Abstract
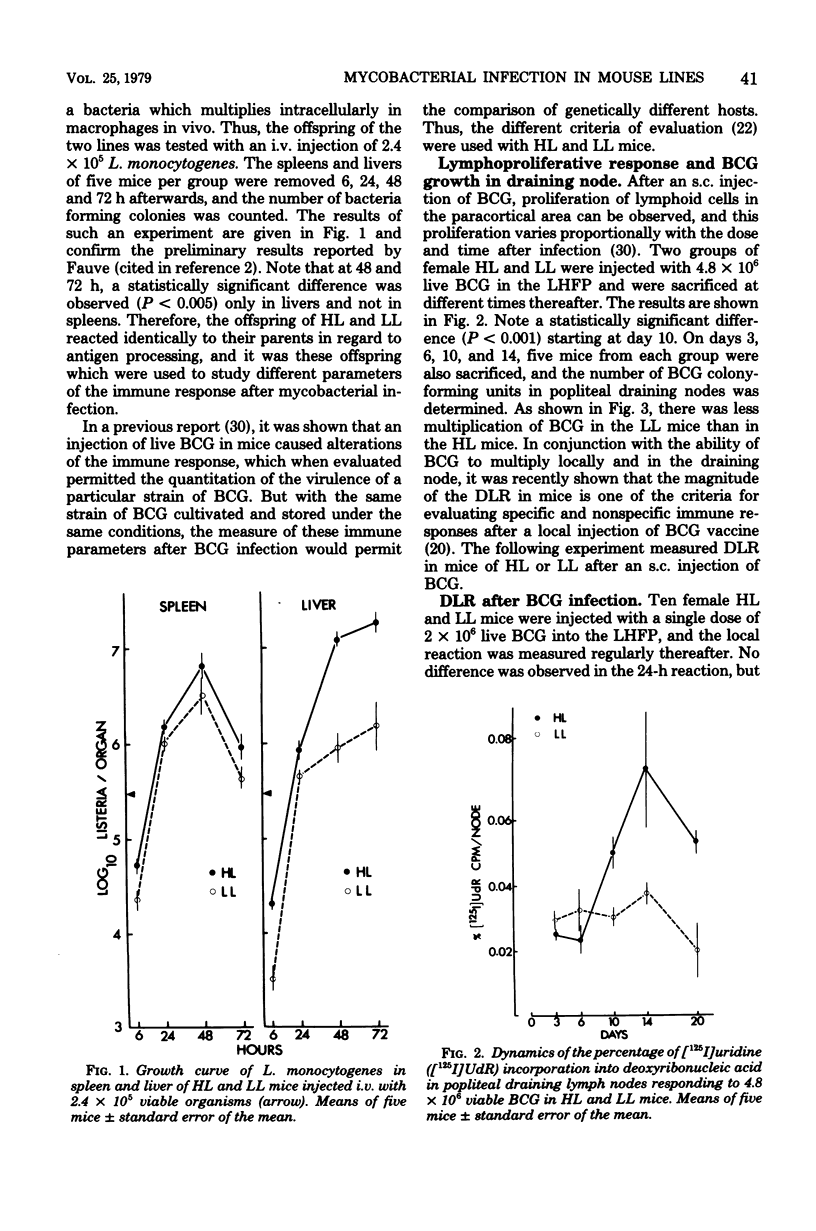
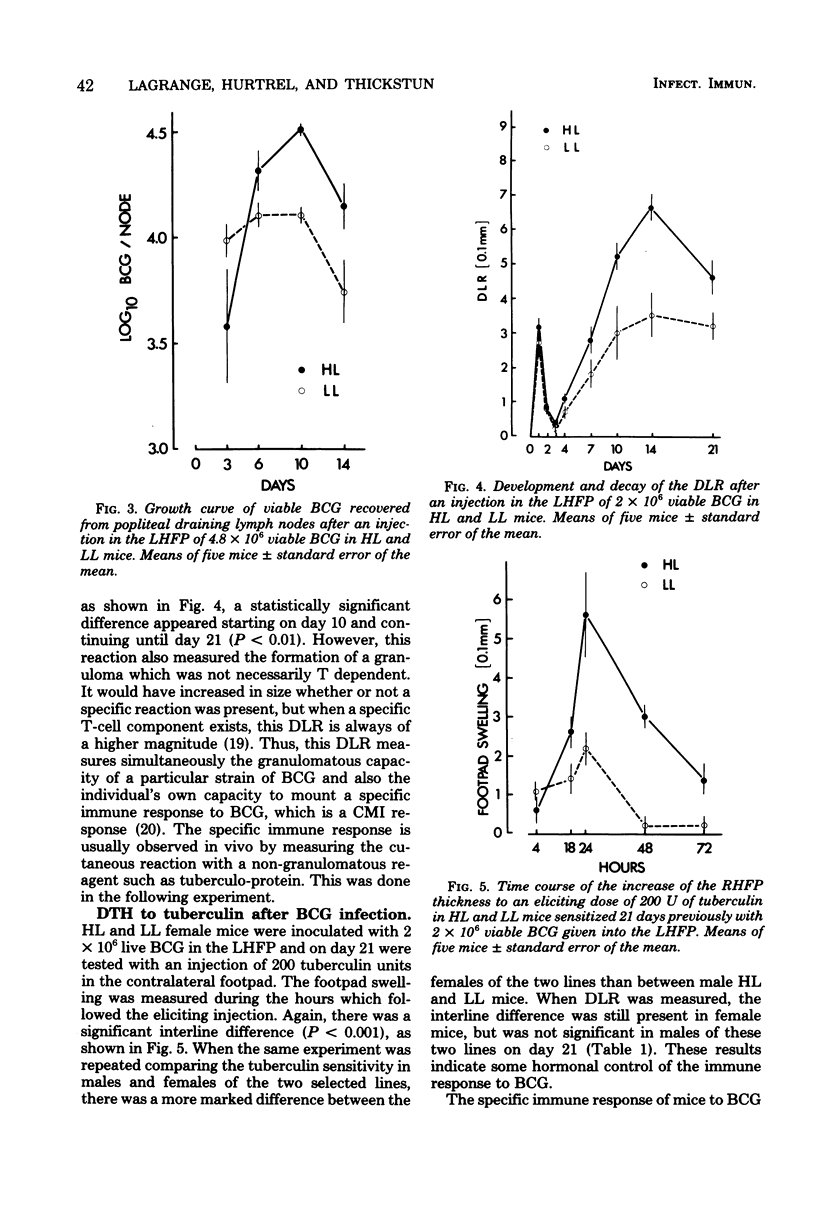
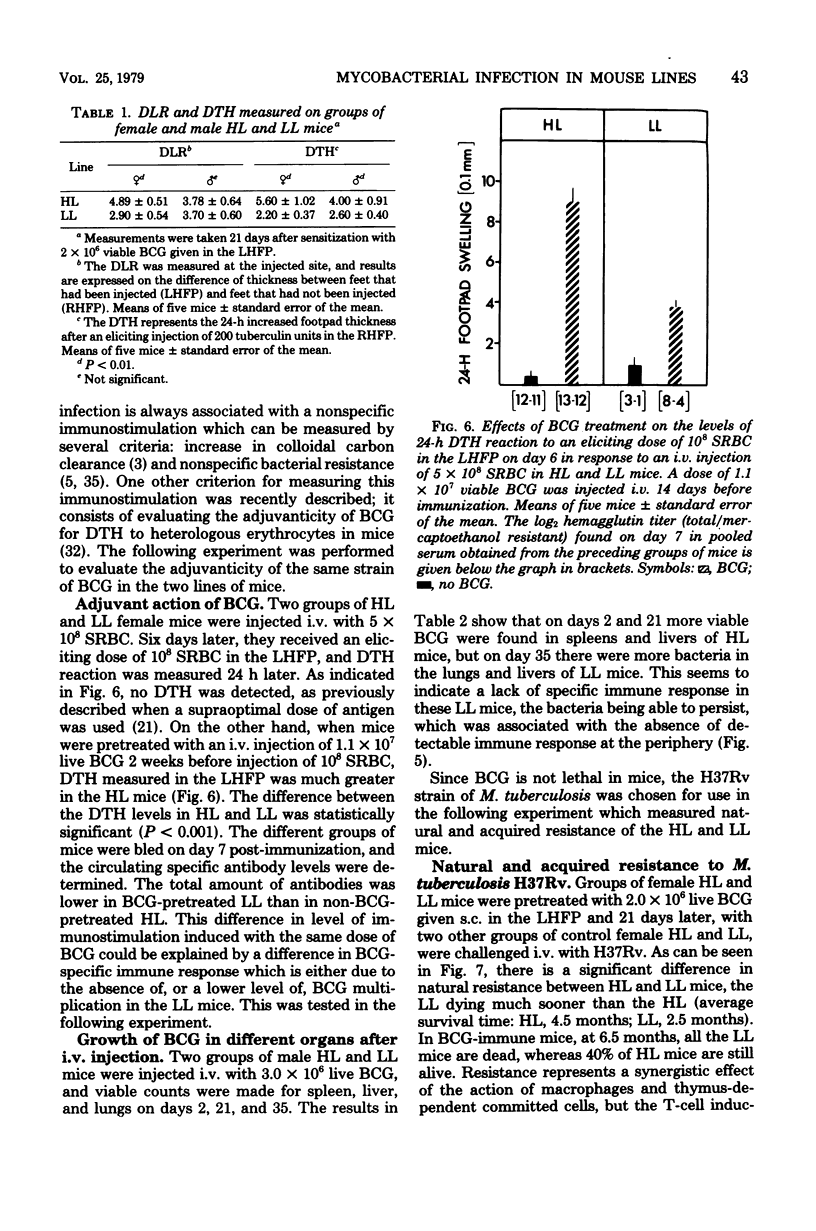
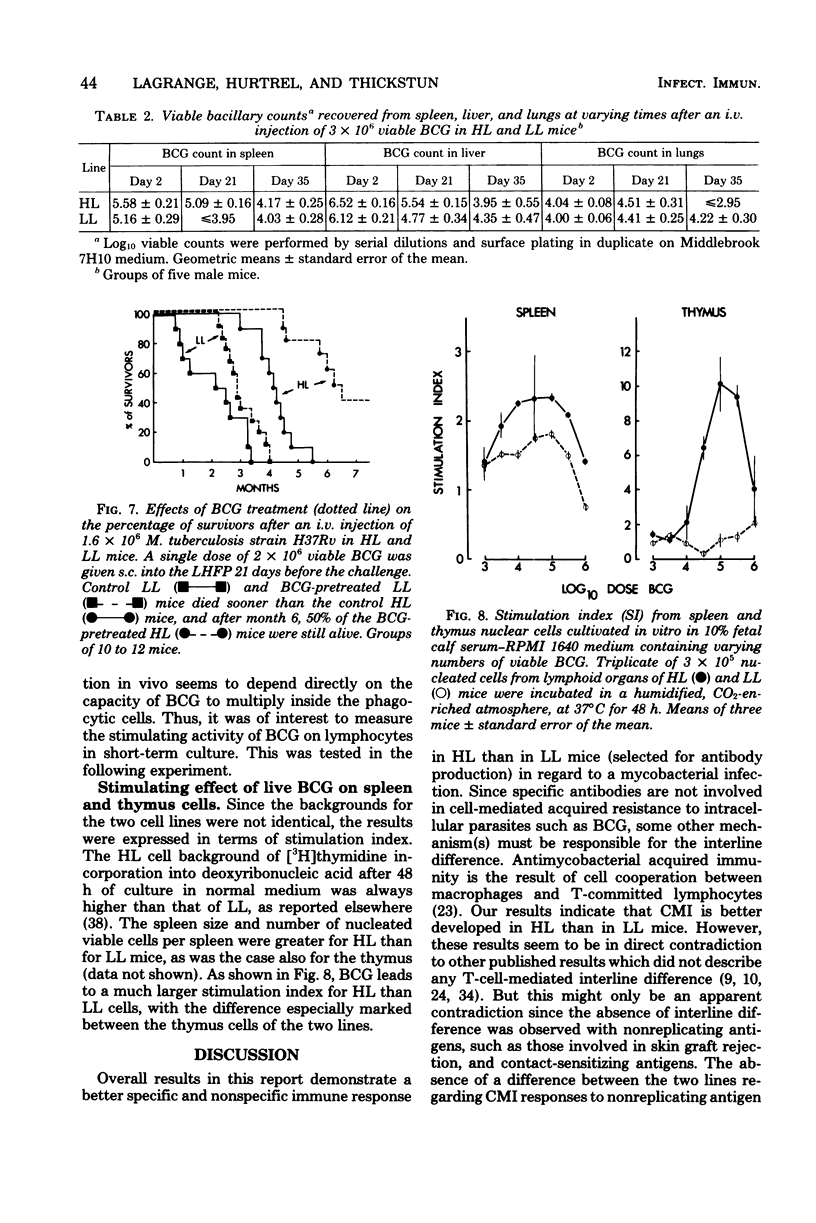
Resistance and susceptibility to mycobacterial infection in the Biozzi high and low lines of mice which were genetically selected for their responses to heterologous erythrocytes have been found to be related to the innate ability of nonimmune macrophages to kill or inhibit the growth of the organisms during the first two weeks after infection and to their ability to mount specific and nonspecific immune responses. High antibody-producer mice were more capable of expressing cell-mediated immune parameters than low antibody-producer mice. A direct relationship was observed between the ability of bacteria (BCG vaccine) to multiply inside the reticuloendothelial system and the development of cell-mediated immunity, as measured by the delayed local reaction at the injection site, the lymphoproliferative response in the draining nodes, the tuberculin delayed-type hypersensitivity, the acquired resistance, and the adjuvant effect after BCG inoculation. In high line mice, apart from the inability of their macrophages to inhibit the early growth of bacteria, their lymphocytes in spleen and thymus were more capable of being stimulated in vitro by varying concentrations of living BCG. The data presented in this report are compatible with the hypothesis that a group of genes segregated in each line during the selective breeding controls the innate microbicidal activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. Thymic maturation in vitro by a secretory product from macrophages. J Immunol. 1977 May;118(5):1780–1787. [PubMed] [Google Scholar]
