Abstract
Recent studies provide a functional link between kallikrein 6 (Klk6) and the development and progression of disease in multiple sclerosis patients and in its murine models. To evaluate the involvement of additional kallikrein family members, we compared Klk6 expression to 4 other kallikreins (Klk1, Klk7, Klk8 and Klk10) in the brain and spinal cord of mice infected with Theiler’s murine encephalomyelitis virus (TMEV), an experimental model of progressive MS. The robust upregulation of Klk6 and Klk8 in the brain during the acute phase of viral encephalitis and in the spinal cord during disease development and progression points to their participation in inflammation, demyelination and progressive axon degeneration. More limited changes in Klk1, Klk7, and Klk10 were also observed. In addition, Klk1, Klk6, and Klk10 were dynamically regulated in T-cells in vitro as a recall response to viral antigen and in activated monocytes pointing to their activities in the development of adaptive and innate immune function. Together, these results point to overlapping and unique roles for multiple kallikreins in the development and progression of virus-mediated central nervous system inflammatory demyelinating disease, including activities in the development of the adaptive and innate immune response, in demyelination and in progressive axon degeneration.
Keywords: Axon Injury, Demyelination, Inflammation, Multiple Sclerosis, Serine Protease, Theiler’s murine encephalomyelitis virus
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) and represents the most common cause of non-traumatic neurologic disability in young adults in North America and Europe. Although considerable progress has been made in understanding the genetic susceptibility and pathogenesis of MS, there is still no uniformly effective treatment strategy, particularly for progressive forms of the disease. Multiple lines of evidence suggest that the secreted serine protease KLK6 is associated with the pathogenesis of CNS inflammatory demyelinating conditions. For example, KLK6 (capitalized to denote the human form) is localized to active MS lesions (Scarisbrick et al. 2002), and is elevated in the serum (Scarisbrick et al. 2008) and cerebrospinal fluid (Hebb et al. 2011) of progressive MS patients. KLK6 is also detected at increased levels in the cerebrospinal fluid of patients with post-polio syndrome (Gonzalez et al. 2009). Moreover, Klk6 (small case to denote the non-human ortholog) is elevated at sites of inflammatory demyelination in all experimental animal models of MS examined to date, including murine (Blaber et al. 2004) and marmoset (Scarisbrick et al. 2002) experimental autoimmune encephalomyelitis, as well as in Theiler’s murine encephalomyelitis virus (TMEV)-induced chronic progressive inflammatory demyelinating disease (Scarisbrick et al. 2002; Christophi et al. 2004; Scarisbrick et al. 2012).
The first potential link between KLK6 and demyelinating disease were studies showing its dense expression by myelin producing oligodendrocytes (Scarisbrick et al. 1997; Scarisbrick et al. 2000; Scarisbrick et al. 2001) coupled with its ability to rapidly hydrolyze major myelin and blood brain barrier proteins (Bernett et al. 2002; Blaber et al. 2002; Scarisbrick et al. 2002; Blaber et al. 2004; Angelo et al. 2006), and to promote oligodendrogliopathy (Scarisbrick et al. 2002; Burda et al. 2013), neuronal injury (Scarisbrick et al. 2008; Radulovic et al. 2013; Yoon et al. 2013), and astrogliosis (Vandell et al. 2008; Scarisbrick et al. 2012). Furthermore, KLK6 is elevated in activated T-cells, and those treated with steroid hormones such as glucocorticoids, androgens or progesterone (Scarisbrick et al. 2002; Blaber et al. 2004; Christophi et al. 2004; Scarisbrick et al. 2006; Scarisbrick et al. 2012). More recently, the cellular effects of KLK6 across the neural-immune axis were linked to activation of protease activated receptors 1 (PAR1) and 2 (PAR2), including its actions in axonopathy (Radulovic et al. 2013; Yoon et al. 2013), oligodendrogliopathy (Burda et al. 2013), astrogliosis (Vandell et al. 2008; Scarisbrick et al. 2012) and lymphocyte survival (Scarisbrick et al. 2011). Of critical importance, KLK6-neutralizing antibodies attenuate neuroinflammatory and neurodegenerative changes in the spinal cord of mice with proteolipid protein- or myelin oligodendrocyte glycoprotein-mediated experimental autoimmune encephalomyelitis (Blaber et al. 2004), in addition to those with TMEV-induced inflammatory demyelinating disease (Scarisbrick et al. 2012).
Human kallikreins are now a well-recognized family of 15 secreted serine proteases tightly aligned on chromosome 19q13.3-13.4. Thirteen of the 15 human kallikreins are found in mouse, all except KLK2 and KLK3. Also, it is known that duplications of the mouse Klk1 gene have generated 13 additional new functional genes and 10 new non-functional pseudogenes. There is also a Klk2 pseudogene at the mouse locus (Lundwall 2013). The entire murine Klk gene family is localized on chromosome 7. Studies of functional kallikreins across human biospecimens and rodent models demonstrate important roles for select kallikreins in cancer (Sotiropoulou et al. 2009; Lawrence et al. 2012; Sotiropoulou et al. 2012), inflammation (Bhoola et al. 2001; Scarisbrick et al. 2002; Blaber et al. 2004; Scarisbrick et al. 2006; Briot et al. 2009; Scarisbrick et al. 2011; Scarisbrick 2012; Hovnanian 2013; Oikonomopoulou et al. 2013), and neurologic conditions (Komai et al. 2000; Shimizu-Okabe et al. 2001; Scarisbrick et al. 2002; Iwata et al. 2003; Diamandis et al. 2004; Scarisbrick et al. 2006; Attwood et al. 2011; Scarisbrick 2012; Drucker et al. 2013; Radulovic et al. 2013; Spencer et al. 2013).
To further define the potential scope of action of kallikreins in CNS inflammatory demyelinating diseases, we compared the expression patterns of Klk6 in the brain and spinal cord of mice with TMEV-induced demyelinating disease (Scarisbrick et al. 2012) to that of Klk1 and Klk8, which have also been implicated in CNS inflammation. For example, in relation to MS, KLK1 is elevated in the serum of patients with relapsing remitting or primary progressive MS (Scarisbrick et al. 2008). Also, Klk8 knockout mice show attenuated signs of inflammation and demyelination in myelin oligodendrocyte glycoprotein-35–55 induced experimental autoimmune encephalomyelitis (Terayama et al. 2005). We assessed Klk7 and Klk10 in parallel, since each of these is expressed across the CNS-immune axis (Scarisbrick et al. 2006). The transcriptional changes observed, together with experiments demonstrating participation of select kallikreins in lymphocyte recall responses to viral antigen, and in the pathophysiologic response of activated monocytes, offer new insights into the potential effector functions and therapeutic usefulness of multiple kallikreins in CNS inflammatory demyelinating disorders.
