Abstract
Oncologists now favor more personalized treatment strategies in breast cancer patients. Gene expression analysis has been widely used, but less is known about epigenetic factors, for example, microRNAs (miRNAs). The aim of this study was to determine the relationship between selected miRNAs and receptor status in core biopsies sampled before preoperative chemotherapy in stage III locally advanced breast cancer (LABC) patients. In 37 LABC core biopsies, three miRNAs per sample were analyzed: hsa-miR-93-5p, hsa-miR-190a, and hsa-miR-200b-3p, and hsa-miR-103a-3p as an endogenous control (TaqMan® RT-PCR; Applied Biosystems). Receptor status was determined by a dedicated pathologist. The Mann–Whitney U, Shapiro–Wilk, and Levene's tests were used to compare related samples. Levels of miRNA-93 differed significantly in core biopsies of LABC patients with different expressions of ER (estrogen receptor) and PR (progesterone receptor). Higher levels of miRNA-93 were found in ER-negative (p=0.0027) and PR-negative patients (p=0.0185). Levels of miRNA-190 and 200b did not differ significantly in core biopsies of LABC patients who expressed ER and PR differently (p=0.7727, p=0.9434, p=0.6213, and p=0.1717). Levels of miRNA-93, 190, and 200b were not significantly different in core biopsies of LABC patients with different HER2 (human epidermal growth factor 2) expressions (p=0.8013, p=0.2609, and p=0.3222). The assessment of core biopsy miRNA profiles and receptor-based subtypes may identify new signaling pathways for improved breast cancer classification.
Introduction
Breast cancer is a heterogeneous disease encompassing a broad spectrum of entities, with variable prognosis, treatment options, and outcomes (Kaufmann et al., 2013; Mayer et al., 2014). Over time, understanding of the underlying disease mechanisms has evolved, beginning as a simple clinical description in Egyptian papyri in 1550 BC (Saber, 2010), through to pioneering research on estrogen dependency in the late 19th century (Schinzinger, 1889; Beatson, 1896), the discovery of estrogen receptors (ER) (Jensen and Jacobsen, 1960), the introduction of progesterone receptors (PR) (Horwitz and McGuire, 1975), human epidermal growth factor 2 (HER2) (Hudziak et al., 1987; Natali et al., 1990), identification of BRCA1 (breast cancer type 1 susceptibility protein) in the early 1990s (Black and Solomon, 1993; Rowell et al., 1994), and in recent years (1999/2000), cDNA microarray-based intrinsic classifications of molecular subtypes—luminal, basal like, HER2 enriched, or normal like (Perou et al., 1999, 2000), and the development of high-throughput gene expression platforms (Harbeck et al., 2014).
The key to understanding the heterogeneity of breast cancer is to decipher the underlying complex molecular interactions (Perou et al., 1999; Kaufmann et al., 2013; Harbeck et al., 2014; Mayer et al., 2014). Breast cancer-related genes have been extensively investigated (Ellis and Perou, 2013), but much less is known about epigenetic alterations such as microRNAs (miRNAs), DNA methylation, or histone modifications (Lehmann, 2014; Li et al., 2014; McDermott et al., 2014). MiRNAs are single-stranded, small noncoding RNAs, ∼18–25 nucleotides in length that potentially may modify up to one-third of the human genome (Fang et al., 2012; Li et al., 2014; McDermott et al., 2014). They were first identified over a decade ago and in February 2014, a total of 2578 human miRNAs were registered in mirbase.org (www.mirbase.org). It has recently been recognized that deregulation of certain miRNAs is associated with carcinogenesis by means of oncogenes, while others act as tumor suppressors (Fang et al., 2012; Li et al., 2014; McDermott et al., 2014). It has also been speculated that certain miRNAs perform different functions in early and advanced stages of cancer (Li et al., 2014). There is increasing evidence to suggest that miRNAs may be responsible for a large proportion of breast cancer heterogeneity (Li et al., 2014). The question is whether selected miRNAs may act as adjuncts to well-established ER, PR, and HER2 receptor-based classification.
Aim
The aim of the study was to examine the associations between expression levels of selected miRNAs and receptor status in core biopsies sampled before preoperative chemotherapy in patients with stage III locally advanced breast cancer (LABC).