- Biozzi G., Siqueira M., Mouton D., Sant'anna O. A., Stiffel C., Esteves M. B., Bouthillier Y. La régulation polygénique non spécifique de la synthèse des anticorps. Ann Immunol (Paris) 1977 Jan-Mar;128(1-2):393–399. [PubMed] [Google Scholar]
- Biozzi G., Stiffel C., Mouton D., Bouthillier Y., Decreusefond C. Cytodynamics of the immune response in two lines of mice genetically selected for "high" and "low" antibody synthesis. J Exp Med. 1972 May 1;135(5):1071–1094. doi: 10.1084/jem.135.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biozzi G., Stiffel C., Mouton D., Bouthillier Y., Decreusefond C. Importance de l'immunité spécifique et non spécifique dans la défense antitumorale. Ann Inst Pasteur (Paris) 1972 Apr;122(4):685–694. [PubMed] [Google Scholar]
- Byfield P. E., Howard J. G. Equivalent graft-versus-host reactivity of spleen cells from two lines of mice genetically selected for high and low humoral antibody formation. Transplantation. 1972 Jul;14(1):133–135. doi: 10.1097/00007890-197207000-00023. [DOI] [PubMed] [Google Scholar]
- Cannat A., Bousquet C., Serre A. Response of high and low antibody producer to Brucella. Ann Immunol (Paris) 1978 Jul-Sep;129 100(5):669–683. [PubMed] [Google Scholar]