Results
Spatial and temporal expression of kallikreins across the brain-spinal cord axis in virus-induced demyelinating disease
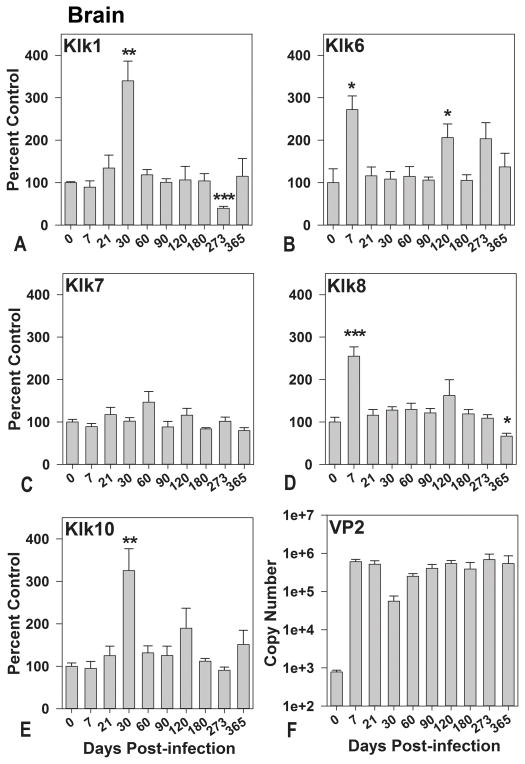
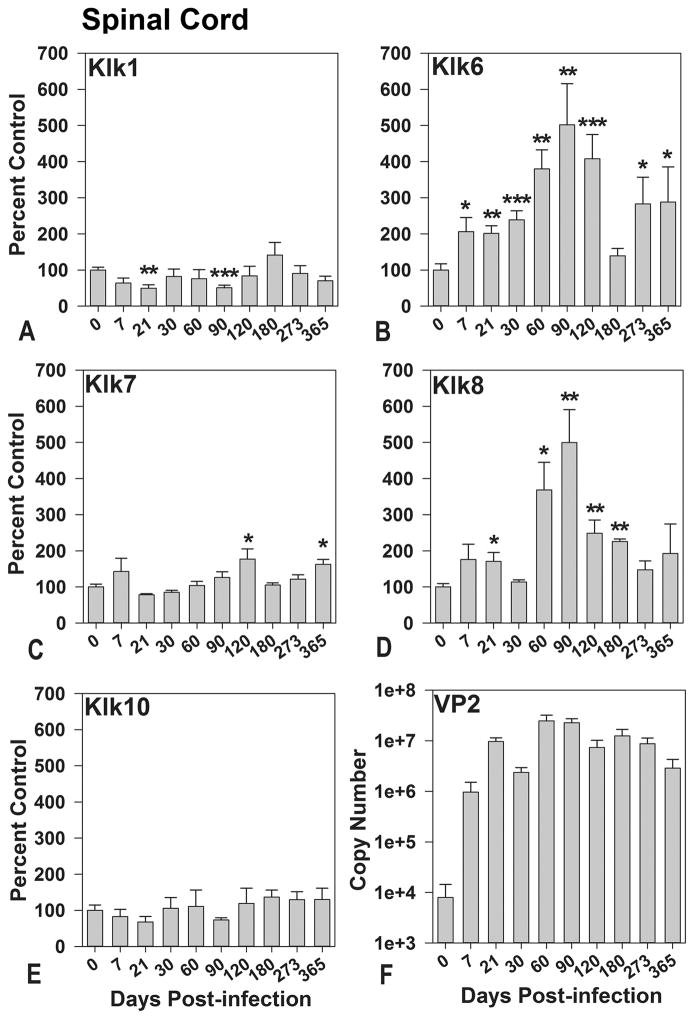
In a recent study, Klk6 was functionally linked to the development of TMEV-inflammatory demyelinating disease in the brain and spinal cord through the early stages of demyelination (Scarisbrick et al. 2012). The well characterized stages of viral encephalitis, demyelination and axon injury that distinguish the TMEV model are highlighted in Figure 1 (McGavern et al. 1999; McGavern et al. 2000). To begin addressing the potential coordinate contribution of additional kallikreins to the development and progression of inflammatory demyelinating disease, we compared transcriptional changes in Klk6 with that of 4 other kallikrein family members (Klk1, Klk7, Klk8, and Klk10) in the brain (Figure 2) and spinal cord (Figure 3) of mice from 0 through 365 days after TMEV-infection. The most dynamic and sustained elevations in kallikrein expression occurred in the spinal cord, reflecting the persistent virulent infection that TMEV establishes at this site soon after CNS infection (Figure 1) (Tsunoda et al. 2010). Among the kallikreins examined, Klk6 and Klk8 were associated with the most significant transcriptional changes across the greatest number of time points in the TMEV-infected spinal cord, with significant elevations at the acute, subacute and chronic phases (Figure 3). As we previously reported (Scarisbrick et al. 2012), changes in Klk6 RNA expression in the spinal cord coincide with inflammation and demyelination being elevated by 2-fold from 7 through 30 days post-infection (dpi) (P ≤ 0.04, Students t-test) and by 4- to 5-fold at early (60 dpi) through mid-chronic phases (90 and 120 dpi) (P ≤ 0.008, Students t-test). We now report for the first time that Klk6 RNA expression levels in the TMEV-infected spinal cord are also more than 2.5-fold higher than baseline, even at the most chronic stages of inflammatory demyelinating disease examined coinciding with the development of progressive axon dropout and the development of permanent functional deficits (273 and 365 dpi; P ≤ 0.04, Students t-test).
Figure 1. The Theiler’s murine encephalomyelitis virus model of demyelination.

The early stages of disease include inflammation and demyelination and the later stages present with progressive axon loss and the development of functional deficits (McGavern et al. 1999; McGavern et al. 2000).
Figure 2. Kallikrein gene transcription is differentially regulated in the brain during the acute, subacute and chronic phases of TMEV-induced demyelinating disease.
A–E, Histograms show real-time PCR for Klk1, Klk6 to Klk8, and Klk10 RNA isolated from the brain of SJL mice during acute- through- chronic phases of TMEV infection (n = 5 or 6 per time point). Kallikrein RNA copy number in each case was determined by real-time PCR, normalized to GAPDH copy number, and expressed as a percent of the uninfected control. F, Histogram shows quantification of viral VP2 RNA copy number in the brain at each time point after infection. PCR denotes polymerase chain reaction; TMEV, Theiler’s murine encephalomyelitis virus. The data pertaining to Klk6 expression in the TMEV infected brain extend the observations reported in (Scarisbrick et al. 2012). Asterisks indicate significant differences from baseline levels (*P < 0.05, ***P ≤ 0.001, Students t-test; **P ≤ 0.008, Mann Whitney U test).
Figure 3. Kallikrein gene transcription is differentially regulated in the spinal cord during the acute, subacute and chronic phases of TMEV-induced demyelinating disease.
A to E, Histograms show real-time PCR for Klk1, Klk6 to Klk8, and Klk10 RNA isolated from the spinal cord of SJL mice during acute- through- chronic phases of infection with TMEV (n = 5 or 6 per time point). Kallikrein RNA copy number in each case was determined by real-time PCR, normalized to GAPDH copy number, and expressed as a percent of the uninfected control. F, Histogram shows quantification of viral VP2 RNA copy number in the spinal cord at each time point after infection. PCR denotes polymerase chain reaction; TMEV, Theiler’s murine encephalomyelitis virus. The data regarding Klk6 expression in the TMEV infected spinal cord extend the data reported in (Scarisbrick et al. 2012). Asterisks indicate significant differences from baseline levels (*P < 0.05, **P ≤ 0.008, ***P ≤ 0.002, Students t-test).