Materials and Methods
The study was conducted under the Institutional Review Board protocol no. RNN/226/11/KE/13/12/2011, Medical University of Lodz. All patients provided written informed consent. Before treatment, ultrasound-guided 14-gauge core needle biopsies using an ultra-automatic biopsy instrument (Pro-Mag TM; Angiotech) were collected from 37 white female patients with LABC (stage III), aged between 31 and 81, median 54.44 years, and from five healthy control patients who had breast reduction procedures when normal breast tissue was sampled intraoperatively at the Cancer Center between December 2011 and April 2012. Four to five tissue samples were obtained from each lesion. Half were frozen immediately at −80°C for subsequent miRNA profiling. The remaining samples were paraffin embedded, stained, and reviewed by specialist breast pathologists in the Department of Cancer Pathology. The ER and PR status was determined by immunohistochemistry (IHC) using the Allred score.
The human epidermal growth factor receptor 2 (HER2) status was evaluated by IHC or by fluorescence in situ hybridization (FISH). Samples were considered ER/PR positive if more than 1% of the tumor cells were immunoreactive. Samples were considered HER2 positive with IHC 3+ staining or with a score of 2+ and HER2 gene amplification when tested by FISH. TNM clinical staging was assessed by mammography, ultrasound of the breast, axilla, and abdomen, bone scan, and chest X-ray or CT scan. In selected cases at the surgeon's discretion, MRI of the breast was performed. Preoperative chemotherapy with anthracycline and taxane regimens in standard dosage was used.
Total RNA isolation
Total RNA was extracted using the mirVana™ miRNA isolation kit (Ambion) according to the manufacturer's instruction. Frozen samples were homogenized in 300 μL of Lysis/Binding Solution using a TissueRuptor homogenizer (Qiagen). RNA was eluted in 100 μL RNase-free water and quantified using the PicoDrop spectrophotometer. The quality of RNA samples was analyzed by measuring the ratio of absorptions at 260/280 nm. The purified total RNA was immediately used for cDNA synthesis or stored at −80°C.
miRNA expression
Reverse transcription was carried out on 10 ng of total RNA in 15 μL reactions using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's instruction. miRNA quantification was performed using standard TaqMan MicroRNA Assays (Applied Biosystems): hsa-miR-93 (Assay ID 000432), hsa-miR-190 (Assay ID 000489), hsa-miR-200b (Assay ID 002251), and hsa-miR-103 (Assay ID 000439) as an endogenous control. These particular miRNAs were selected on the basis of previous reports in the literature, the miRNA database, and communications from breast cancer conferences. They represent a broad range of epigenetic pathways involved in migration, invasion, receptor status, epithelial-to-mesenchymal transition, cancer dormancy, switch to the fast growing phenotype, drug resistance, etc (Blenkiron et al., 2007; Lowery et al., 2008; Fang et al., 2012; Liu et al., 2012; Manavalan et al., 2013; Lehmann, 2014; Li et al., 2014; McDermott et al., 2014; www.mirbase.org). We also used unpublished data of TaqMan Low Density Arrays from our previous research. The 20 μL PCR included 1.33 μL RT product, 10 μL TaqMan Universal PCR Master Mix, and 1 μL TaqMan miRNA Assay (20×). These reactions were incubated in a 96-well plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. All reactions were run in triplicate.
Real-time PCR analysis
TaqMan PCR assays were performed on 7900HT Fast Real-Time PCR System (Applied Biosystems) and analyzed using the Sequence Detection System 2.3 Software. Fold induction values (RQ) were calculated according to the equation 2−ΔΔCt, where ΔCt represents the differences in cycle threshold numbers between the target gene and endogenous control, and ΔΔCt represents the relative change in these differences between examined and control groups.
Statistical analysis
Continuous variables between two groups were compared using the Mann–Whitney U test or Student's t-test adequately to variable distribution and homogeneity of variances. Normal distribution fitting was assessed with the usage of Shapiro–Wilk test, and homoscedasticity was assessed with Levene's test.