- Collins F. M., Montalbine V. Relative immunogenicity of streptomycin-susceptible and -resistant strains of BCG. II. Effect of the route of inoculation on growth and immunogenicity. Am Rev Respir Dis. 1975 Jan;111(1):43–51. doi: 10.1164/arrd.1975.111.1.43. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEFER W. B., PIERCE C. H. Antituberculous immunity in mice vaccinated with killed tubercle bacilli. J Exp Med. 1953 Feb 1;97(2):221–233. doi: 10.1084/jem.97.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYT A., MOORE F. J., KNOWLES R. G., SMITH C. R. Sex differences of normal and immunized mice in resistance to experimental tuberculosis. Am Rev Tuberc. 1957 Apr;75(4):618–623. doi: 10.1164/artpd.1957.75.4.618. [DOI] [PubMed] [Google Scholar]
- Han S. H., Weiser R. S. Systemic tuberculin sensitivity in mice. I. Factors contributing to active tuberculin shock. J Immunol. 1967 Jun;98(6):1152–1157. [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M., Biozzi G. Studies on immunological paralysis. 8. Pneumococcal polysaccharide tolerance and immunity differences between the Biozzi high and low responder lines of mice. Eur J Immunol. 1972 Jun;2(3):269–273. doi: 10.1002/eji.1830020315. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Courtenay B. M., Desaymard C. Equivalent responsiveness to branched polysaccharides and their dinitrophenyl conjugates in the Biozzi high and low responder lines of mice. Eur J Immunol. 1974 Jul;4(7):453–457. doi: 10.1002/eji.1830040702. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Hurtrel B. La réaction locale granulomateuse après injection sous-cutanée de BCG chez la souris. II.