Abstract
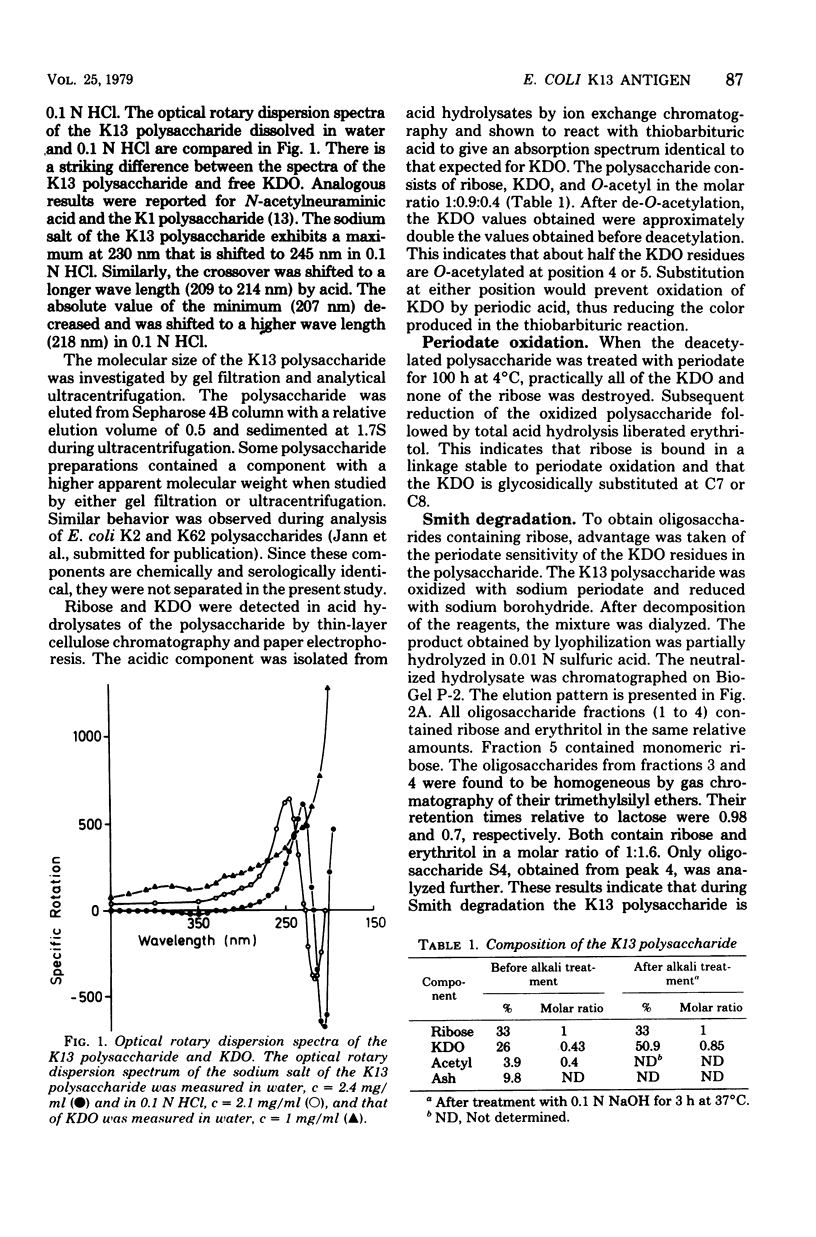
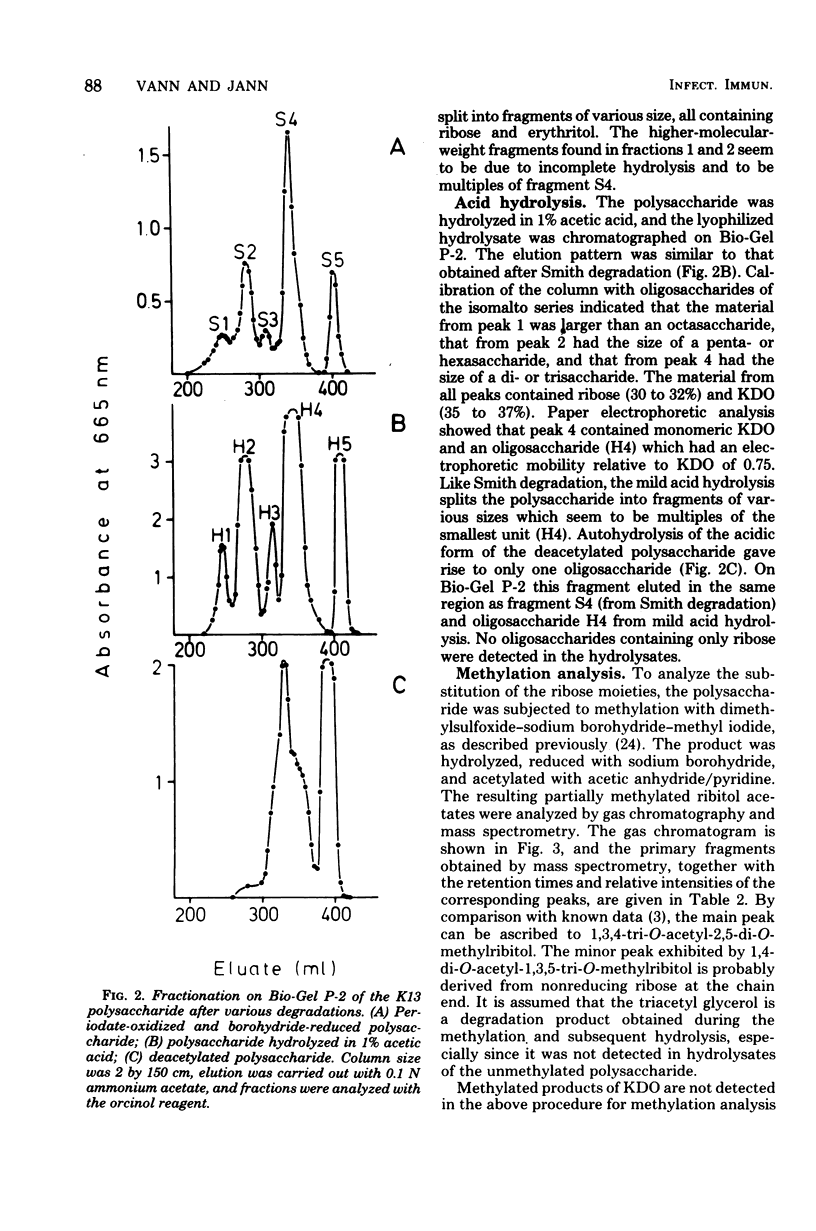
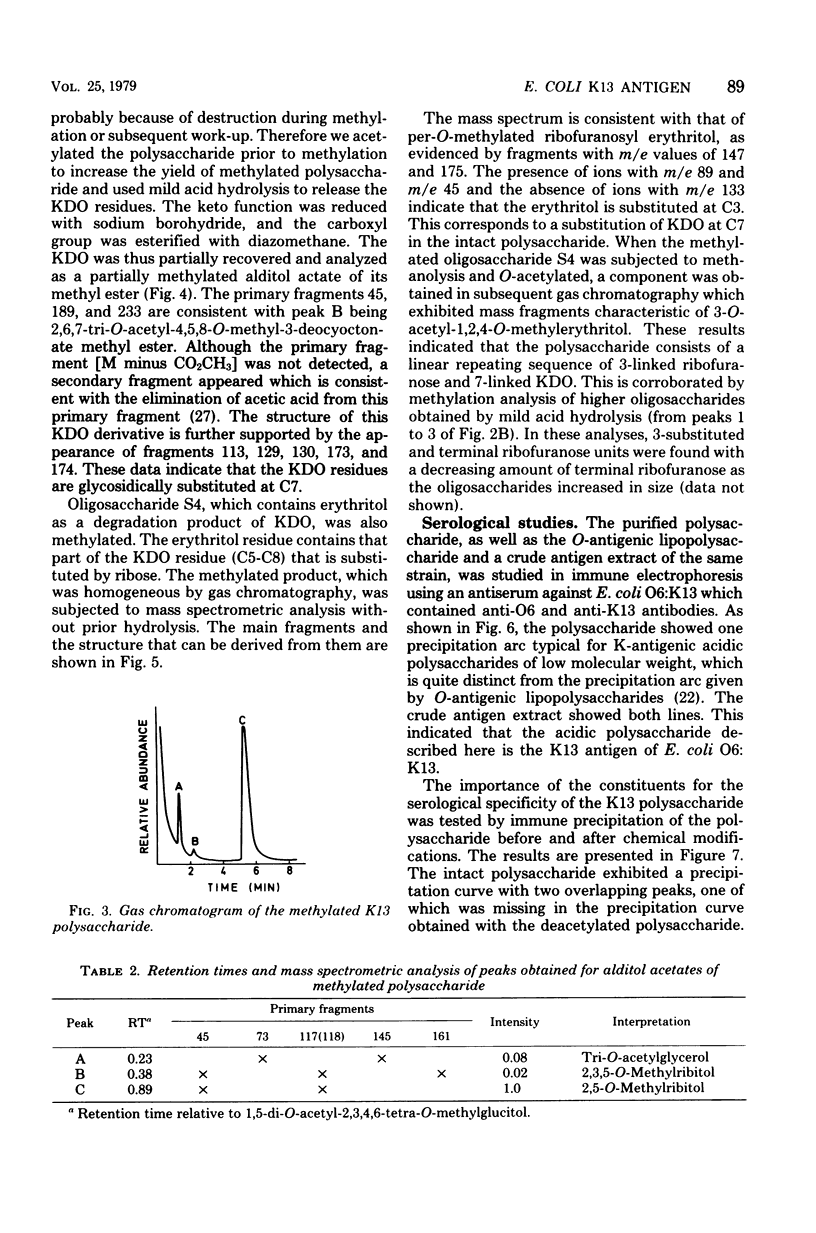
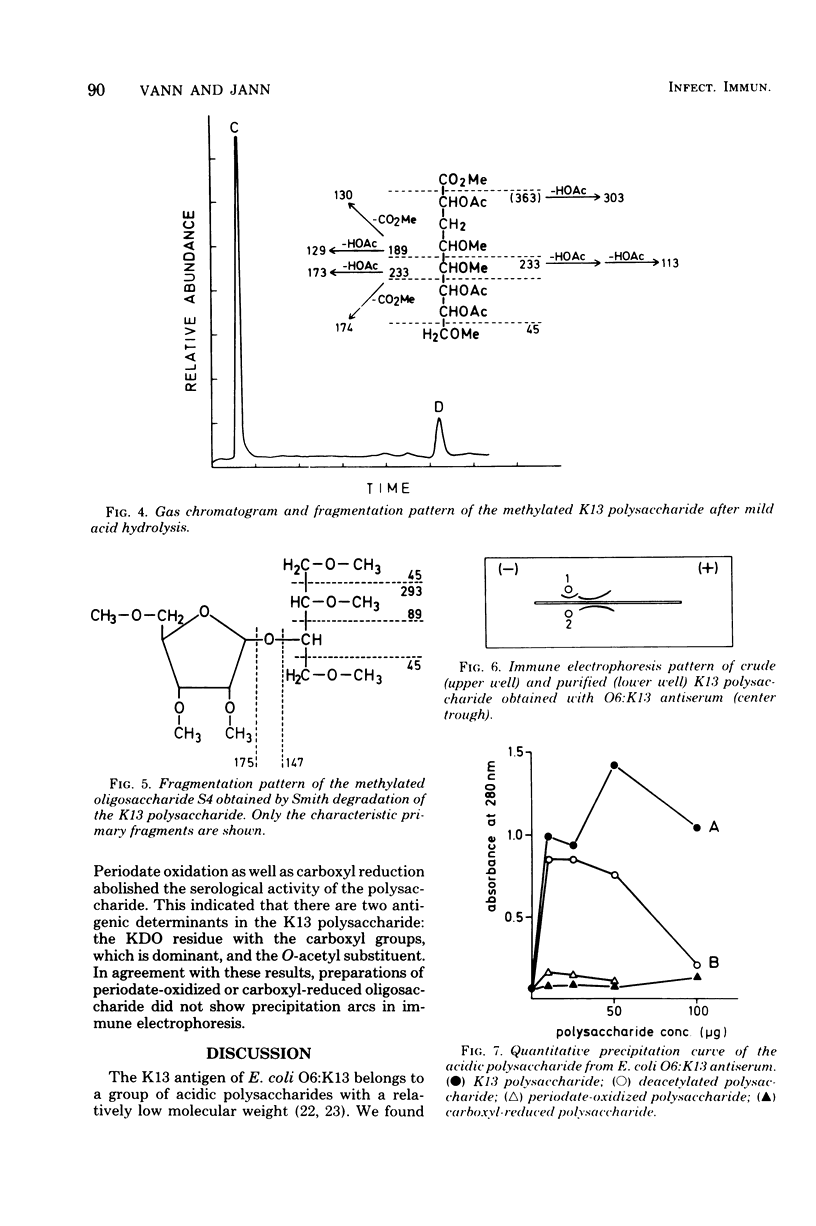
The primary structure of the K13-antigenic polysaccharide (K13 antigen) of Escherichia coli O6:K13:H1 was elucidated by composition, periodate oxidation, Smith degradation, and methylation analysis. The polysaccharide consists of a repeating sequence of 3-linked ribofuranose and 7-linked 3-deoxymannooctulosonic acid (KDO). About 50% of the KDO residues are O-acetylated at position 4 or 5. Measurement of the optical rotary dispersion indicated that in aqueous solution the K13 polysaccharide assumes a secondary structure in which the carboxyl groups of KDO are engaged. The serological specificity of the K13 polysaccharide is expressed through KDO and its O-acetyl substituent, the ribose unit being antigenically silent. There are two populations of anti-K13 antibodies one directed against the charged region of the KDO and the other against the O-acetyl groups.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J. A sensitive reagent for detecting 2-deoxysugars and 3-deoxypolyols. J Chromatogr. 