Transcriptional elevations in Klk8 RNA within the TMEV-infected spinal cord were similar but not identical to those observed for Klk6. Elevated Klk8 RNA levels were first observed at the beginning stages of demyelination (21 dpi), when expression was 1.7-fold higher than baseline (P = 0.03, Students t-test). Klk8 RNA expression also was elevated at 60, 90, 120, and 180 dpi (P ≤ 0.02, Students t-test), with the highest expression seen at 90 dpi (5-fold higher than baseline), coinciding with progressive increases in demyelination, axon injury and the development of functional deficits. However, unlike Klk6, KLK8 expression was not elevated at the very late chronic stages (273 and 365 dpi) examined.
Smaller and more temporally restricted elevations were also observed in Klk7 RNA expression in the TMEV-infected spinal cord, with 1.6-fold elevations seen at the early and late chronic time points (120 and 365 dpi) (P ≤ 0.03, Students t-test). Uniquely, Klk1 RNA expression was significantly reduced in the spinal cord at 21 and 90 dpi. Klk10 RNA levels also showed reduced expression at subacute and early chronic time points in spinal cord, but these changes were not statistically significant from baseline.
Reflecting the potential importance of Klk6 and Klk8 during the peak stages of acute polioencephalomyelitis, levels of Klk6 (P = 0.03, Mann Whitney U test) and Klk8 (P ≤ 0.001, Students t-test) RNA were elevated by more than 2-fold in the brain at 7 days after TMEV infection (Figure 2). By contrast, the first significant elevations in Klk1 and Klk10 gene transcription in the brain were observed at the early demyelinating stage (30 dpi) (P = 0.008, Mann Whitney U test), when levels were approximately 2.8-fold higher. A second peak in Klk6 RNA transcription occurred at 120 dpi, when levels were again 2-fold higher than baseline (P = 0.04, Students t-test). At the most chronic stages of TMEV infection examined, transcription of Klk1 RNA was below baseline at 273 dpi (P ≤ 0.001, Students t-test) and Klk8 RNA levels were below baseline at 365 dpi (P = 0.02, Students t-test). Klk7 gene transcription in the brain did not change significantly at any stage of infection examined.
To determine the relative abundance of the kallikreins examined across the brain-spinal cord axis, we included amplification of serially diluted plasmid DNA of known copy number for each kallikrein in all polymerase chain reaction (PCR) experiments. As expected from our prior studies (Scarisbrick et al. 1997; Scarisbrick et al. 2001; Christophi et al. 2004; Scarisbrick et al. 2006; Radulovic et al. 2013) Klk6 RNA levels were the most abundant in the brain and spinal cord of all of the kallikreins examined, with approximately 2.5×105 ± 7.9×104 and 1.0×106 ± 1.3×105 copies detected in 0.5 μg of RNA isolated from the brain and spinal cord, respectively. Similarly, copies of Klk1 (7.1×104 ± 1.2×104), Klk7 (9.1×103 ± 1.9×102), and Klk8 (4.7×104 ± 2.7×103) were each approximately 10-fold more abundant in the spinal cord than the whole brain (Klk1, 5.1×103 ± 1.5×102; Klk7, 1.9×102 ± 1.2×101; Klk8, 5.1×103 ± 4.8×102). Klk10 RNA expression was detected at similar levels in the brain (3.1×104 ± 2.3×103) and spinal cord (1.5×104 ± 3.3×103).
Regulated expression of kallikreins in activated T-cells
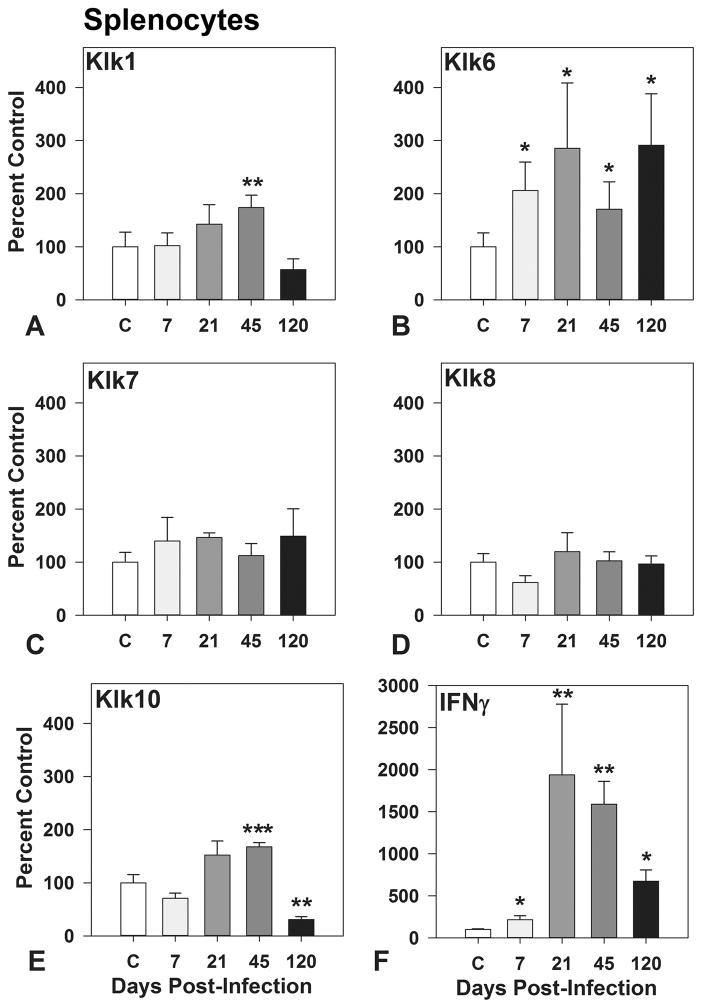
Klk6 was recently shown to be dynamically upregulated in whole-splenocyte cultures as a recall response to viral antigen (Scarisbrick et al. 2012). To elucidate the potential contribution of additional kallikreins to the TMEV-driven adaptive immune response, we compared changes in gene transcription of Klk6 to that of Klk1, Klk7, Klk8, and Klk10 in lymphocytes treated with viral capsid proteins in vitro (Figure 4). Of the kallikreins examined, the transcriptional-recall response to viral antigen was the most robust in terms of magnitude and temporal framework for Klk6, with 2 to 3-fold elevations observed in lymphocytes derived from the spleen of mice at 7, 21, or 120 dpi (P ≤ 0.05, Students t-test). Viral antigen-induced increases in kallikrein RNA transcription were also observed for Klk1 (P = 0.008, Mann Whitney U test) and Klk10 (P ≤ 0.001, Students t-test) in lymphocytes derived from TMEV-infected mice at 45 dpi, but Klk10 RNA levels were reduced relative to baseline by 120 dpi. No changes in Klk7 or Klk8 RNA levels were observed. The pro-inflammatory response was confirmed by examination of changes in interferon-γ gene transcription, which was upregulated by 2-to 19-fold in virus antigen pulsed splenocytes from 7 through120 dpi (P ≤ 0.3, Mann Whitney U test).
Figure 4. VP1 and VP2 viral capsid proteins drive kallikrein gene transcription in whole-splenocyte cultures derived from TMEV-infected mice.
