-h following termination of nicotine vapor exposure (i.e., testing occurred during weeks 6 & of vapor exposure). Prior to each of the four tests, rats were injected with either R121919 (10 mg/2 ml/kg, s.c.) or vehicle (2 ml/kg), such that each rat was injected once with each dose at each test time point. A 60-min pre-treatment time was used to allow the drug to reach peak efficacy (Roberto et al 2010). We chose the 10 mg/kg R121919 dose based on preliminary Hargreaves studies conducted in our laboratory. For the Hargreaves test, rats were placed in Plexiglas holding chambers for a 5-min acclimation period prior to Hargreaves testing. At the completion of testing each day, animals were returned to their home cages.
Experiment 2
The purpose of Experiment 2 was to measure CRF and CRF1R gene expression in the brains of nicotine-dependent rats; we measured gene expression in the CeA, our brain region of interest for this study, as well as the nucleus accumbens (NAc) shell, another component region of the extended amygdala, used here as a control region. Rats were exposed to 3 weeks of nicotine vapor, because recent published data suggest that <3 weeks nicotine vapor is sufficient to produce somatic withdrawal (George et al., 2010; Gilpin et al., 2013) and escalated nicotine self-administration (i.e., motivational withdrawal; Gilpin et al., 2013), the latter of which has been attributed to altered CRF/CRF1R signaling in amygdala (George et al., 2007). On day 21 of nicotine vapor exposure, rats were sacrificed 16 h following termination of vapor exposure (time point chosen based on preliminary data from our lab) and their brains collected and stored at −80 °C until processed for quantitative real-time PCR. Briefly, rats were anesthetized with isoflurane and decapitated; brains were quickly removed and sliced coronally in a cryostat, with punches of the brain regions of interest collected on an ice-cold stage. Punches were taken with 14-17 gauge needles guided by an atlas and stored at −80°C until they were processed for quantitative real time PCR. Total RNA was prepared from each punch using the RNeasy lipid mini kit (Qiagen, Valencia, CA) as recommended for animal tissue. Total RNA (1 μg), quantified by Nanodrop 1000 (Thermo Scientific, Wilmington, DE), was reverse transcribed with QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA), which included a DNA removal step. For quantitative real-time PCR, Roche Light Cycler 480 Master-plus SYBR Green mix (Roche Applied Science, Indianapolis, IN) was used. Reactions (10 μl) were carried out in a 96-well plate Realplex2 machine (Eppendorf). The primers for each target (0.5 μM final concentration, Sigma, St. Louis, MO) were synthesized with a standard desalting purification: Targets included CRF and the CRF1 receptor. Standard curves were constructed using sequenced PCR products. Results were analyzed by second derivative methods and expressed in arbitrary units, normalized to Cyclophilin A expression levels. Standards and samples were run in duplicate, and all reactions for a given brain region performed concurrently. Gene-specific amplification was determined by melting curve analysis as one peak at the expected melting temperature and by agarose gel electrophoresis.
Experiment 3
Rats were exposed to chronic intermittent (14-h/day) nicotine vapor (n=8) or ambient air (n=8) for 4 weeks, at which point nicotine dependence was confirmed by observing somatic withdrawal signs after mecamylamine injection. All rats then underwent stereotaxic surgery and were implanted bilaterally with cannulae aimed at the CeA. Following surgery, rats were returned to previous housing conditions (nicotine vapor or ambient air), monitored during five days of recovery, then tested for baseline thermal withdrawal latencies. In order to minimize the effects of surgery and infusion procedures on thermal sensitivity, all rats were habituated to the infusion procedure with two sham infusion days (i.e., nothing infused into brain) and one vehicle infusion day to ensure that thermal withdrawal latencies were representative of pre-surgery baselines, at which point R121919 infusions were conducted during weeks 6-7 of vapor exposure.
On test days, rats were infused with either vehicle or R121919 (0.125, 0.250, or 0.500 μg) in a within-subjects Latin-square design, and all tests occurred 6 hrs into nicotine withdrawal. The 6-h withdrawal test time point was chosen because (1) nicotine-dependent rats exhibited hyperalgesia at this time point in Experiment 1, and (2) to allow for behavioral testing in the context of daily withdrawals without the need for multiple 72-h periods of nicotine abstinence. A Harvard 33 microinfusion pump was used for all drug infusions at a rate of 0.2μl/minute for a period of 2.5 minutes, and the injection cannula was left in the guide cannula for one additional minute to allow for adequate diffusion of the solution. Infusions were delivered to the cannula via polyethylene tubing (PE 20) that was connected to a Hamilton 10 μl syringe. Immediately following infusions, rats were placed directly into the Plexiglas enclosures on the Hargreaves apparatus for 5 minutes to acclimate before testing began. At the completion of the testing period, animals were returned to their home cages. Each test was separated by 48 hours to avoid any residual effects of R121919 from the previous test day.