1966 Jan;21(1):163–164. doi: 10.1016/s0021-9673(01)91287-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J., Kenny C. P. Structural elucidation of the 3-deoxy-D-manno-octulosonic acid containing meningococcal 29-e capsular polysaccharide antigen using carbon-13 nuclear magnetic resonance. Biochemistry. 1978 Feb 21;17(4):645–651. doi: 10.1021/bi00597a013. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D., Heath E. C. Isolation of colitose-containing oligosaccharides from the cell wall lipopolysaccharide of Escherichia coli. Biochem Biophys Res Commun. 1965 Dec 21;21(6):638–643. doi: 10.1016/0006-291x(65)90534-6. [DOI] [PubMed] [Google Scholar]
- Fromme I., Beilharz H. Gas chromatographic assay of total and O-acetyl groups in bacterial lipopolysaccharides. Anal Biochem. 1978 Feb;84(2):347–353. doi: 10.1016/0003-2697(78)90051-9. [DOI] [PubMed] [Google Scholar]
- Glode M. P., Robbins J. B., Liu T. Y., Gotschlich E. C., Orskov I., Orskov F. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J Infect Dis. 1977 Jan;135(1):94–104. doi: 10.1093/infdis/135.1.94. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Etienne J., Sanborn W. R., Triau R., Cvjetanović B. The immunological responses observed in field studies in Africa with group A meningococcal vaccines. Prog Immunobiol Stand. 1971;5:485–491. [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Jann K., Jann B., Orskov F., Orskov I., Westphal O. Immunchemische Untersuchungen an K-Antigenen von Escherichia Coli. II. Das K-Antigen von E. coli 08:K42(A):H-. Biochem Z. 1965 Jun 3;342(1):1–22. [PubMed] [Google Scholar]