Histograms show changes in Klk1, Klk6 to Klk8, and Klk10 RNA expression in whole-splenocyte cultures derived from uninfected control mice or mice during acute through chronic phases of TMEV infection treated with a combination of VP1 and VP2 viral capsid proteins (each at 5 μg/ml) for 72 hr (n = 3 to 5 per time point). All experiments were repeated at least twice, with parallel results. TMEV denotes Theiler murine encephalomyelitis virus. The findings related to Klk6 expression in viral capsid protein stimulated splenocytes (Scarisbrick et al. 2012) are shown to facilitate comparison with Klk1, 7, 8 and 10. Asterisks indicate significant differences from baseline (*P < 0.05, ***P ≤ 0.001, Students t-test; **P ≤ 0.008, Mann Whitney U test).
To begin addressing the potential significance of the kallikreins examined to the immunobiology of whole-splenocyte cultures, we estimated the relative abundance of each kallikrein in 0.125 μg of RNA derived from splenocytes before treatment with viral capsid proteins. In cultures prepared from the spleens of TMEV infected mice on 7 dpi, Klk1 (7.6×105 ± 3.4×105) and Klk10 (1.8×105 ± 6.6×104) RNA transcripts were most abundant, followed by Klk6 (4.9×104 ± 6.6×103), Klk8 (2.0×104 ± 5.5×102) and Klk7 (1.9×104 ± 2.1×103). These levels were similar to those observed in splenocytes derived from uninfected mice across several mouse strains examined to date, including C57BL6/J and B6129 (Christophi et al. 2004) and unpublished observations.
Regulated expression of kallikreins in activated monocytes
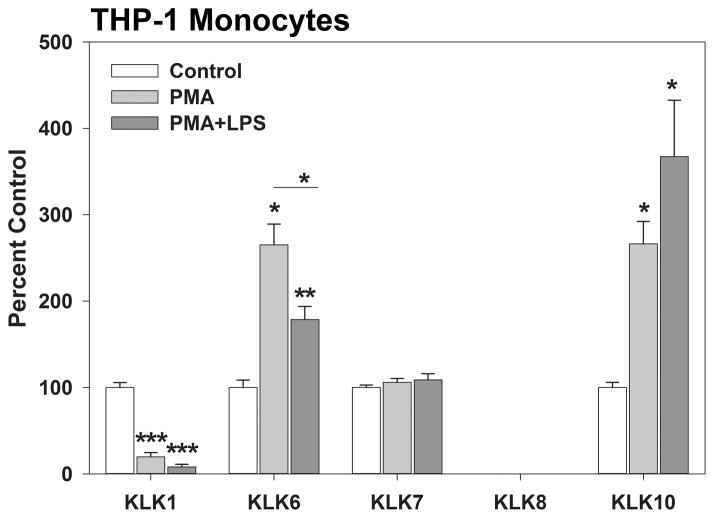
Treatment of the THP-1 human monocyte cell line with phorbol 12-myristate-13-acetate (PMA), or PMA plus lipopolysaccharide (LPS) increases KLK6 RNA expression (P ≤ 0.02, Mann Whitney U test) (Scarisbrick et al. 2012). Here, we additionally show that these known activators of monocyte immune function also significantly increase transcription of KLK10 RNA (P = 0.02, Mann Whitney U test). By contrast, PMA or PMA plus LPS, significantly reduced KLK1 RNA expression. KLK7 RNA levels were not altered by these monocyte activators (Figure 5).
Figure 5. Kallikrein gene transcription is dynamically regulated in activated human THP-1 monocytes.
Activation of THP-1 macrophages using PMA or PMA plus LPS resulted in significantly elevated levels of transcription of KLK6 and KLK10 RNA, but significantly reduced KLK1. (n = 3 to 5 per condition). All experiments were repeated at least twice, with parallel results. LPS denotes lipopolysaccharide; PMA, phorbol 12-myristate-13-acetate; TMEV, Theiler murine encephalomyelitis virus. Data related to KLK6 expression in activated monocytes (Scarisbrick et al. 2012) are shown to permit direct comparison with that of KLK1, 7, 8 and 10. Asterisks indicate significant differences from baseline (*P < 0.05, ***P ≤ 0.001, Students t-test; **P ≤ 0.008, Mann Whitney U test).
In terms of abundance, similar levels of KLK1 (7.0×106 ± 4.0×106), KLK6 (1.0×106 ± 1.6×105), and KLK10 (2.2×106 ± 5.8×105) transcripts were detected in 0.5 μg of RNA isolated from nonactivated THP-1 monocytes. Klk7 was detected at approximately 10-fold lower levels (1.2×105 ± 2.4×104). Under these conditions, KLK8 RNA levels were below the detection limit of our assay.
Discussion
TMEV induces an acute encephalomyelitis followed by a chronic progressive inflammatory demyelinating disease in the spinal cord of susceptible mice resulting in demyelination, axon injury and paraplegia; it is an important model of progressive MS (Tsunoda et al. 2010). The current study links Klk1, Klk6, Klk7, Klk8, and Klk10 to the pathophysiology of viral encephalitis and virus-induced chronic progressive inflammatory demyelinating disease of the spinal cord. Of the kallikreins examined, Klk6 and Klk8 expression were most prominent in the brain during acute TMEV-induced encephalomyelitis and in the spinal cord during the acute and chronic phases of virus-induced inflammatory demyelination and axon dropout. Our results also suggest that Klk1, Klk6 and Klk10 have integral roles in T-cell recall responses to viral antigen and in activated monocytes.
The distinct and overlapping patterns of kallikrein expression in the brain and spinal cord from acute through chronic phases of TMEV infection and upon activation of T-cells or monocytes in vitro indicate only partially overlapping cellular sources and activities in virus-induced brain encephalomyelitis and spinal cord inflammatory demyelinating disease. For example, evidence suggests that the robust elevations in Klk6 gene expression observed acutely in the brain and over the course of TMEV inflammatory demyelinating disease in the spinal cord involve both CNS endogenous cells and infiltrating immune cells. The likely important contribution of CNS endogenous cells to virus-driven Klk6 expression is suggested by prior studies demonstrating Klk6 induction in reactive astrocytes in active MS lesions and at sites of demyelination in animal models (Scarisbrick et al. 2002; Blaber et al. 2004; Scarisbrick et al. 2012). In this context, Klk6 can promote key facets of astrogliosis, including astrocyte stellation and secretion of the proinflammatory cytokine interleukin 6 (Scarisbrick et al. 2012). Moreover, Klk6 is expressed at significant levels by myelin-producing oligodendroglia and neurons in the uninjured brain, and it is upregulated in each cell type by injury (Scarisbrick et al. 1997; Scarisbrick et al. 2000; Scarisbrick et al. 2001; Scarisbrick et al. 2006; Radulovic et al. 2013). Importantly, elevated Klk6 promotes oligodendrogliopathy (Scarisbrick et al. 2002), suppresses myelin gene expression (Burda et al. 2013), and rapidly hydrolyzes myelin proteins in vitro (Bernett et al. 2002; Blaber et al. 2004) and in vivo (Burda et al. 2013). Moreover, Klk6 is toxic toward cultured neurons in vitro (Scarisbrick et al. 2008; Radulovic et al. 2013; Yoon et al. 2013). Therefore, elevated levels of Klk6 are positioned to participate directly in multiple neurodegenerative cascades that promote progressive functional deficits in TMEV-infected mice, including failure of remyelination.