Statistical Analysis
All behavioral data are expressed as mean ± SEM with the number of animals per group indicated in the figure legends. Statistical analysis of differences in thermal withdrawal latencies for the Hargreaves test was determined by three-way and two-way repeated measures (RM) analyses of variance (ANOVA) where nicotine vapor history was the between-subjects factor, and drug dose and/or withdrawal time point were the within-subjects factors. For Experiment 3 data, two rats were excluded from all data analyses due to inaccurate placement of one or both cannula. Molecular data are expressed as a percentage of control group mean with the number of animals per group indicated in the figure legends. Statistical analysis of differences in CRF and CRF1R mRNA expression was determined by one-way ANOVAs where nicotine vapor history was the treatment factor. Post-hoc comparisons were conducted using the Student Newman-Keuls test and statistical significance was set at p<0.05.
RESULTS
Nicotine Dependence-Induced Hyperalgesia Attenuated by Systemic CRF1R Antagonist
A 3-way (Vapor condition × withdrawal time point × R121919 dose) RM ANOVA revealed that nicotine-dependent rats exhibited significant reductions in paw withdrawal latencies in response to a thermal nociceptive stimulus at both 6 and 72 hours withdrawal, F(1,13)=6.59, p<0.05, indicative of hyperalgesia in those rats (Figure 1) There was also a significant main effect of withdrawal timepoint, F(1,13)=6.21, p<0.05, such that all rats exhibited shorter paw withdrawal latencies at 6-h v.s 72-h withdrawal. Because nicotine-dependent rats exhibited shorter paw withdrawal latencies at 6-h withdrawal (M=6.59 ± 0.41) than at 72-h withdrawal (M=6.90 ± 0.54), we decided to test rats at 6-h withdrawal in Experiment 3. There was a non-significant trend toward a dose × vapor exposure interaction effect (p=.115; Figure 1), suggesting the possibility of altered CRF1R number/function in nicotine-dependent rats.
Figure 1. Nicotine-dependent rats exhibit heightened thermal sensitivity during nicotine withdrawal.
Mean (+/− SEM) hind paw withdrawal latencies in response to a thermal stimulus by nicotine-dependent rats (n=7; black bars) and nicotine-naïve rats (n=8; white bars) following systemic administration of R121919 (10 mg/kg) or an equivalent volume of vehicle. *p<0.05 nicotine-dependent rats vs. controls; #p<0.05 6 hrs vs. 72 hrs withdrawal time points.
Nicotine Dependence Increases CRF and CRF1R mRNA in the CeA
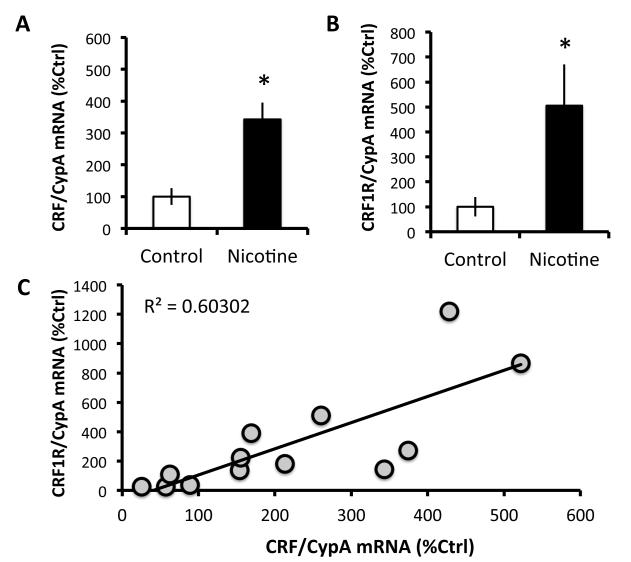
As shown in Figure 2A & 2B, paired-samples t-tests revealed that nicotine-dependent rats have higher levels of CRF (242.8 ± 52.5% increase) mRNA, t(6)=−4.183, p<0.01, and CRF1R (405.4 ± 164.7% increase) mRNA, t(5)=−2.545, p=0.05, in the CeA than controls at 16-h withdrawal from nicotine vapor. Figure 2C shows that CRF mRNA expression was significantly and positively correlated with CRF1R mRNA expression in CeA, r(11)=0.78, p<0.01. These effects were neuroanatomically specific, as we did not observe any change in CRF (7.2 ± 20.7% increase from controls) or CRF1R (4.6 ± 7.7% increase from controls) mRNA in the NAc shell.