Results
Patients enrolled in the study were aged between 31 and 81, median 54.44 years. Histopathological tumor types were as follows: no special type (invasive ductal) breast cancer in 34 patients, mucinous in 1, metaplastic in 1, and lobular in 1 patient. The tumor grade was G2 in 8 patients and G3 in 29 patients. The tumor stage was III in all patients. The receptor status was ER positive in 22, ER negative in 15, PR positive in 14, PR negative in 23, HER2 positive in 12, and HER2 negative in 25 patients (Table 1).
Table 1.
Patient Characteristics
| No. | Sex | Race | Age | Histopathology | Stage | ER | PR | HER2 | RQmiR93 | RQmiR190 | RQmiR200b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | W | 64 | NST | III | pos | pos | neg | 1.034 | 0.238 | 0.268 |
| 2 | F | W | 56 | NST | III | pos | neg | neg | 1.252 | 0.424 | 1.898 |
| 3 | F | W | 51 | NST | III | pos | pos | neg | 1.526 | 1.518 | 0.150 |
| 4 | F | W | 61 | NST | III | pos | neg | pos | 1.932 | 0.305 | 1.037 |
| 5 | F | W | 47 | NST | III | neg | neg | neg | 4.836 | 0.160 | 1.303 |
| 6 | F | W | 38 | NST | III | pos | pos | pos | 1.690 | 0.470 | 1.166 |
| 7 | F | W | 52 | NST | III | neg | neg | pos | 2.372 | 0.627 | 0.232 |
| 8 | F | W | 52 | NST | III | neg | neg | pos | 2.199 | 0.634 | 0.348 |
| 9 | F | W | 66 | NST | III | pos | pos | neg | 1.204 | 0.553 | 0.811 |
| 10 | F | W | 68 | NST | III | pos | neg | neg | 0.894 | 0.308 | 0.612 |
| 11 | F | W | 47 | NST | III | neg | neg | neg | 0.748 | 0.609 | 0.522 |
| 12 | F | W | 34 | NST | III | pos | pos | pos | 0.963 | 0.395 | 0.554 |
| 13 | F | W | 55 | NST | III | neg | neg | neg | 3.997 | 0.459 | 0.742 |
| 14 | F | W | 55 | NST | III | neg | neg | neg | 0.843 | 0.274 | 0.624 |
| 15 | F | W | 48 | NST | III | pos | pos | pos | 0.684 | 0.491 | 0.571 |
| 16 | F | W | 42 | NST | III | pos | pos | neg | 0.860 | 0.360 | 0.717 |
| 17 | F | W | 43 | NST | III | pos | neg | pos | 1.252 | 0.280 | 1.303 |
| 18 | F | W | 81 | NST | III | neg | neg | neg | 2.245 | 0.374 | 0.685 |
| 19 | F | W | 49 | NST | III | pos | neg | neg | 1.058 | 0.179 | 0.798 |
| 20 | F | W | 72 | NST | III | pos | pos | neg | 1.146 | 0.290 | 0.801 |
| 21 | F | W | 52 | NST | III | pos | pos | pos | 2.323 | 1.556 | 0.249 |
| 22 | F | W | 66 | muc | III | pos | neg | pos | 1.546 | 1.207 | 0.621 |
| 23 | F | W | 42 | NST | III | pos | pos | neg | 1.774 | 0.210 | 0.844 |
| 24 | F | W | 36 | NST | III | neg | neg | neg | 3.683 | 0.264 | 0.769 |
| 25 | F | W | 52 | metapl | III | pos | neg | neg | 1.482 | 0.293 | 0.910 |
| 26 | F | W | 55 | NST | III | neg | neg | neg | 8.791 | 1.324 | 1.680 |
| 27 | F | W | 80 | NST | III | neg | neg | neg | 1.990 | 0.527 | 0.881 |
| 28 | F | W | 66 | NST | III | neg | neg | neg | 1.380 | 0.197 | 0.390 |
| 29 | F | W | 67 | lob | III | pos | pos | neg | 0.518 | 0.310 | 0.401 |
| 30 | F | W | 51 | NST | III | pos | neg | pos | 1.188 | 0.749 | 0.849 |
| 31 | F | W | 44 | NST | III | neg | neg | neg | 2.911 | 0.520 | 1.738 |
| 32 | F | W | 68 | NST | III | pos | pos | pos | 0.767 | 0.274 | 0.386 |
| 33 | F | W | 31 | NST | III | neg | neg | neg | 1.084 | 0.489 | 0.471 |
| 34 | F | W | 48 | NST | III | pos | pos | neg | 1.540 | 0.632 | 0.982 |
| 35 | F | W | 69 | NST | III | pos | pos | neg | 0.831 | 0.500 | 0.497 |
| 36 | F | W | 45 | NST | III | neg | neg | neg | 1.587 | 1.264 | 0.563 |
| 37 | F | W | 58 | NST | III | neg | neg | pos | 2.534 | 0.316 | 0.744 |
F, female; lob, lobular; metapl, metaplastic; muc, mucinous; neg, negative; NST, ductal invasive, no special type; pos, positive; RQ, relative quotient; W, white.