Considerable evidence suggests that infiltrating immune cells also contribute to increased Klk6 expression in response to TMEV. For example, in vitro studies document increased Klk6 transcription as a recall response to viral antigen in T-cells and in activated monocytes (Scarisbrick et al. 2012). Also, prior studies showed Klk6 localization to monocytes or microglia and CD3+ T-cells at sites of neuroinflammation (Scarisbrick et al. 2002; Blaber et al. 2004). Because Klk6 was recently discovered to also promote lymphocyte survival (Scarisbrick et al. 2011), this protease is uniquely positioned to promote and prolong virus-induced CNS inflammation and to participate in demyelination and axon dropout.
Like Klk6, Klk8 was also robustly elevated at acute and early chronic time points in the TMEV-infected spinal cord and is well positioned to participate in inflammatory demyelination. It is likely that elevations in Klk8 occurring in the TMEV infected CNS originate largely from endogenous cells since this protease is already known to be expressed by neurons in the uninjured hippocampus (Chen et al. 1995) and spinal cord (Radulovic et al. 2013) and to be upregulated in axons and in reactive astroglia after injury (Radulovic et al. 2013). Of interest, and unlike Klk6, recombinant Klk8 is not toxic towards cortical neurons in vitro (Radulovic et al. 2013) and is reported to participate in neurite outgrowth and fasciculation (Oka et al. 2002). Notably, Klk8 is best known for roles in activity-dependent synaptic plasticity within the hippocampus (Davies et al. 2001; Matsumoto-Miyai et al. 2003; Tamura et al. 2006; Ishikawa et al. 2008). Also in contrast to Klk6, Klk8 expression was not altered in T-cells as part of the viral recall response and KLK8 RNA was not detected in the THP-1 monocyte cell line in vitro. Monocytes contribute markedly to the inflammatory response in TMEV inflammatory demyelinating disease, but the fact that KLK8 was not expressed by monocytes in vitro or detected in macrophages/microglia in human spinal cord injury (Radulovic et al. 2013) further supports the idea that elevations in Klk8 in the TMEV-infected CNS largely arise from-CNS endogenous cells. Although the role of Klk8 in CNS inflammatory disease remains to be fully established, Klk8 knockout mice show attenuated signs of inflammation and demyelination in myelin oligodendrocyte glycoprotein-35–55 induced experimental autoimmune encephalomyelitis (Terayama et al. 2005). The results presented here strongly support the need for additional studies using Klk8-specific antibodies, and Klk8-specific activity assays, to further elucidate the sites of Klk8 action in TMEV- inflammatory demyelinating disease and potentially in MS lesions.
More limited elevations in Klk1 and Klk10 were observed subacutely in the TMEV-infected brain, and Klk7 showed small elevations in the spinal cord at chronic time points. Although these kallikreins are less well studied in the CNS relative to Klk6 and Klk8, some evidence suggests a role in neurodegeneration. For example, elevated or reduced levels of KLK7 and KLK10, in addition to KLK6 and KLK8, have been specifically linked to neurodegenerative conditions, including Alzheimers (Shimizu-Okabe et al. 2001), frontotemporal dementia (Diamandis et al. 2004) and Parkinsons (Iwata et al. 2003). A recent study established that KLK7 is highly degradative and neurotoxic in vitro (Radulovic et al. 2013). Importantly, prior studies show KLK1 is elevated in the serum of patients with relapsing remitting or primary progressive MS (Scarisbrick et al. 2008) and KLK1 promotes neurotoxicity in vitro (Scarisbrick et al. 2008; Radulovic et al. 2013). Altered expression of KLK1, KLK6, KLK7 and KLK8 were also recently demonstrated in cases of human traumatic spinal cord injury (Scarisbrick et al. 2006; Radulovic et al. 2013). More detailed analysis of the expression patterns of these and other kallikreins, as well as their specific enzymatic activity, in CNS inflammatory disease and in vitro will be needed to fully interpret the significance of the transcriptional changes seen in the TMEV model of inflammatory demyelinating disease.
Virus antigen-directed T-cell responses in vitro suggest that Klk1, Klk6, and Klk10 participate in the development of proinflammatory responses mediated by the adaptive immune system. VP1 and VP2 viral capsid proteins are known to exacerbate disease in susceptible SJL mice (Yauch et al. 1998) and when transgenically expressed in resistant strains (Denic et al. 2011). High levels of Klk6 were previously identified in infiltrating CD4 and CD8 T cells in the TMEV and experimental autoimmune encephalomyelitis models of MS (Scarisbrick et al. 2002; Blaber et al. 2004), suggesting a role for Klk6 in adaptive immune responses. In addition, proinflammatory stimuli such as interleukin-2, concanavalin A, or T-cell receptor cross-linking antibodies also increase Klk6 transcriptional activity (Blaber et al. 2004; Christophi et al. 2004). In the human Jurkat T-cell line, one or more of these inflammatory mediators also promoted an increase in the expression of KLK8 and KLK10, whereas levels of KLK7 were suppressed (Christophi et al. 2004). Further supporting the role of select kallikreins in the adaptive immune response, Klk1 promotes lymphocyte proliferation, whereas Klk6 promotes survival of T and B cells (Scarisbrick et al. 2011). Reduced proinflammatory responses, including interferon-γ production and T-cell proliferative responses, in the experimental autoimmune encephalomyelitis model of MS after treatment with Klk6-neutralizing antibodies further implicates Klk6 in key activities within the adaptive immune system (Blaber et al. 2004). Klk6-neutralizing antibodies also reduce TMEV-driven delayed type-2 hypersensitivity responses (Scarisbrick et al. 2012). Thus, at sites of viral infection, viral capsid-induced elevations in T-cell kallikreins may act in an autocrine or paracrine fashion to enhance and prolong the chronic inflammatory response. The current results suggest additional study regarding the roles of Klk1, Klk6, Klk10, and potentially other kallikrein family members (Scarisbrick 2008; Scarisbrick 2012), as well as potential regulatory elements (Scarisbrick et al. 2006) in the development of virus-directed T-cell responses is needed, with results likely to shed new light on novel immune regulatory mechanisms relevant to inflammatory demyelinating disease.
The host response to TMEV involves activation of the innate and adaptive arms of the immune system and here we show that like T-cells, monocytes respond to activation by increasing transcription of KLK6 and KLK10 RNA. In contrast, levels of KLK1 transcription were suppressed in activated monocytes. Possibly in relation to this, Klk1 RNA levels were also reduced at select time points in the TMEV-infected brain and spinal cord. These results suggest proinflammatory roles for KLK6 and KLK10, but a possible antiinflammatory role for KLK1 in the innate immune response, although additional functional studies are needed for clarification. Elevated levels of Klk6 have been previously observed in infiltrating monocytes and microglia at sites of CNS inflammation in the TMEV (Blaber et al. 2002; Scarisbrick et al. 2002) and experimental autoimmune encephalomyelitis (Blaber et al. 2004) models of MS, in human MS lesions (Scarisbrick et al. 2002), and in traumatic spinal cord injury (Scarisbrick et al. 2006). Importantly, in a recent study of TMEV-infected mice with high levels of circulating Klk6-neutralizing antibodies, the number of monocytes/microglia within the parenchyma of the spinal cord was significantly reduced (Scarisbrick et al. 2012). Taken together, these findings indicate key roles for KLK6 and potentially KLK1 and KLK10 in monocyte/microglial-driven processes that govern the innate immune response in TMEV- inflammatory demyelinating disease.