Figure 2. Nicotine dependence increases CRF and CRF1R mRNA in CeA.
(A) Mean (+/− SEM) CRF mRNA expression in CeA as percent of control for nicotine-dependent (n=7; black bars) and nicotine-naïve (n=7; white bars) rats. (B) Mean (+/− SEM) CRF1R mRNA expression in CeA as percent of control nicotine-dependent (n=6; black bars) and nicotine-naïve (n=6; white bars) rats. (C) Scatter plot for individual rats (n=13 rats with values for both CRF and CRF1R) shows that CRF mRNA expression in CeA is strongly and positively correlated with CRF1R mRNA expression in CeA. *p≤0.05 nicotine-dependent rats vs. controls.
Nicotine Dependence-Induced Hyperalgesia is Attenuated by CRF1R Antagonist in CeA
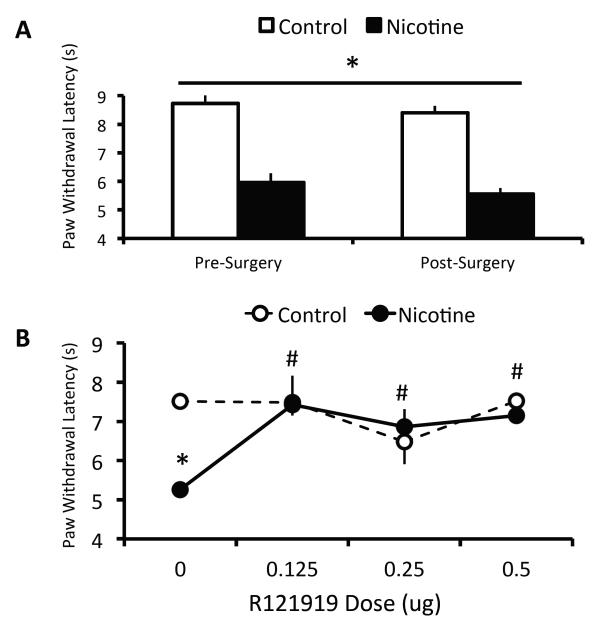
To determine the effect of intracranial cannulation on thermal nociception, pre- and post-surgery baseline Hargreaves values were compared with a two-way RM (vapor condition × time) ANOVA that yielded no effect of surgery on baseline thermal withdrawal latencies (Figure 3A). As shown in Figure 3B, a two-way RM (vapor condition × R121919 dose) ANOVA indicated a significant vapor x dose interaction, F(3,30)=6.74, p=0.001, as well as a main effect of dose on thermal withdrawal latencies, F(3,30)=5.33, p=0.01. Post-hoc analyses revealed that nicotine-dependent rats exhibited significantly lower thermal withdrawal latencies relative to nicotine-naïve controls after intra-CeA vehicle infusions (p<0.001). All three doses of the CRF1R antagonist R121919 rescued this effect, since nicotine-dependent rats exhibited significantly longer latencies at all three R121919 doses (0.125, 0.250, 0.500 μg) relative to vehicle (p≤0.001 in all three cases), and also since the paw withdrawal latencies of nicotine-dependent rats was not different from controls at any of the three R121919 doses (p>0.05 in all cases). Intra-CeA infusion did not affect thermal withdrawal latencies in nicotine-naïve rats.
Figure 3. Antagonism of CRF1Rs in CeA Abolishes Nicotine Dependence-Induced Hyperlagesia.
(A) Mean (+/− SEM) baseline thermal withdrawal latencies before and after bilateral cannulation of CeA in nicotine-dependent (n=6; black bars) and nicotine-naïve (n=6; white bars) rats. (B) Mean (+/− SEM) thermal withdrawal latencies in nicotine-dependent (n=6; black circles) and nicotine-naïve (n=6; white circles) rats. *p<0.01 nicotine-dependent rats vs. controls; #p<0.01 R121919 dose vs. vehicle condition for nicotine-dependent rats.