Statistical analysis showed that
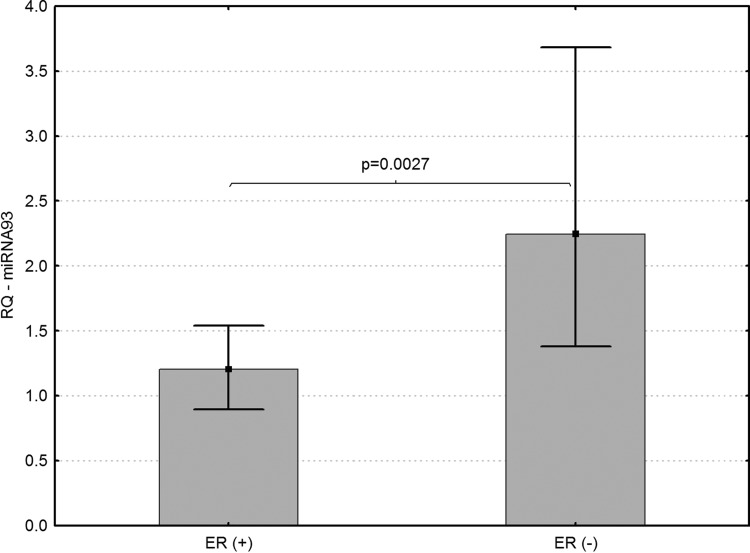
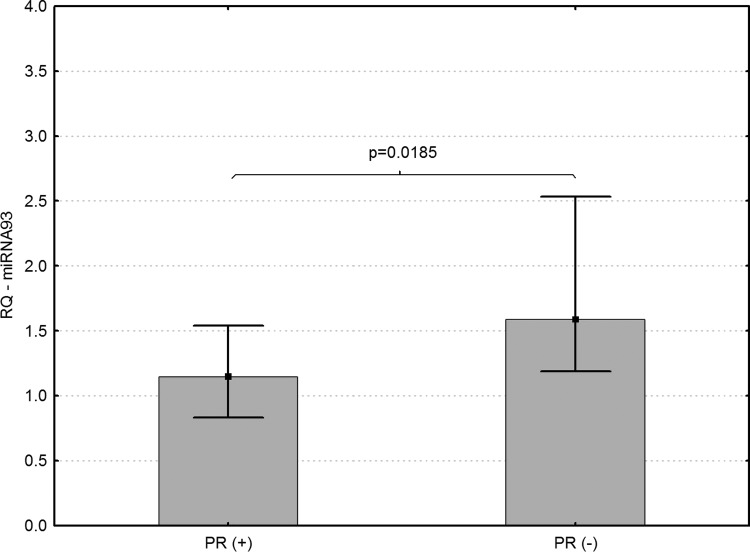
(1) Levels of miRNA-93 differed significantly in core biopsies of LABC patients with different expression of ER and PR. Higher levels of miRNA-93 were found in ER-negative (p=0.0027) and PR-negative breast cancer patients (p=0.0185) (Figs. 1 and 2).
(2) Levels of miRNA-190 and 200b did not differ significantly in core biopsies of LABC patients with different expressions of ER (p=0.7727 and p=0.9434, respectively) and PR (p=0.6213 and p=0.1717, respectively).
(3) Levels of miRNA-93, 190, and 200b did not differ significantly in core biopsies of LABC patients who had different expressions of HER2 (p=0.8013, p=0.2609, and p=0.3222, respectively).
FIG. 1.