- Jennings H. J., Williams R. E. The circular dichroic spectra of several sialic acid-containing polysaccharides isolated from Neisseria meningitidis. Carbohydr Res. 1976 Sep;50(2):257–265. doi: 10.1016/s0008-6215(00)83857-4. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Lloyd K. O., Beychok S. Optical activity and conformation of carbohydrates. II. Opitical rotatory dispersion and circular dichroism studies on immunochemically reactive oligo- and polysaccharides containing amino sugars and their derivatives. Biochemistry. 1969 Mar;8(3):747–756. doi: 10.1021/bi00831a001. [DOI] [PubMed] [Google Scholar]
- Kaijser B., Hanson L. A., Jodal U., Lidin-Janson G., Robbins J. B. Frequency of E. coli K antigens in urinary-tract infections in children. Lancet. 1977 Mar 26;1(8013):663–666. doi: 10.1016/s0140-6736(77)92111-0. [DOI] [PubMed] [Google Scholar]
- Kaijser B., Olling S. Experimental hematogenous pyelonephritis due to Escherichia coli in rabbits: the antibody response and its protective capacity. J Infect Dis. 1973 Jul;128(1):41–49. doi: 10.1093/infdis/128.1.41. [DOI] [PubMed] [Google Scholar]
- MCGUIRE E. J., BINKLEY S. B. THE STRUCTURE AND CHEMISTRY OF COLOMINIC ACID. Biochemistry. 1964 Feb;3:247–251. doi: 10.1021/bi00890a017. [DOI] [PubMed] [Google Scholar]
- Nicholson A. M., Glynn A. A. Investigation of the effect of K antigen in Escherichia coli urinary tract infections by use of a mouse model. Br J Exp Pathol. 1975 Dec;56(6):549–553. [PMC free article] [PubMed] [Google Scholar]
- Orskov F. Agarose electrophoresis combined with second dimensional Cetavlon precipitation. A new method for demonstration of acidic polysaccharide K antigens. Acta Pathol Microbiol Scand B. 1976 Oct;84B(5):319–320. [PubMed] [Google Scholar]
- Orskov F., Orskov I., Jann B., Jann K. Immunoelectrophoretic patterns of extracts from all Escherichia coli O and K antigen test strains: correlation with pathogenicity. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(2):142–152. doi: 10.1111/j.1699-0463.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske K., Jann K. The O8 antigen of Escherichia coli. Structure of the polysaccharide chain. Eur J Biochem. 1972 Dec 4;31(2):320–328. doi: 10.1111/j.1432-1033.1972.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Taylor P. W. An unusual acidic polysaccharide produced by a rough strain of Escherichia coli. Biochem Biophys Res Commun. 1974 Nov 6;61(1):148–154. doi: 10.1016/0006-291x(74)90546-4. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]