In summary, the data presented suggest that multiple kallikreins are positioned to participate in the development and progression of virus-induced encephalomyelitis and chronic progressive virus-mediated inflammatory demyelinating disease in the spinal cord. Importantly, we previously established that Klk6-neutralizing antibodies attenuate neurobehavioral deficits in proteolipid protein- and myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (Blaber et al. 2004) and the early stages of inflammatory demyelination in the spinal cord of TMEV-infected mice (Scarisbrick et al. 2012). The current findings also link Klk1, Klk7, Klk8, and Klk10 to discrete stages and to unique inflammatory and pathogenic events in TMEV inflammatory demyelinating disease, therefore highlighting potential new therapeutic opportunities. Specifically, we propose that additional efforts to target unique or multiple sets of kallikreins at different stages of CNS- inflammatory demyelinating disease progression are needed to further elucidate the likely complex cascade of kallikreins involved and to functionally evaluate their roles and potential utility as therapeutic targets.
Materials and Methods
All studies were accomplished according to the guidelines of the Institutional Animal Care and Use Committee at the Mayo Clinic. Unless otherwise indicated, all reagents were obtained from Sigma (St. Louis, MO).
TMEV Model
Eight-week-old female SJL/J (H-2S) mice (Jackson Laboratories, Bar Harbor, ME) were injected intracerebrally with 2×105 plaque-forming units of the Daniel’s strain of TMEV in 10 μl. Analyses of Klk1, Klk6, Klk7, Klk8, and Klk10 RNA expression levels in the brain and spinal cord were performed at acute (7 dpi), subacute (21 and 30 dpi), early chronic (60 and 90 dpi), mid-chronic (120 and180 dpi), and late chronic (273 and 365 dpi) phases of infection. The immunopathologic features characterizing each phase of disease are provided in Figure 1.
Quantification of CNS kallikreins in TMEV-induced demyelinating disease
Quantitative real-time reverse-transcription (RT)-PCR was used to evaluate the expression of Klk1, Klk6, Klk7, Klk8, and Klk10 in the brain and spinal cord from at least 5 to 6 mice in acute through late chronic phases of TMEV infection. Samples were snap-frozen in liquid nitrogen before RNA isolation using RNA STAT-60 (Tel-Test, Inc. Friendswood, TX). We used 0.5 μg of total RNA for RT-PCR, using the Light Cycler-RNA Amplification Kit SYBR Green I in a Roche Light Cycler apparatus following the manufacturer’s instructions (Roche Diagnostics, Mannheim Germany). Primers specific for Mus musculus Klk1, Klk6, Klk7, Klk8, and Klk10 are detailed in Table 1. To gauge viral replication over the disease course, RNA coding for the Daniel strain VP2 capsid protein was amplified in the same RNA samples (Rodriguez et al. 2008; Scarisbrick et al. 2012). Levels of the housekeeping gene glyceraldehyde phosphate 3-dehydrogenase (GAPDH) were amplified to control for loading. Relative expression levels for each gene were quantified based on a standard curve created by parallel amplification of serially diluted nucleic acid templates containing known copy numbers.
Table 1.
PCR Primers
Summary of amplified sequences. Note lower-case kallikrein symbols indicate murine form, whereas upper-case indicates human form.
| Accession Number | Base Pairs | Forward Primer | Reverse Primer |
|---|---|---|---|
|
Klk1 NM_010639 |
363-471 | CAGGAGATATGGATGGAGGCAAAGA | CCCGGCACATTGGGTTTAC |
|
Klk6 NM_011177.1 |
749-949 | CCTACCCTGGCAAGATCA | GGATCCATCTGATATGAGTGC |
|
Klk7 NM_011872.2 |
143-245 | ACCGCTGGACAAGGAGAAA | CTCCACAGTGAAGCTGATTGC |
|
Klk8 NM_008940.2 |
1004-1236 | GTGCGGAAGTGAAAATCTAT | GGTAGTGTAGCGGCAG |
|
Klk10 NM_133712.1 |
418-496 | CAAGCAGACGAGGACTAC | CGCACTCATCATTAGGCA |
|
KLK1 NM_002257.3 |
370-592 | CAAGCAGACGAGGACTAC | CGCACTCATCATTAGGCA |
|
KLK6 NM_002774.3 |
62-176 | TGCCAGGGTGATTCTGGG | TGCAGACGTTGGTGTAGACT |
|
KLK7 NM_001207053.1 |
453-638 | GCTCGTGAAGCTCAATAG | CCTTGTAAACCTTCGTGC |
|
KLK8 NM_007196 |
725-951 | AAGTGTGAGGATGCTTACC | TGCCTATGATCTTCTTGATCCA |
|
KLK10 NM_001077500.1 |
890-1097 | ATAAAGTCATACGCTCCAAC | GTACAGGCAGAGAATGGG |
| IFN-γ | Amplicon Length: 100 | The assay probe spans Exon Boundary 3–4 (Applied Biosystems) | Assay ID: Mm01168134_m1 |
|
TMEV (DA) JX443418 |
1595-1813 | TGGTCGACTCTGTGGTTACG | GCCGGTCTTGCAAAGATAGT |
|
GAPDH NM_008084.2 |
351-584 | ACCACCATGGAGAAGGC | GGCATGGACTGTGGTCATGA |
In vitro analysis of kallikrein expression in response to viral capsid proteins
To determine whether the transcription of each kallikrein gene was regulated in T cells in a virus-dependent manner, whole-splenocyte cultures were prepared from TMEV-infected mice at 7, 21, 45, or 120 dpi and stimulated with a combination of VP1 and VP2 viral capsid proteins, each at 5 μg/ml for 72 hr (n = 3 to 5 per time point). The constructs encoding TMEV VP1 and VP2 capsid proteins were expressed in Escherichia coli and purified as previously detailed (Lin et al. 2002; Pavelko et al. 2007; Scarisbrick et al. 2012). All proteins were purified over a His-tag column and dialyzed into PBS before use. The VP1 construct encoded 274 amino acids of VP1 and the VP2 construct encoded 276 amino acids of VP2. Cultured cells were grown in RPMI 1640 with 10% heat-inactivated fetal calf serum, 2mM glutamine, 1 mM sodium pyruvate, 10 mM HEPES, and 10 μM 2-mercaptoethanol. Murine kallikreins were quantified as described above, and levels of interferon-γ were also assessed in the same samples to gauge the proinflammatory response.
In vitro analysis of kallikrein expression in activated monocytes
To determine whether KLK1, KLK6, KLK7, KLK8 or KLK10 RNA expression was regulated in activated monocytes, levels of RNA encoding the human forms of each kallikrein were quantified in untreated THP-1 monocyte cultures and in parallel cultures activated with PMA (10 ng/ml, Promega), or a combination of PMA plus LPS (10 μg/ml). THP-1 cells are a human monocytic leukemia cell line (American Type Culture Collection). Cells were cultured in RPMI 1640, with all supplements listed above. Expression of RNA encoding human kallikreins was quantified as described above using human-specific primers (Table 1) (Scarisbrick et al. 2006). All experiments were performed in triplicate and repeated at least twice.