Association of miRNA-93 expression and ER levels in core biopsies sampled from patients with locally advanced breast cancer (LABC) (p=0.0027).
FIG. 2.
Association of miRNA-93 expression and PR levels in core biopsies sampled from patients with LABC (p=0.0185).
Discussion and Conclusions
miRNA-93 and ER, PR expression
ER, PR, and HER2 are well established and useful predictive factors in breast cancer (Schinzinger, 1889; Beatson, 1896; Jensen and Jacobson, 1960; Horwitz and McGuire, 1975; Hudziak et al., 1987; Natali et al., 1990). Additional genetic and epigenetic biomarkers are needed because of cancer heterogeneity (Ellis and Perou, 2013; Harbeck et al., 2014; Li et al., 2014). In the present study we analyzed miRNAs that are examples of epigenetic factors. Statistical analysis showed that miRNA-93 levels differed significantly in core biopsies of groups of patients with different ER and PR expression. Higher levels of this particular miRNA were found in ER- and PR-negative cohorts (p=0.0027 and p=0.0185, respectively). Fang et al. (2012) demonstrated higher expression of miRNA-93 in MT-1 human breast carcinoma cell lines with enhanced angiogenesis and in a lung metastasis mouse model. Furthermore, the same authors showed that higher levels of miRNA-93 were present in tumors with strong Ki-67 immunoreactivity, consistent with extensive cell proliferation.
These observations concord with our own human clinical model, because ER- and PR-negative breast cancers lead a more aggressive clinical course with greater adverse outcomes.
Fang et al. (2012) showed in breast cancer cell lines that the MCF-7 cells expressed significantly lower levels of miRNA-93 than MB-468 and MB-231. MCF-7 cells are characterized by positive ER and PR expression, negative HER2 amplification, and a luminal epithelial phenotype; MB-468 cells have no ER; and MB-231 cells exhibit cancer stem cell-like properties (Fang et al., 2012). This is in agreement with our own results, whereby ER- and PR-positive tumors were characterized by the lowest levels of miRNA-93. Blenkiron et al. (2007) revealed striking differences in miRNA expression between ER-negative and ER-positive tumors. MiRNA-93 was among those miRNAs correlating with tumor subtype (p<0.05), ER status (p<0.01), and grade (p<0.001).
Liu et al. (2012) using a series of breast cancer cell lines representing different stages of differentiation and mouse xenograft models, showed convincingly that miRNA-93 modulates the fate of breast cancer stem cells (BCSCs). Recent evidence suggests that BCSCs may contribute to tumor metastasis, treatment resistance, and relapse. The effect of miRNA-93 on the BCSC population is dependent on the cellular differentiation status. Specifically, this miRNA-93 increases BCSCs in MCF-7 cells with a better differentiated luminal ER-positive phenotype. Conversely, in claudin low SUM159 cells, expression of miRNA-93 induces mesenchymal-to-epithelial transition, resulting in BCSC depletion (Liu et al., 2012). In our study, although we did not analyze miRNA-93 levels in the context of BCSCs, the receptor status is a surrogate for intrinsic subtypes.
We used two software TargetScan Human 6.2 (www.targetscan.org) and miRTarBase (www.mirtarbase.mbc.nctu.edu.tw) to identify target genes of miRNA-93. ER was a target gene of miRNA-93 according to TargetScan. We did not find such an association between PR and miRNA-93 in these bioinformatics programs.
In summary, in-depth analysis of data from cell lines, mouse and human clinical studies produced further evidence that miRNA-93 seems to be a therapeutic target in breast cancer although these observations need to be validated (Blenkiron et al., 2007; Fang et al., 2012; Liu et al., 2012).
miRNA-190, miRNA-200b, and ER, PR, and HER2 expression
In this study, we failed to demonstrate statistically significant differences in miRNA-190 and 200b levels in core biopsies of breast cancer patients with a variety of ER, PR, and HER2 expression. In the literature, there is a paucity of data of associations among these particular miRNAs with receptor subtypes. Lowery et al. (2008) have demonstrated an association between miRNA-190 expression and ER status, but these studies were conducted on 29 early breast cancer specimens, in contrast to our own study examining more advanced stages of the disease. Lowery et al. (2008) showed using artificial neural network analysis that miRNA signatures were associated with ER, PR, and HER2 status.