--Corrélations avec la réponse immune. Ann Immunol (Paris) 1978 Apr-Jun;129 100(4):547–558. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Induction and expression of immunity after BCG immunization. Infect Immun. 1977 Dec;18(3):646–653. doi: 10.1128/iai.18.3.646-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacopoulos-Briot M., Bouthillier Y., Mouton D., Lambert F., Decreusefond C., Stiffel C., Biozzi G. Comparison of skin allograft rejection and cytotoxic antibody production in two lines of mice genetically selected for "high" and "low" antibody synthesis. Transplantation. 1972 Nov;14(5):590–596. doi: 10.1097/00007890-197211000-00010. [DOI] [PubMed] [Google Scholar]
- Liacopoulos-Briot M., Mouton D., Stiffel C., Lambert F., Decreusefond C., Bouthillier Y., Biozzi G. Réponse a la phytohémagglutinine des lymphocytes de deux lignées de souris génétiquement sélectionnées pour une forte et une faible synthèse d'anticorps. Ann Immunol (Paris) 1974 Mar-Apr;125(3):393–404. [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974 Jun 1;139(6):1540–1552. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Miller T. E., Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974 Mar 1;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton D., Bouthillier Y., Oriol R., Decreusefond C., Stiffel C., Biozzi G. Intensité de la réaction d'hypersensibilité retardée chez les souris des lignées sélectionnées "bonnes" et "mauvaises" productrices d'anticorps. Ann Immunol (Paris) 1974 Jun;125 100(4):581–588. [PubMed] [Google Scholar]