DISCUSSION
The present study examined the effects of nicotine dependence on thermal nociception during withdrawal from nicotine vapor and the role of brain CRF/CRF1Rs in this effect. Nicotine-withdrawn rats exhibited a significant reduction in hindpaw withdrawal latency, indicative of thermal hyperalgesia, which may have important implications for humans that abuse not only traditional nicotine products (cigarettes, etc.) but also e-cigarettes, since those products are marketed as “safe” because they deliver “only” nicotine to the user in the absence of other harmful compounds. In our study, nicotine-withdrawn rats also exhibited robust increases in CRF and CRF1R mRNA in CeA. Because CRF/CRF1R signaling has been implicated in both nicotine dependence and nociceptive processing, we examined whether systemic and brain site-specific antagonism of CRF1Rs (with R121919) would attenuate withdrawal-induced hyperalgesia in nicotine-dependent rats. Both systemic and intra-CeA antagonism of CRF1Rs markedly attenuated thermal hyperalgesia during nicotine withdrawal. These data suggest that augmented CRF/CRF1R signaling in the CeA mediates withdrawal-induced thermal hyperalgesia, in much the same way that CRF1Rs in CeA mediate nicotine withdrawal-induced increases in mechanical allodynia, anxiety-like behavior, and nicotine self-administration (Cohen et al., in press; George et al., 2007).
Here, we identify a role for CRF1Rs in nicotine withdrawal-induced thermal hyperalgesia. CRF1Rs play a central role in the negative affective state produced by withdrawal from chronic high-dose nicotine (Bruijinzeel et al., 2009), and this withdrawal state is thought to drive subsequent excessive nicotine use (Markou et al., 2008). The hypothesis that CRF1Rs are important in mediating escalated nicotine use driven by the aversive aspects of withdrawal is supported by findings that CRF1R antagonism blocks the nicotine deprivation effect (i.e., transient increase in nicotine self-administration following a period without nicotine access) in rats with 23-h/day nicotine access, but does not affect low intake levels by rats with 1-h/day nicotine access (George et al., 2007). Furthermore, systemic administration or ventricular infusion of a CRF1R antagonist blocks reinstatement of previously extinguished nicotine responding elicited by exposure to a stressor (Bruijnzeel et al., 2009; Zislis et al., 2007). Antagonism of CRF1Rs or reduction of CRF1R expression attenuates hyperalgesia associated with inflammatory and neuropathic pain in mice (Hummel et al., 2010). The lack of a dose-response effect of intra-CeA R121919 in Experiment 3 of the current report is likely due to the choice of a dose range that was too high. Nevertheless, our finding that CRF1Rs in CeA mediate nicotine withdrawal-induced hyperalgesia suggests that excessive nicotine use by dependent individuals may be driven in some cases by increased nociceptive processing. These findings also identify a neuroanatomical substrate for CRF1R effects on pain, whether it is related to drug dependence or not (e.g., inflammatory pain).
A recent study that implanted adult male rats with osmotic nicotine mini-pumps confirms our observed increases in CRF mRNA in CeA, but that study suggests these increases may actually be driven by chronic nicotine exposure rather than acute withdrawal from nicotine, since nicotine-intoxicated rats exhibit more robust increases in CRF mRNA in amygdala than nicotine-withdrawn rats (Torres et al., 2013). The finding that synthesis of CRF and CRF1Rs are both up-regulated in nicotine-dependent rats may seem counterintuitive, since one might expect CRF1Rs to be down-regulated in response to elevated CRF levels. However, this finding is not without precedent, since it has been shown previously that nicotine-withdrawn rats exhibit increased CRF-like immunoreactivity in CeA, as well as increased sensitivity to the behavioral effects of a CRF1R antagonist (indicative of up-regulated number and/or function of CRF1Rs) during nicotine withdrawal (George et al., 2007). Furthermore, rats that are made dependent on alcohol via chronic intermittent vapor inhalation exhibit increased CRF mRNA in CeA during withdrawal, as well as increased sensitivity to the effects of a CRF1R antagonist on inhibitory transmission in CeA (again suggesting up-regulated number and/or function of CRF1Rs; Roberto et al., 2010). One potential molecular pathway for mediating nicotine effects on nociception is the MAPK/ERK signaling pathway, particularly since chronic nicotine alters ERK phosphorylation in the amygdala (Brunzell et al., 2003), MAPK/ERK signaling is attributed a role in the regulation of CRF1R expression (Meng et al., 2011), and inhibition of the MAPK/ERK signaling pathway attenuates both inflammatory and neuropathic pain in various animal models (Ji et al., 2009).