Even though we performed our analysis at a different stage of breast cancer (stage III) in comparison with Lowery et al.'s study (early breast cancer, stage I and II) and there were discrepancies in miRNA-190 results, our conclusions clearly support the hypothesis that the precise regulation of hormones (ER and PR) and HER2 receptors, vitally important in the current classification and management of breast cancer, is unknown. Therefore, identification of miRNAs associated with these receptors will add to the characterization of such a heterogeneous disease.
Manavalan et al. (2013) demonstrated that lower expression of the miRNA-200 family contributes to endocrine resistance in LY2 human breast cancer cells. Peurala et al. (2011), as in our own research, included ER, PR, and HER2 levels in their analysis, although they used miRNA-34a as a biomarker. The latter authors noted higher levels of miRNA-34a in ER-negative and HER2-positive tumors in a large clinical series of breast tumor samples. Anfossi et al. (2014) demonstrated higher expression of miRNA-21 in the serum of nonmetastatic HER2-positive breast cancer patients compared with nonmetastatic HER2-negative disease. They found higher median serum miRNA-10b levels in metastatic HER2-positive cancers compared with metastatic HER2-negative disease. Furthermore, higher miRNA-19a levels were found in inflammatory breast cancer (IBC).
Higher expression of miRNA-19a was associated with a better outcome in this very aggressive form of breast cancer (Anfossi et al., 2014). Our research in locally advanced cancer, including IBC, found no statistically significant link between miRNA-190 and 200b.
Almog et al. (2012, 2013) emphasized the role of miRNA-190 in the reversal of fast-growing angiogenic tumors toward prolonged tumor dormancy. Pecot et al. (2013) and Li et al. (2013) emphasized the critical role of miRNA-200 in regulating epithelial-to-mesenchymal transition, which are key processes in invasion, progression, and metastasis. Lim et al. (2013), in a study of pleural effusion or ascites samples in stage IV breast cancer patients, added to these observations and demonstrated the involvement of the miRNA-200 family in the process of nonstem to a stem-like phenotypic conversion.
In conclusion, we have demonstrated that miRNA-93 can distinguish between ER- and PR-positive and ER- and PR-negative breast cancers and may act as an adjunct to well-established receptor-based classifications. Although these results were from a small-sample cohort, they provide an important basis for larger, prospective multicenter studies to investigate the potential role of miRNAs in breast cancer (Dvinge et al., 2013).
Acknowledgments
The study was supported by grants 2011/01/B/NZ4/03345 and 2012/05/B/NZ5/01852 from the National Center of Science.
Disclosure Statement
No competing financial interests exist.
References
- Almog N., Briggs C., Beheshti A., Ma L., Wilkie K.P., Vajkoczy P., Folkman J., Hlatky L., and Abdollahi A. (2013). Transcriptional changes induced by the tumor dormancy-asociated microRNA-190. Transcription 1,4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog N., Ma L., Schwager C., Brinkmann B.G., Beheshti A., Rietman E., and Hlatky L. (2012). Consensus microRNAs governing the switch of dormant tumors to the fast-growing angiogenic phenotype. PLoS One 7,e44001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfossi S., Giordano A., Gao H., Cohen E.N., Tin S., Wu Q., Garza R.J., Debeb B.G., Alvarez R.H., Valero V., Hortobagyi G.N., Callin G.A., Ueno N.T., Woodward W.A., and Reuben J.M. (2014). High serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+ inflammatory breast cancer. PLoS One 9,e83113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatson E. (1896). On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet 2,104–107 [PMC free article] [PubMed] [Google Scholar]
- Black D.M., and Solomon E. (1993). The search for familial breast and ovarian cancer gene. Trends Genet 9,22–26 [DOI] [PubMed] [Google Scholar]