Statistical analysis
All measurements were made without knowledge of treatment groups. Pairwise comparisons were made using the Students t-test; if data were not normally distributed, the Mann Whitney U Rank Sum test was applied. The level for significance was set as P < 0.05 for all statistical comparisons.
Acknowledgments
These studies were supported by the National Institutes of Health R01NS052741 and a Collaborative MS Research Award CA1060A11 and Pilot Project PP2009 from the National Multiple Sclerosis Society. The data pertaining to kallikrein 6 extend findings reported in Scarisbrick et al., 2012.
References
- Angelo PF, Lima AR, Alves FM, Blaber SI, Scarisbrick IA, Blaber M, Juliano L, Juliano MA. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan activation effects. J Biol Chem. 2006;281:3116–3126. doi: 10.1074/jbc.M510096200. [DOI] [PubMed] [Google Scholar]
- Attwood BK, Bourgognon JM, Patel S, Mucha M, Schiavon E, Skrzypiec AE, Young KW, Shiosaka S, Korostynski M, Piechota M, Przewlocki R, Pawlak R. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature. 2011;473:372–375. doi: 10.1038/nature09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernett MJ, Blaber SI, Scarisbrick IA, Dhanarajan P, Thompson SM, Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals that a trypsin-like kallikrein is expressed in the central nervous system. J Biol Chem. 2002;277:24562–24570. doi: 10.1074/jbc.M202392200. [DOI] [PubMed] [Google Scholar]
- Bhoola K, Ramsaroop R, Plendl J, Cassim B, Dlamini Z, Naicker S. Kallikrein and kinin receptor expression in inflammation and cancer. Biol Chem. 2001;382:77–89. doi: 10.1515/BC.2001.013. [DOI] [PubMed] [Google Scholar]
- Blaber SI, Ciric B, Christophi GP, Bernett MJ, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6-proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;19:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- Blaber SI, I, Scarisbrick A, Bernett MJ, Dhanarajan P, Seavy MA, Jin Y, Schwartz MA, Rodriguez M, Blaber M. Enzymatic properties of rat myelencephalon-specific protease. Biochemistry. 2002;41:1165–1173. doi: 10.1021/bi015781a. [DOI] [PubMed] [Google Scholar]
- Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, Dubus P, Hovnanian A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Radulovic M, Yoon H, Scarisbrick IA. Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy. Glia. 2013;61:1456–1470. doi: 10.1002/glia.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Yoshida S, Kato K, Momota Y, Suzuki J, Tanaka T, Ito J, Nishino H, Aimoto S, Kiyama H, et al. Expression and activity-dependent changes of a novel limbic-serine protease gene in the hippocampus. J Neurosci. 1995;15:5088–5097. doi: 10.1523/JNEUROSCI.15-07-05088.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophi GP, Isackson PJ, Blaber SI, Blaber M, Rodriguez M, Scarisbrick IA. Distinct promoters regulate tissue-specific and differential expression of kallikrein 6 in CNS demyelinating disease. J Neurochem. 2004;91:1439–1449. doi: 10.1111/j.1471-4159.2004.02826.x. [DOI] [PubMed] [Google Scholar]
- Davies B, I, Kearns R, Ure J, Davies CH, Lathe R. Loss of hippocampal serine protease BSP1/neuropsin predisposes to global seizure activity. J Neurosci. 2001;21:6993–7000. doi: 10.1523/JNEUROSCI.21-18-06993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic A, Zoecklein L, Kerkvliet J, Papke L, Edukulla R, Warrington A, Bieber A, Pease LR, David CS, Rodriguez M. Transgenic expression of viral capsid proteins predisposes to axonal injury in a murine model of multiple sclerosis. Brain Pathol. 2011;21:501–515. doi: 10.1111/j.1750-3639.2011.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamandis EP, Scorilas A, Kishi T, Blennow K, Luo LY, Soosaipillai A, Rademaker AW, Sjogren M. Altered kallikrein 7 and 10 concentrations in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. Clin Biochem. 2004;37:230–237. doi: 10.1016/j.clinbiochem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Drucker KD, Paulsen AP, Giannini C, Decker PA, Blaber IS, Blaber M, Uhm J, O’Neill BP, Jenkins R, Scarisbrick IA. Clinical Significance and Novel Mechanism of Action of Kallikrein 6 in Glioblastoma. Neuro-Oncology. 2013;15(3):305–18. doi: 10.1093/neuonc/nos313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Ottervald J, Nilsson KC, Sjogren N, Miliotis T, Von Bahr H, Khademi M, Eriksson B, Kjellstrom S, Vegvari A, Harris R, Marko-Varga G, Borg K, Nilsson J, Laurell T, Olsson T, Franzen B. Identification of novel candidate protein biomarkers for the post-polio syndrome - implications for diagnosis, neurodegeneration and neuroinflammation. J Proteomics. 2009;71:670–681. doi: 10.1016/j.jprot.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Bhan V, Wishart AD, Moore CS, Robertson GS. Human kallikrein 6 cerebrospinal levels are elevated in multiple sclerosis. Curr Drug Discov Technol. 2011;7:137–140. doi: 10.2174/157016310793180611. [DOI] [PubMed] [Google Scholar]
- Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell and tissue research. 2013;351:289–300. doi: 10.1007/s00441-013-1558-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Horii Y, Tamura H, Shiosaka S. Neuropsin (KLK8)-dependent and -independent synaptic tagging in the Schaffer-collateral pathway of mouse hippocampus. J Neurosci. 2008;28:843–849. doi: 10.1523/JNEUROSCI.4397-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S, Nukina N. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003;12:2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- Komai S, Matsuyama T, Matsumoto K, Kato K, Kobayashi M, Imamura K, Yoshida S, Ugawa S, Shiosaka S. Neuropsin regulates an early phase of schaffer-collateral long-term potentiation in the murine hippocampus. Eur J Neurosci. 2000;12:1479–1486. doi: 10.1046/j.1460-9568.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- Lawrence MG, Stephens CR, Need EF, Lai J, Buchanan G, Clements JA. Long terminal repeats act as androgen-responsive enhancers for the PSA-kallikrein locus. Endocrinology. 2012;153:3199–3210. doi: 10.1210/en.2012-1267. [DOI] [PubMed] [Google Scholar]
- Lin X, Njenga MK, Johnson AJ, Pavelko KD, David CS, Pease LR, Rodriguez M. Transgenic expression of Theiler’s murine encephalomyelitis virus genes in H-2(b) mice inhibits resistance to virus-induced demyelination. J Virol. 2002;76:7799–7811. doi: 10.1128/JVI.76.15.7799-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundwall A. Old genes and new genes: The evolution of the kallikrein locus. Thrombosis and haemostasis. 2013:109. doi: 10.1160/TH12-11-0851. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Miyai K, Ninomiya A, Yamasaki H, Tamura H, Nakamura Y, Shiosaka S. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J Neurosci. 2003;23:7727–7736. doi: 10.1523/JNEUROSCI.23-21-07727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain : a journal of neurology. 2000;123(Pt 3):519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Murray PD, Rodriguez M. Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. Journal of neuroscience research. 1999;58:492–504. doi: 10.1002/(sici)1097-4547(19991115)58:4<492::aid-jnr3>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomopoulou K, DeAngelis RA, Chen H, Diamandis EP, Hollenberg MD, Ricklin D, Lambris JD. Induction of complement C3a receptor responses by kallikrein-related peptidase 14. Journal of immunology. 2013;191:3858–3866. doi: 10.4049/jimmunol.1202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Akisada M, Okabe A, Sakurai K, Shiosaka S, Kato K. Extracellular serine protease neuropsin (KLK8) modulates neurite outgrowth and fasciculation of mouse hippocampal neurons in culture. Neurosci Lett. 2002;321:141–144. doi: 10.1016/s0304-3940(01)02470-3. [DOI] [PubMed] [Google Scholar]
