Abstract
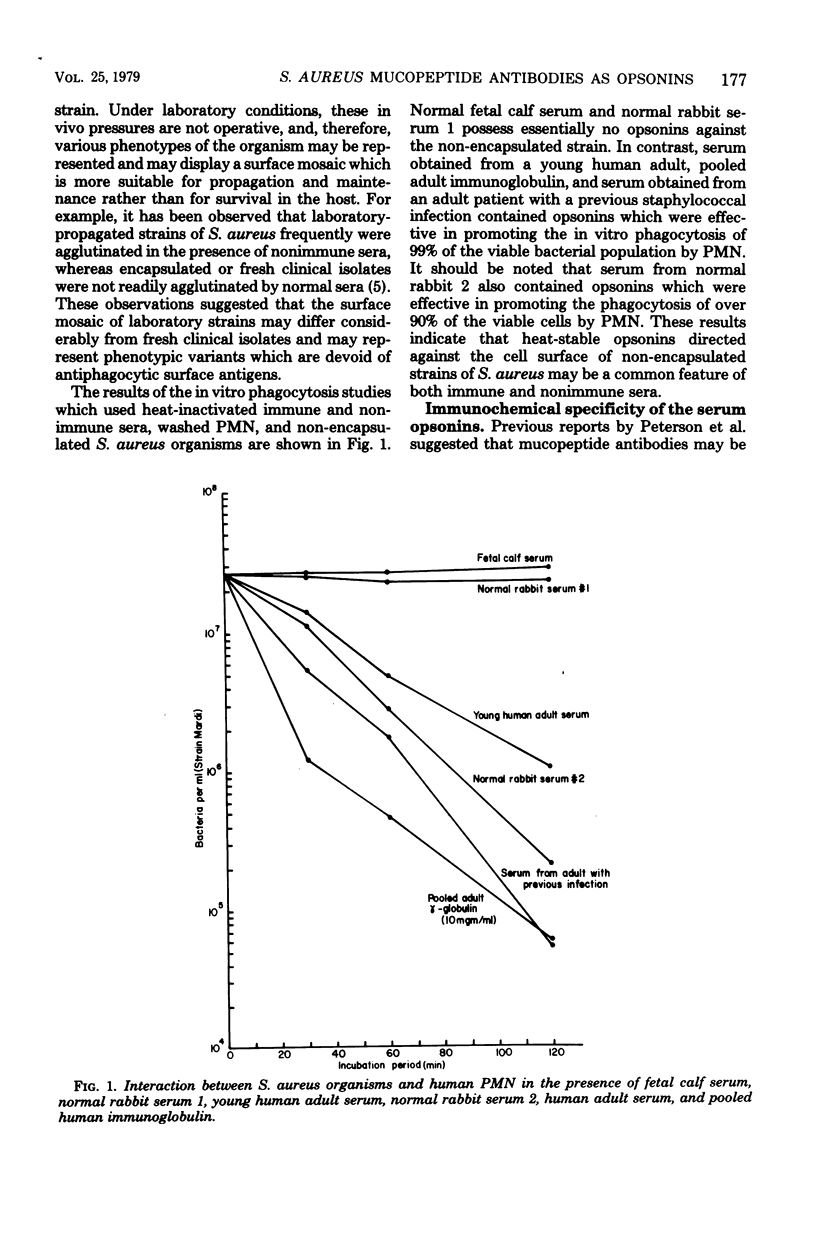
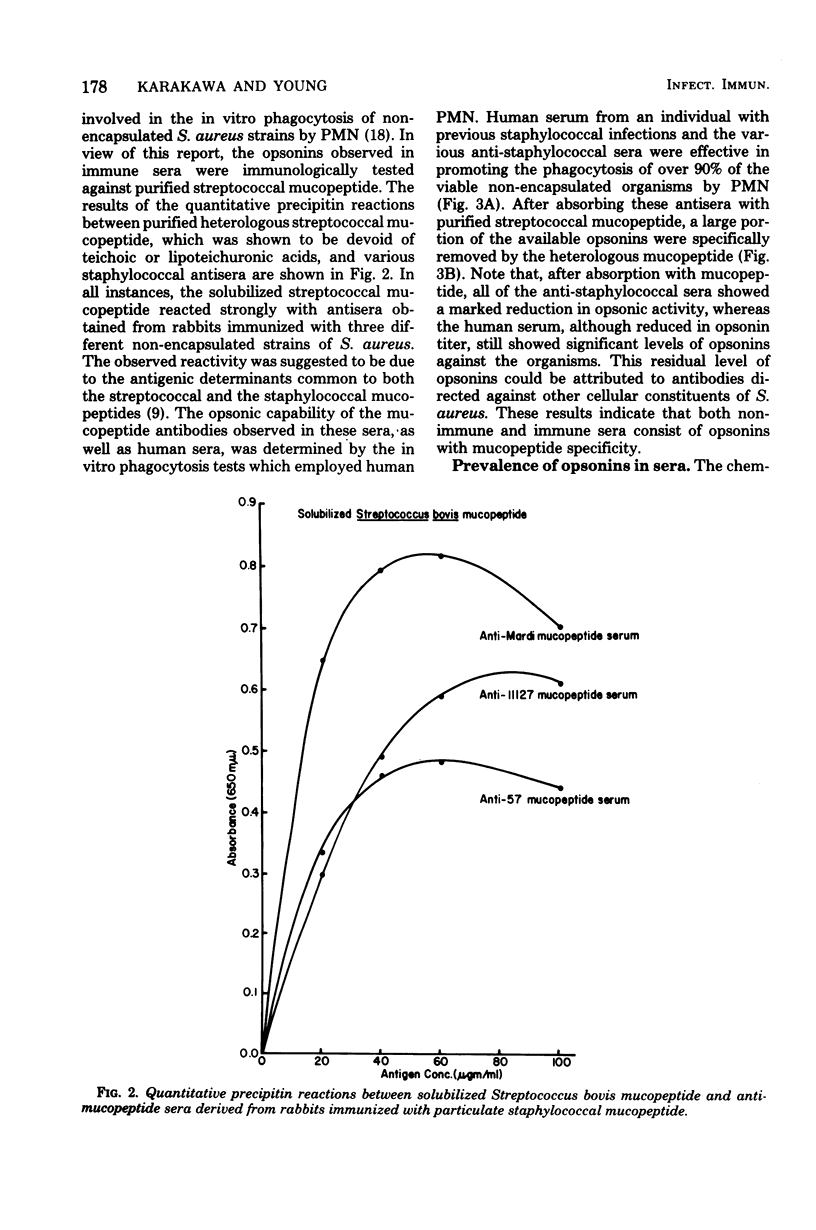
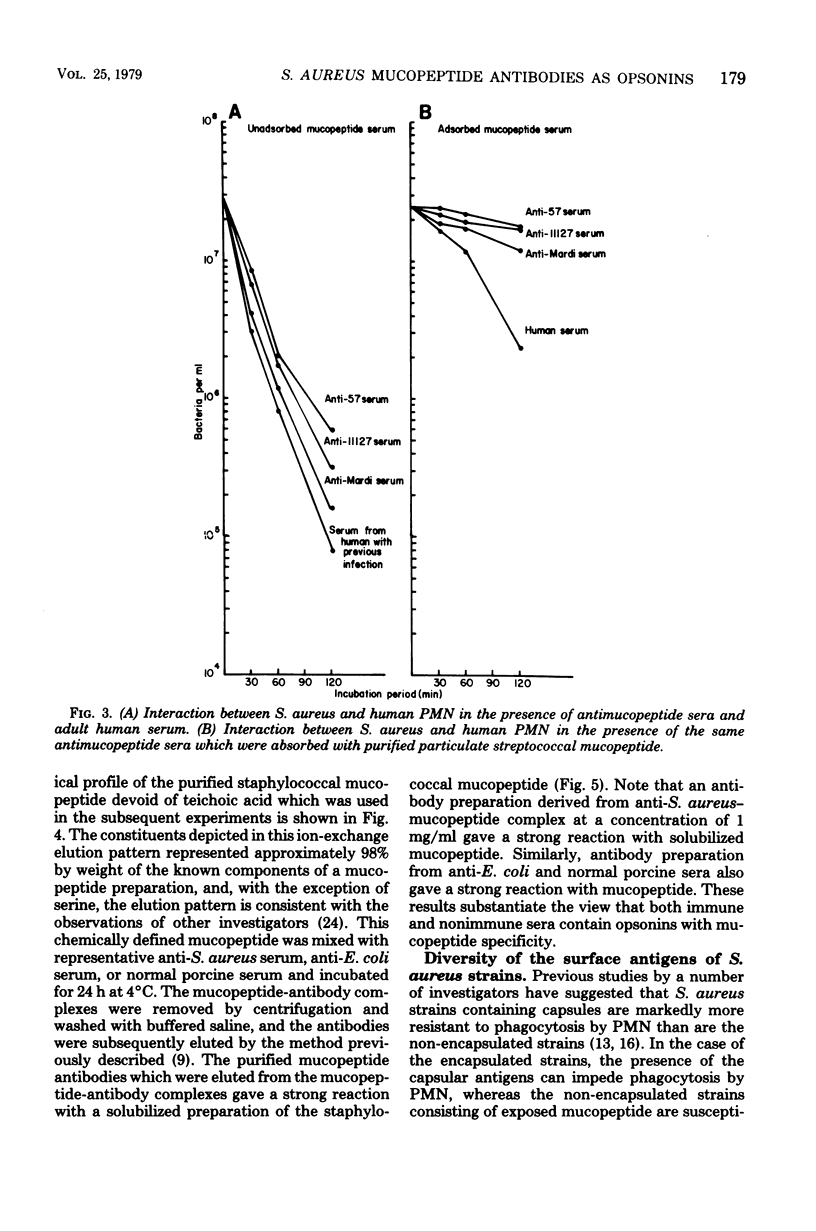
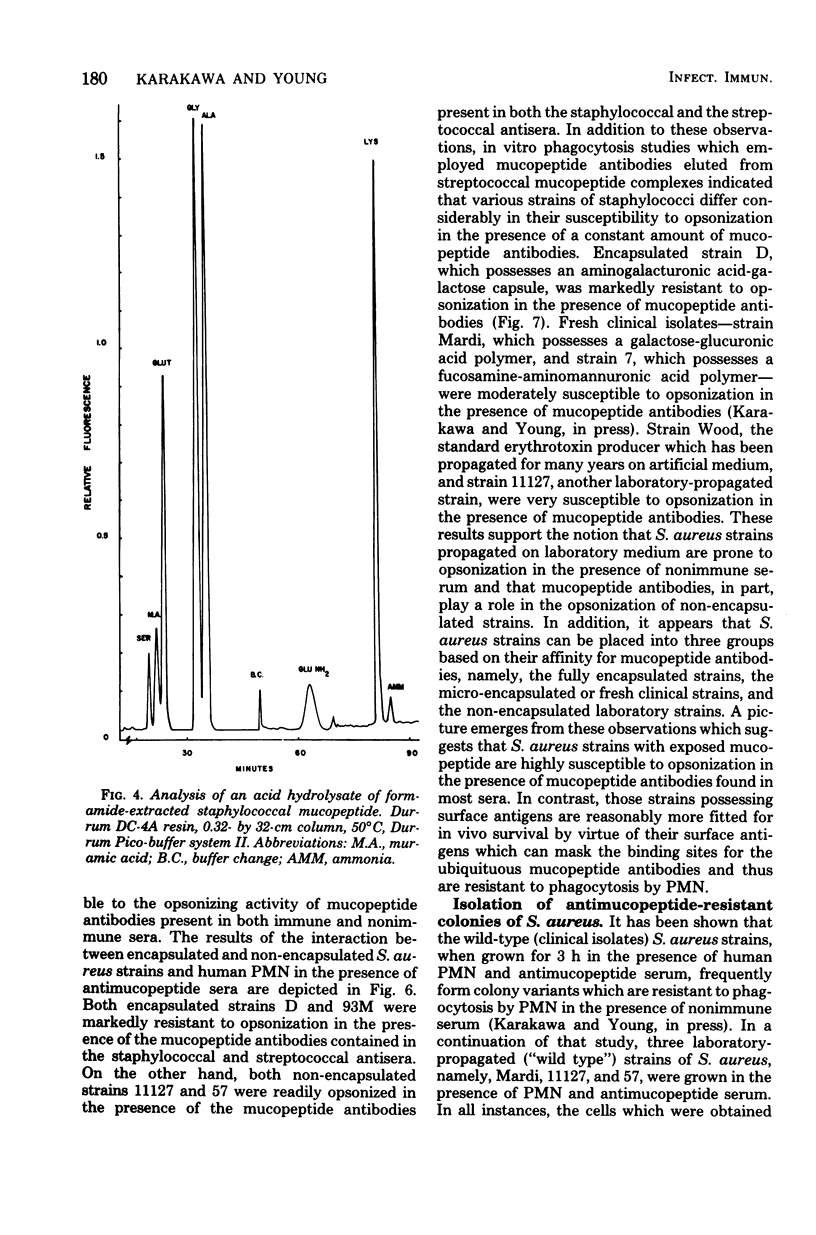
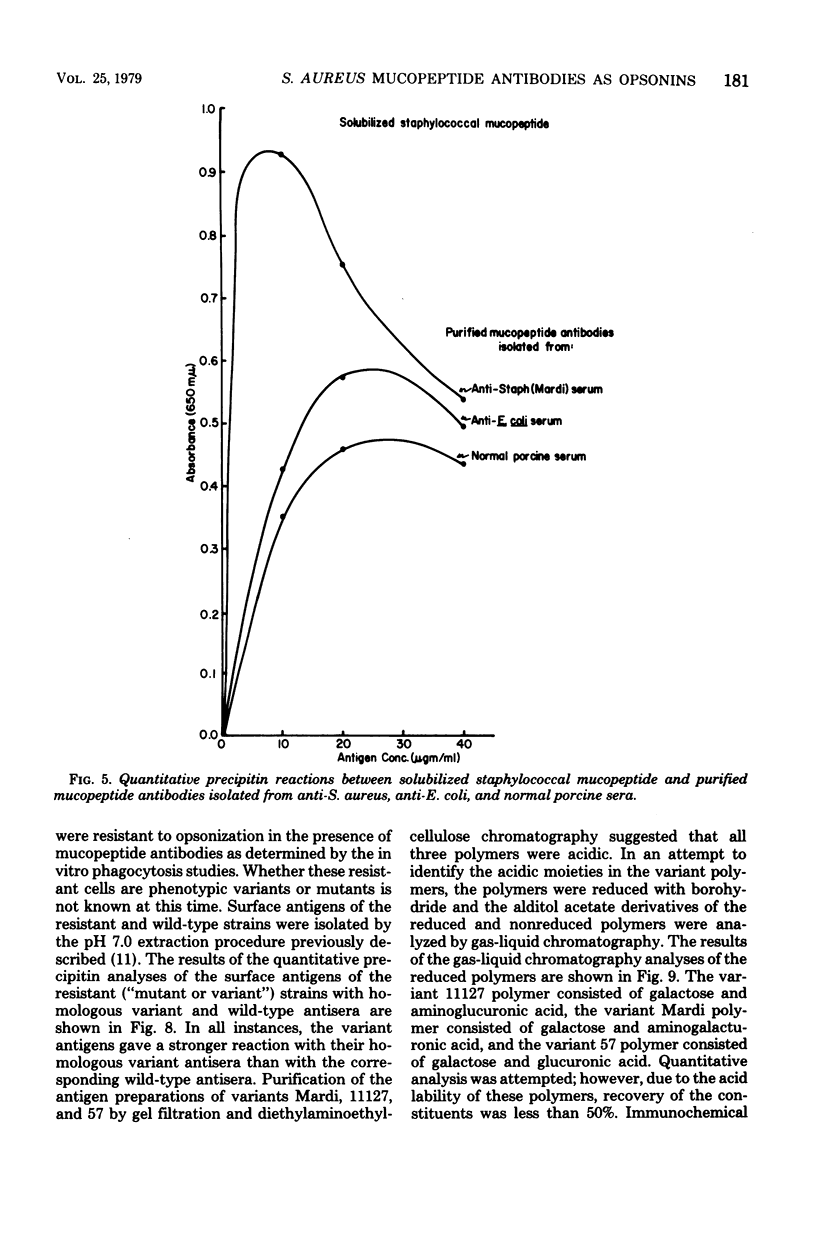
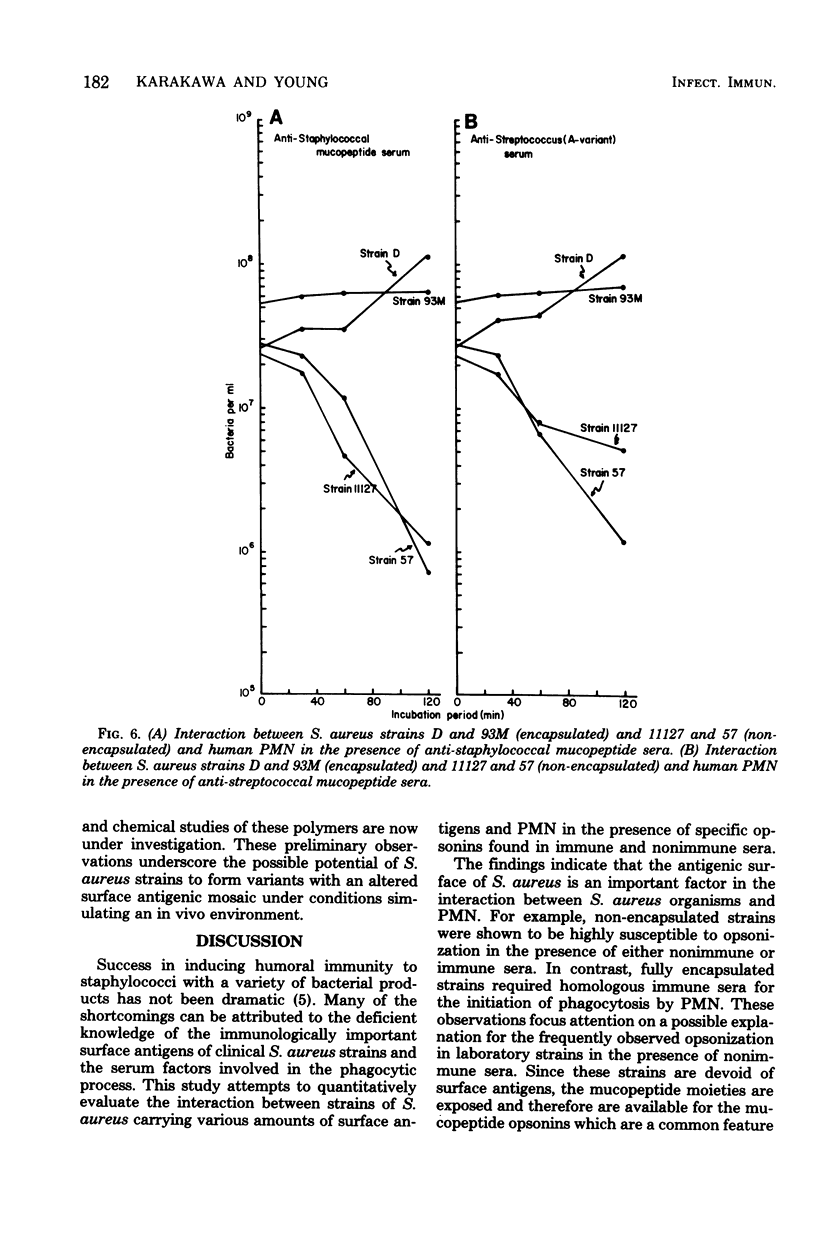
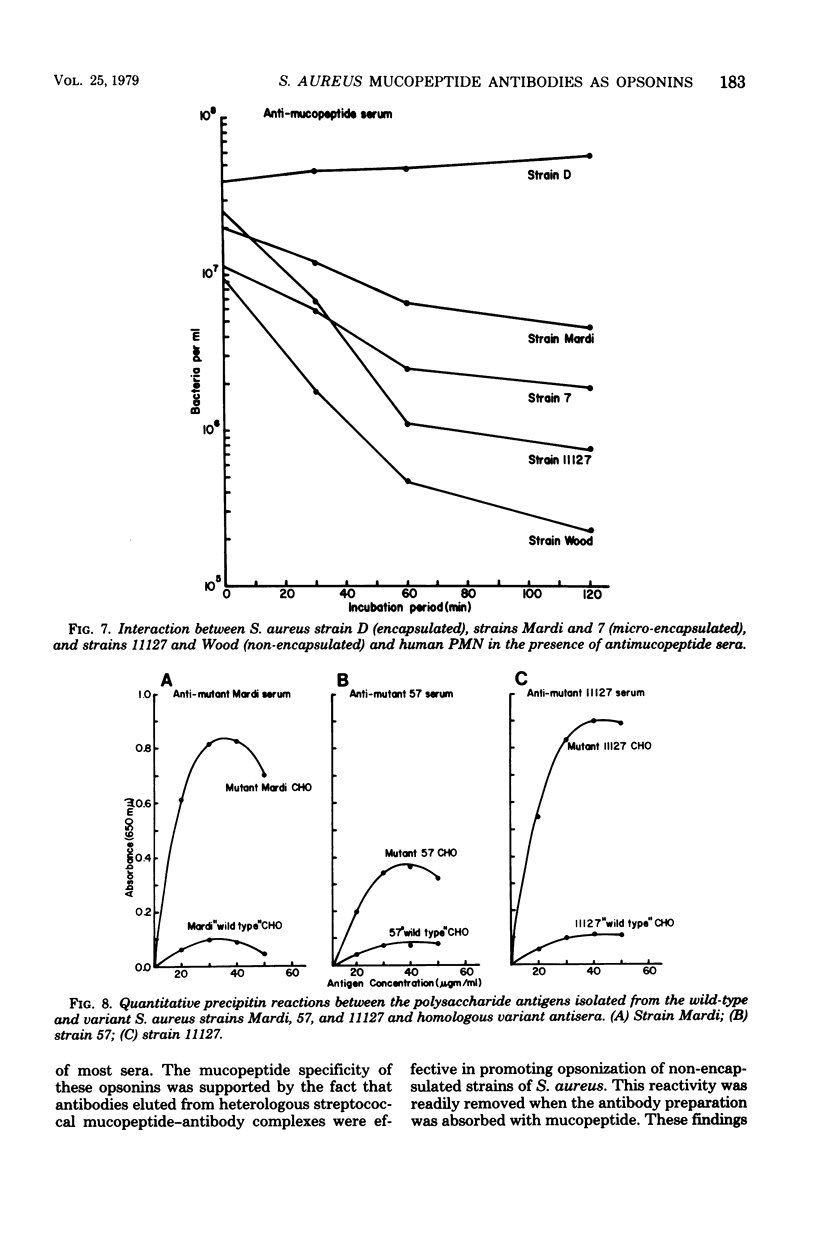
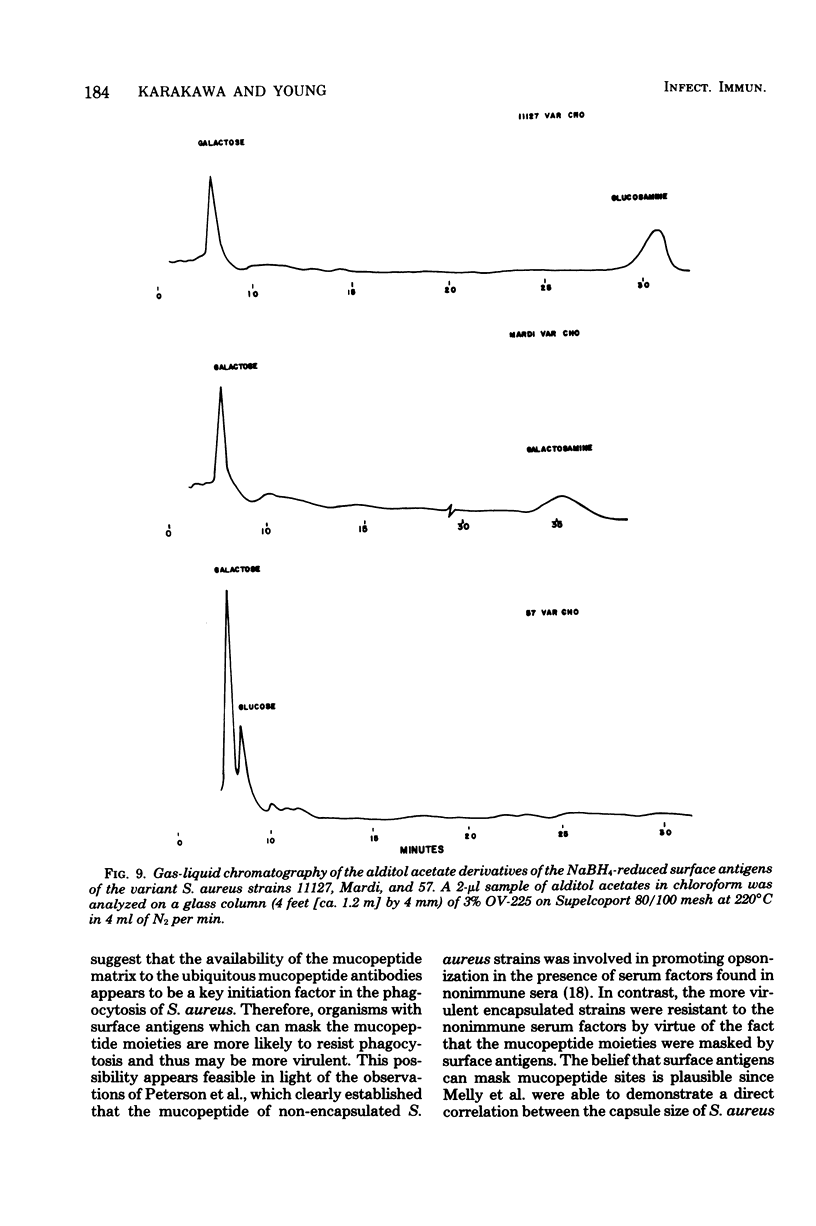
The in vitro interactions between strains of Staphylococcus aureus and human polymorphonuclear leukocytes in the presence of immune and nonimmune sera were studied. Evidence indicated that phagocytosis of encapsulated strains occurred in the presence of specific homologous antiserum, whereas non-encapsulated strains were readily phagocytized by polymorphonuclear