The current study does not allow us to identify whether observed thermal hyperalgesia is a consequence of chronic nicotine exposure or withdrawal associated with termination of that exposure, however, prior work has shown that nicotine intoxication produces analgesia in humans and animals (Fertig et al., 1986; Pomerleau et al., 1984; Tripathi et al., 1982; Anderson et al., 2004). Smokers allowed to self-administer nicotine exhibit higher pain thresholds and higher pain tolerance, and report reduced pain perception, relative to nicotine-deprived smokers (Fertig et al., 1986; Pomerleau et al., 1984). Finally, prior work in rats showed that nicotine intoxication via mini-pumps produces analgesia, that chronic nicotine exposure produces tolerance to this effect, and that termination of nicotine exposure (i.e., withdrawal) produces thermal hyperalgesia (Yang et al., 1992). Therefore, we attribute our thermal hyperalgesia effects to nicotine withdrawal, but future studies should include tests of thermal nociception during nicotine intoxication produced by chronic intermittent nicotine vapor.
The PAG is important for descending behavioral and physiological responses to pain (Reynolds, 1969), and this region receives dense and highly organized GABAergic projections from CeA (Oka et al., 2008; Rizvi et al., 1991) that co-localize CRF and substance P (Gray & Magnuson, 1992), which both play pivotal roles in nicotine withdrawal and pain perception. These projections from CeA to PAG are important for gating the anti-nociceptive pain response mediated by opioids in the PAG (Oliveira et al., 2001; Xu et al., 2003). Data from fear conditioning studies show that the PAG and another amygdaloid nucleus (lateral amygdala) are each activated by a painful stimulus, and the response by each of these regions is dampened by signals predictive of that stimulus (Johansen et al., 2010). Future studies will examine whether the effects of CRF1R manipulation on thermal nociception observed here are mediated by projections from CeA to PAG.
Regardless of drug or alcohol exposure history, CRF/CRF1R brain signaling plays an important role in both the physical and affective components of pain. For example, implantation of corticosterone pellets into the CeA of rats increases CRF mRNA expression in the CeA and anxiety-like behavior, and also produces mechanical allodynia (Myers & Greenwood-Van Meerveld, 2010; Shepard et al., 2000; Tran & Greenwood-Van Meerveld, 2012). Furthermore, infusion of exogenous CRF into the CeA produces a pain-like state that is mediated by CRF1Rs, even when hypothalamic-pituitary-adrenal axis activity is pharmacologically suppressed (Ji et al., 2013). Systemic inflammatory pain is also mediated by brain CRF1Rs since antagonism of CRF1Rs in the CeA reduces anxiety-like behavior produced by arthritic pain in rats (Ji et al., 2007). Finally, formalin-evoked pain enhances CRF release in another constituent region of the extended amygdala, the bed nucleus of the stria terminalis, and antagonism of CRF1Rs in this region attenuates conditioned aversion to a context paired with pain (Ide et al., 2013). These and other empirical studies have led to a recent review article that proposes that CRF promotes hyperalgesia via actions at CRF1Rs in amygdala and analgesia via actions at CRF2Rs in amygdala, although that hypothesis remains speculative at this point (Rouwette et al., 2011). Collectively, these results suggest a role for CRF/CRF1R signaling in the extended amygdala in central processing of pain whether or not it is originally related to drug exposure. That said, CRF1Rs in CeA are attributed an important role in mediating the aversive state that drives excessive use of drugs including nicotine, and the above findings strengthen the notion that pain is a part of the symptomatology that can drive drug abuse. While CRF appears to be integral for the development of affective symptoms (i.e. anxiety, hyperalgesia, etc.) associated with nicotine dependence, there is also evidence for other neuromodulatory systems (e.g., neuropeptide Y and dynorphin; Aydin et al., 2011; Jackson et al., 2010) contributing to these behaviors.