- North R. J., Mackaness G. B., Elliott R. W. The histogenesis of immunologically committed lymphocytes. Cell Immunol. 1972 Apr;3(4):680–694. doi: 10.1016/0008-8749(72)90130-x. [DOI] [PubMed] [Google Scholar]
- Rowley D., Auzins I., Jenkin C. R. Further studies regarding the question of cellular immunity in mouse typhoid. Aust J Exp Biol Med Sci. 1968 Aug;46(4):447–463. doi: 10.1038/icb.1968.38. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Biozzi G., Benacerraf B. Immunocytochemical studies on B lymphocytes from the Biozzi high and low lines of mice selected for their responses to heterologous erythrocytes. Proc Soc Exp Biol Med. 1974 Apr;145(4):1243–1249. doi: 10.3181/00379727-145-37990. [DOI] [PubMed] [Google Scholar]
- Wiener E., Bandieri A. Differences in antigen handling by peritoneal macrophages from the Biozzi high and low responder lines of mice. Eur J Immunol. 1974 Jul;4(7):457–463. doi: 10.1002/eji.1830040703. [DOI] [PubMed] [Google Scholar]
- Youmans G. P. Relation between delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975 Feb;111(2):109–118. doi: 10.1164/arrd.1975.111.2.109. [DOI] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Response of vaccinated and nonvaccinated syngeneic C57B1-6 mice to infection with Mycobacterium tuberculosis. Infect Immun. 1972 Nov;6(5):748–754. doi: 10.1128/iai.6.5.748-754.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