leukocytes in the presence of both normal and immune sera. Immunological analyses demonstrated that normal serum opsonins, which reacted with the non-encapsulated strains, were specifically directed against exposed mucopeptide moieties of the organisms. Sera rich in antimucopeptide antibodies were obtained from rabbits immunized with heterologous bacteria such as Escherichia coli and group A-variant streptococci and were shown to be effective in opsonizing the non-encapsulated strains of S. aureus. Fresh clinical isolates of S. aureus were noticeably more resistant to the opsonizing effects of the antimucopeptide antibodies. Results were presented which suggest that the surface structures of these clinical isolates are more diverse than laboratory-propagated strains and that these antiphagocytic surface antigens may be significant factors in masking the opsonizing effects of the mucopeptide opsonins which are present in most sera.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ekstedt R. D. Immune response to surface antigens of Staphylococcus aureus and their role in resistance to staphylococcal disease. Ann N Y Acad Sci. 1974 Jul 31;236(0):203–220. doi: 10.1111/j.1749-6632.1974.tb41492.x. [DOI] [PubMed] [Google Scholar]
- Fraser B. A., Mallette M. F. An improved isolation method and new composition data for Forssman hapten from sheep erythrocytes. Immunochemistry. 1973 Nov;10(11):745–753. doi: 10.1016/0019-2791(73)90176-6. [DOI] [PubMed] [Google Scholar]