Addiction theories propose that pain drives drug use in some humans, and in the case of opiates, this scenario is well accepted by the field (Turk et al., 2008). Recent studies have shown in rats that alcohol and heroin withdrawal (but not cocaine abstinence) produces an allodynic state that is mediated by CRF/CRF1R signaling (Edwards et al., 2012). Recently, it has been suggested that alcohol dependence may be conceptualized, at least for some individuals, as a chronic pain syndrome, and this may extend to other drugs of abuse including but not limited to nicotine (Egli et al., 2012). Collectively, our results show that nicotine dependence produces a hyperactive CRF/CRF1R system in the CeA that mediates withdrawal-induced increases in sensitivity to a painful stimulus. Our findings identify a potential therapeutic role for CRF1R antagonists in smoking cessation treatment strategies and future work will explore the role of CRF1R-mediated hyperalgesia in driving excessive nicotine self-administration.
ACKNOWLEDGEMENTS
This work was supported by LSUHSC Start-Up funds (NWG) and NIH grants AA018400 (NWG), MH093650 (VS), AA016731 (VS) and the Peter McManus Trust award (VS).
REFERENCES
- World Health Organization . WHO | Data and statistics; 2012. [Google Scholar]
- Adhikari B. Smoking-attributable mortality, years of potential life lost, and productivity. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- Arborelius L, Owens M, Plotsky P, Nemeroff C. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of Endocrinology. 1999;160:1–10. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Effects of a selective Y2R antagonist, JNJ-31020028, on nicotine abstinence-related social anxiety-like behavior, neuropeptide Y and corticotropin releasing factor mRNA levels in the novelty-seeking phenotype. Behav Brain Res. 2011;222:332–41. doi: 10.1016/j.bbr.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual Reviews of Pharmacology and Toxicology. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among. Addiction. 2005;100(6):777–786. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel A. Tobacco addiction and the dysregulation of brain stress systems. Neuroscience and Biobehavioral Reviews. 2012;36(5):1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biological Psychiatry. 2009;66(2):110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel A, Ford J, Rogers JA, Scheick S, Ji Y, Bishnoi M, Alexander JC. Blockade of CRF1 receptors in the central nucleus of the amygdala attenuates the dysphoria associated with nicotine withdrawal in rats. Pharmacology, Biochemistry, and Behavior. 2012;101(1):62–68. doi: 10.1016/j.pbb.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–41. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leão RM, Schulteis G, Koob GF, George O. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addiction Biology. 2013 doi: 10.1111/adb.12077. doi: 10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, et al. Development of mechanical hypersensitivity in rats during heroin and ethanol. Neuropharmacology. 2012;62(2):1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neuroscience and Biobehavioral Reviews. 2012;36(10):2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertig JB, Pomerleau OF, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addiction Behavior. 1986;11(3):239–248. doi: 10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotrophin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol dependent rats. Journal of Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotrophin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological Psychiatry. 2007;61(1):78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proceedings of the National Academy of Sciences. 2007;104(43):17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O. Nicotine vapor inhalation escalates nicotine self-administration. Addiction Biology. 2013 doi: 10.1111/adb.12021. doi:10.1111/adb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, Imad Damaj M. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology. 2005;178(2):183–192. doi: 10.1007/s00213-004-2007-3. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13(3):451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Johnson AC, Schulkin J, Myers DA. Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Research. 2006;1071(1):91–96. doi: 10.1016/j.brainres.2005.11.071. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotrophin-releasing factor and neuropeptide Y: Role in emotional integration. Trends in Neuroscience. 1994;17(2):80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Zaatari GS. Electronic nicotine delivery systems: Emerging science foundation for policy. Tobacco Control. 2010;19(2):89–90. doi: 10.1136/tc.2009.035279. [DOI] [PubMed] [Google Scholar]
- Hogle J, Kaye J, Curtin J. Nicotine Withdrawal Increases Threat-Induced Anxiety but Not Fear: Neuroadaptation in Human Addiction. 2010;68(8):719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. Journal of Neuroscience. 2011;31(35):12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and Symptoms of Tobacco Withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hummel M, Cummons T, Lu P, Mark L, Harrison JE, Kennedy JD, Whiteside GT. Pain is a salient “stressor” that is mediated by corticotropin-releasing factor-1 receptors. Neuropharmacology. 2010;59:160–166. doi: 10.1016/j.neuropharm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Ide S, Hara T, Ohno A, Tomano R, Koseki K, Naka T, et al. Opposing roles of corticotropin-releasing factor and neuropeptide Y within the dorsal bed nucleus of the stria terminalis in the negative affective component of pain in rats. Journal of Neuroscience. 