- Pavelko KD, Pease LR, David CS, Rodriguez M. Genetic deletion of a single immunodominant T-cell response confers susceptibility to virus-induced demyelination. Brain Pathol. 2007;17:184–196. doi: 10.1111/j.1750-3639.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Larson N, Wu J, Linbo R, Burda JE, Diamandis EP, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein cascades in traumatic spinal cord injury: in vitro evidence for roles in axonopathy and neuron degeneration. Journal of neuropathology and experimental neurology. 2013;72:1072–1089. doi: 10.1097/NEN.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Zoecklein L, Kerkvliet JG, Pavelko KD, Papke L, Howe CL, Pease LR, David C. Human HLA-DR transgenes protect mice from fatal virus-induced encephalomyelitis and chronic demyelination. J Virol. 2008;82:3369–3380. doi: 10.1128/JVI.02243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA. The multiple sclerosis degradome: enzymatic cascades in development and progression of central nervous system inflammatory disease. Curr Top Microbiol Immunol. 2008;318:133–175. doi: 10.1007/978-3-540-73677-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA. Kallikrein Activity in the Central Nervous System. In: Schmitt M, Sommerhoff C, Fritz H, Magdolen V, editors. The Kallikreins. Berlin: De Gruyter Publishing; 2012. pp. 349–372. [Google Scholar]
- Scarisbrick IA, Asakura K, Blaber S, Blaber M, Isackson PJ, Beito T, Rodriguez M, Windebank AJ. Preferential expression of myelencephalon specific protease by oligodendrocytes of the adult rat spinal cord white matter. Glia. 2000;30:219–230. doi: 10.1002/(sici)1098-1136(200005)30:3<219::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Blaber SI, Lucchinetti CF, Genain CP, Blaber M, Rodriguez M. Activity of a newly identified serine protease in CNS demyelination. Brain. 2002;125:1283–1296. doi: 10.1093/brain/awf142. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Blaber SI, Tingling JT, Rodriguez M, Blaber M, Christophi GP. Potential scope of action of tissue kallikreins in CNS immune-mediated disease. J Neuroimmunology. 2006;178:167–176. doi: 10.1016/j.jneuroim.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Epstein B, Cloud BA, Yoon H, Wu J, Renner DN, Blaber SI, Blaber M, Vandell AG, Bryson AL. Functional role of kallikrein 6 in regulating immune cell survival. PLoS One. 2011;6:e18376, 18371–18311. doi: 10.1371/journal.pone.0018376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Isackson PJ, Ciric B, Windebank AJ, Rodriguez M. MSP, a trypsin-like serine protease, is abundantly expressed in the human nervous system. J Comp Neurol. 2001;431:347–361. [PubMed] [Google Scholar]
- Scarisbrick IA, Linbo R, Vandell AG, Keegan M, Blaber SI, Blaber M, Sneve D, Lucchinetti CF, Rodriguez M, Diamandis EP. Kallikreins are associated with secondary progressive multiple sclerosis and promote neurodegeneration. Biol Chem. 2008;389:739–745. doi: 10.1515/BC.2008.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Radulovic M, Burda JE, Larson N, Blaber SI, Giannini C, Blaber M, Vandell AG. Kallikrein 6 is a novel molecular trigger of reactive astrogliosis. Biological Chemistry. 2012;393:355–367. doi: 10.1515/hsz-2011-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Sabharwal P, Cruz H, Larsen N, Vandell A, Blaber SI, Ameenuddin S, Papke LM, Fehlings MG, Reeves RK, Blaber M, Windebank AJ, Rodriguez M. Dynamic role of kallikrein 6 in traumatic spinal cord injury. Eur J Neuroscience. 2006;24:1457–1469. doi: 10.1111/j.1460-9568.2006.05021.x. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Towner MD, Isackson PJ. Nervous system specific expression of a novel serine protease: regulation in the adult rat spinal cord by excitotoxic injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8156–8168. doi: 10.1523/JNEUROSCI.17-21-08156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Yoon H, Panos M, Larson N, Blaber SI, Blaber M, Rodriguez M. Kallikrein 6 regulates early CNS demyelination in a viral model of multiple sclerosis. Brain Pathol. 2012;22:709–722. doi: 10.1111/j.1750-3639.2012.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Okabe C, Yousef GM, Diamandis EP, Yoshida S, Shiosaka S, Fahnestock M. Expression of the kallikrein gene family in normal and Alzheimer’s disease brain. Neuroreport. 2001;12:2747–2751. doi: 10.1097/00001756-200108280-00031. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G, Pampalakis G. Targeting the kallikrein-related peptidases for drug development. Trends in pharmacological sciences. 2012;33:623–634. doi: 10.1016/j.tips.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G, Pampalakis G, Diamandis EP. Functional roles of human kallikrein-related peptidases. J Biol Chem. 2009;284:32989–32994. doi: 10.1074/jbc.R109.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Michael S, Shen J, Kosberg K, Rockenstein E, Patrick C, Adame A, Masliah E. Lentivirus mediated delivery of neurosin promotes clearance of wild-type alpha-synuclein and reduces the pathology in an alpha-synuclein model of LBD. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:31–41. doi: 10.1038/mt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tamura H, Ishikawa Y, Hino N, Maeda M, Yoshida S, Kaku S, Shiosaka S. Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo. J Physiol. 2006;570:541–551. doi: 10.1113/jphysiol.2005.098715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama R, Bando Y, Yamada M, Yoshida S. Involvement of neuropsin in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 2005;52:108–118. doi: 10.1002/glia.20226. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Fujinami RS. Neuropathogenesis of Theiler’s murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J Neuroimmune Pharmacol. 2010;5:355–369. doi: 10.1007/s11481-009-9179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandell AG, Larson N, Laxmikanthan G, Panos M, Blaber SI, Blaber M, Scarisbrick IA. Protease Activated Receptor Dependent and Independent Signaling by Kallikreins 1 and 6 in CNS Neuron and Astroglial Cell Lines. J Neurochem. 2008;107:855–870. doi: 10.1111/j.1471-4159.2008.05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Palma JP, Yahikozawa H, Koh CS, Kim BS. Role of individual T- cell epitopes of Theiler’s virus in the pathogenesis of demyelination correlates with the ability to induce a Th1 response. J Virol. 1998;72:6169–6174. doi: 10.1128/jvi.72.7.6169-6174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Wu J, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein 6 signals through PAR1 and PAR2 to promote neuron injury and exacerbate glutamate neurotoxicity. Journal of neurochemistry. 2013;127:283–298. doi: 10.1111/jnc.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]