- Greenberg L. Staphylococcus vaccines. Bull N Y Acad Med. 1968 Oct;44(10):1222–1226. [PMC free article] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. A., Karakawa W. W. Multiple polysaccharide antigens of group B streptococcus, type Ia: emphasis on a sialic acid type-specific polysaccharide. J Immunol. 1977 Jun;118(6):2155–2160. [PubMed] [Google Scholar]
- Karakawa W. W., Braun D. G., Lackland H., Krause R. M. Immunochemical studies on the cross-reactivity between streptococcal and staphylococcal mucopeptide. J Exp Med. 1968 Aug 1;128(2):325–340. doi: 10.1084/jem.128.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Kane J. A. Immunochemical analysis of a Smith-like antigen isolated from two human strains of Staphylococcus aureus. J Immunol. 1975 Aug;115(2):564–568. [PubMed] [Google Scholar]
- Karakawa W. W., Young D. A., Kane J. A. Structural analysis of the cellular constituents of a fresh clinical isolate of Staphylococcus aureus, and their role in the interaction between the organisms and polymorphonuclear leukocytes in the presence of serum factors. Infect Immun. 1978 Aug;21(2):496–505. doi: 10.1128/iai.21.2.496-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M. G., Melly M. A. The importance of surface antigens in staphylococcal virulence. Ann N Y Acad Sci. 1965 