2013;33(14):5881–5894. doi: 10.1523/JNEUROSCI.4278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine E, Cheeta S, File S. Tolerance to nicotine’s effects in the elevated plus-maze and increased anxiety during withdrawal. 2001;68(2):319–325. doi: 10.1016/s0091-3057(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, Caroll FI, Negus SS, Damaj MI. Effect of selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210(2):285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R-R, Gereau RW, IV, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Research Reviews. 2009;60:135–48. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Molecular Pain. 2007;3(13) doi: 10.1186/1744-8069-3-13. doi:10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Fu Y, Adwanikar H, Neugebauer V. Non-pain-related CRF1 activation in the amygdala facilitates the synaptic transmission and pain responses. Molecular Pain. 2013;9(2) doi: 10.1186/1744-8069-9-2. doi: 10.1186/1744-8069-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. Journal of Neurophysiology. 2008;102:2253–2264. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learining in the amygdala and periaqueductal gray. Nature Neuroscience. 2010;13(8):979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Risbrough VB, Geyer MA, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2008;33(9):2131–2138. doi: 10.1038/sj.npp.1301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Markou A, Weiss F, Schulteis G. Opponenet process and drug dependence: Neurobiological mechanisms. Seminars in Neuroscience. 1993;5:351–358. [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84(1):1–12. doi: 10.1016/S0304-3959(99)00193-1. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotrophin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34(7):1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Neurobiology of nicotine addiction. Phil. Trans. R. Soc. B. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H. Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience. 2002;112(3):605–617. doi: 10.1016/s0306-4522(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B. Elevated corticosterone in the amygdala leads to persistent increases in anxiety-like behavior and pain sensitivity. Behavioural Brain Research. 2010;214(2):465–469. doi: 10.1016/j.bbr.2010.05.049. [DOI] [PubMed] [Google Scholar]
- Oka T, Tsumori T, Yokota S, Yasui Y. Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neuroscience Research. 2008;62(4):286–298. doi: 10.1016/j.neures.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Oliveira MA, Prado WA. Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Research Bulletin. 2001;54(1):55–63. doi: 10.1016/s0361-9230(00)00420-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed Academic Press; San Diego, CA: 1998. [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fleming MF, Bittrich AA, Brown JL, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence. J Consult Clin Psychol. 2010;78(1):13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addiction Behavior. 1984;9(3):265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zhoa Y, Fekete EM, Funk CK, Wirsching P, Janda KD, et al. MPZP: A novel small molecule corticotrophin-releasing factor type 1 receptor (CRF1)antagonist. Pharmacology, Biochemistry, and Behavior. 2007;88(4):497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, 3rd, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain. 2009;10(9):944–952. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough V. Role of corticotropin releasing factor in anxiety disorders: A translational research perspective. 2006;50(4):550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. Journal of Comparative Neurology. 1991;303(1):121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotrophin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biological Psychiatry. 2010;67(9):831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouwette T, Vanelderen P, Roubos EW, Kozicz T, Vissers K. The amygdala, a relay station for switching on and off pain. European Journal of Pain. 2012;16(6):782–792. doi: 10.1002/j.1532-2149.2011.00071.x. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Research. 2000;861(2):288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by. Psychopharmacology (Berl) 1986;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Tran L, Greenwood-Van Meerveld B. Altered expression of glucocorticoid receptor and corticotropin-releasing factor in the central amygdala in response to elevated corticosterone. Behavioural Brain Research. 2012;234(2):380–385. doi: 10.1016/j.bbr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Trtchounian A, Talbot P. Electronic nicotine delivery systems: Is there a need for regulation? Tobacco Control. 2011;20:47–52. doi: 10.1136/tc.2010.037259. [DOI] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clinical Journal of Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotrophin-releasing factor. Alcoholism: Clinical and Experimental Research. 2002;26(10):1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type-2 corticotrophin-releasing factor agonist. Brain Research. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- Xu W, Lundeberg T, Wang YT, Yu LC. Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of the amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience. 2003;118(4):1015–1022. doi: 10.1016/s0306-4522(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Yang CY, Wu WH, Zbuzek VK. Antinociceptive effect of chronic nicotine and nociceptive effect of its. Psychopharmacology. 1992;106(3):417–420. doi: 10.1007/BF02245428. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF antagonist D-Phe CRF (12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53(8):958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]