Jul 23;128(1):231–250. doi: 10.1111/j.1749-6632.1965.tb11641.x. [DOI] [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly M. A., Duke L. J., Liau D. F., Hash J. H. Biological properties of the encapsulated Staphylococcus aureus M. Infect Immun. 1974 Aug;10(2):389–397. doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Shaw D. R., Chatterjee A. N., Mirelman D., Wu T. Mutants of staphylococci with altered cell walls. Ann N Y Acad Sci. 1974 Jul 31;236(0):54–62. doi: 10.1111/j.1749-6632.1974.tb41481.x. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B. The relationship between group A and group C meningococcal polysaccharides and serum opsonins in man. J Exp Med. 1970 Mar 1;131(3):499–513. doi: 10.1084/jem.131.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. E., Melly M. A. Speculations on the immunology of staphylococcal infections. Ann N Y Acad Sci. 1965 Jul 23;128(1):274–284. doi: 10.1111/j.1749-6632.1965.tb11644.x. [DOI] [PubMed] [Google Scholar]
- Shayegani M. Failure of Immune Sera to Enhance Significantly Phagocytosis of Staphylococus aureus: Nonspecific Adsorption of Phagocytosis-Promoting Factors. Infect Immun. 1970 Dec;2(6):742–749. doi: 10.1128/iai.2.6.742-749.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L., Ensign J. C. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. VII. Mode of action of the bacteriolytic peptidase from Myxobacter and the isolation of intact cell wall polysaccharides. Biochemistry. 1967 Mar;6(3):906–920. doi: 10.1021/bi00855a035. [DOI] [PubMed] [Google Scholar]
- Weber P., Winzler R. J. Determination of hexosaminitols by ion-exchange chromatography and its application to alkali-labile glycosidic linkages in glycoproteins. Arch Biochem Biophys. 1969 Feb;129(2):534–538. doi: 10.1016/0003-9861(69)90211-2. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Smith M. R., Naito Y. Biological and Immunological Properties of Encapsulated Strains of Staphylococcus aureus from Human Sources. Infect Immun. 1970 Nov;2(5):528–532. doi: 10.1128/iai.2.5.528-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