- Blenkiron C., Goldstein L.D., Thorne N.P., Spiteri I., Chin S.F., Dunning M.J., Barbarosa-Morais N.L., Teschendorff A.E., Green A.R., Ellis I.O., Tavare S., Caldas C., and Miska E.A. (2007). MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol 8,R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvinge H., Git A., Graf S., Salmon-Divon M., Curtis S., Sottoriva A., Zhao Y., Hirst M., Armisen J., Miska E.A., Chin S.F., Provenzano E., Turashvili G., Green A., Ellis I., Aparicio S., and Caldas C. (2013). The shaping and functional consequences of the microRNA landscape in breast cancer. Nature 497,378–382 [DOI] [PubMed] [Google Scholar]
- Ellis M.J., and Perou C.M. (2013). The genomic landscape of breast cancer as a therapeutic roadmap. Cancer Discov 3,27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Du W.W., Yang W., Rutnam Z.J., Peng C., Li H., O'Malley Y.Q., Askeland R.W., Suggs S., Liu M., Mehta T., Deng Z., and Yang B.B. (2012). MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle 11,4352–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck N., Sotlar K., Wuerstlein R., and Doisneau-Sixou S. (2014). Molecular and protein markers for clinical decision making in breast cancer. Cancer Treat Rev 40,434–444 [DOI] [PubMed] [Google Scholar]
- Horwitz K.B., and McGuire W.L. (1975). Specific progesterone receptors in human breast cancer. Steroids 25,497–505 [DOI] [PubMed] [Google Scholar]
- Hudziak R.M., Schlessinger J., and Ullrich A. (1987). Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 353 cells. Proc Natl Acad Sci U S A 84,7159–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E.V., and Jacobson H.I. (1960). Fate of steroid estrogens in target tissues. In Biological Activities of Steroids in Relations to Cancer. Pincus G., and Vollmer E.P., eds. (Academic Press, New York: ), pp. 161–174 [Google Scholar]
- Kaufmann M., von Minckwitz G., Berg J., Conte P.F., Darby S., Eiermam W., Howell A., Kiechle M., Mauri D., Senn H.J., Viale G., and Loibl S. (2013). Breakthroughs in research and treatment of early breast cancer: an overview of the last three decades. Arch Gynecol Obstet 288,1203–1212 [DOI] [PubMed] [Google Scholar]
- Lehmann U. (2014). Aberrant DNA methylation of microRNA genes in human breast cancer- a critical appraisal. Cell Tissue Res [Epub ahead of print]; DOI: 10.1007/s00441-014-1793-0 [DOI] [PubMed] [Google Scholar]
- Li L., Liu C., Wang F., Miao W., Zhang J., Kang Z., Chen Y., and Peng L. (2014). Unraveling the hidden heterogeneities of breast cancer based on functional miRNA cluster. PLoS One 9,e87601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Roslan S., Johnstone C.N., Wright J.A., Bracken C.P., Anderson M., Bert A.G., Selth L.A., Anderson R.L., Goodall G.J., Gregory P.A., and Khew-Goodall Y. (2013). MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathway. Oncogene [Epub ahead of print]; DOI: 10.1038/onc.2013.370 [DOI] [PubMed] [Google Scholar]
- Lim Y.Y., Wright J.A., Attema J.L., Gregory P.A., Bert A.G., Smith E., Thomas D., Lopez A.F., Drew P.A., Khew-Goodall Y., and Goodall G.J. (2013). Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci 126,2256–2266 [DOI] [PubMed] [Google Scholar]
- Liu S., Patel S.H., Ginestier C., Ibarra I., Martin-Trevino R., Bai S., McDermott S.P., Shang L., Ke J., Ou S.J., Heath A., Zhang K.J., Korkaya H., Clouthier S.G., Charafe-Jauffret E., Birnbaum D., Hannon G.J., and Wicha M.S. (2012). MicroRNA93 regulates proliferation and differentiation of normal and malignant breast stem cells. PLoS Genet 8,e1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery A.J., Miller N., Devaney A., McNeill R.E., Davoren P.A., Lemetre C., Benes V., Schmidt S., Blake J., Ball G., and Kerin M.J. (2008). Breast Cancer Res 11,R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan T.T., Teng Y., Litchfield L.M., Muluhngwi P., Al.-Rayyan N., and Klinge C.M. (2013). Reduced expression of miR-200 family members contributes to antiestrogen resistance in LY2 human breast cancer cells. PLoS One 8,e62334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer I.A., Abramson V.G., Lehmann B.D., and Pietenpol I.A. (2014). New strategies for triple-negative breast cancer-deciphering the heterogeneity. Clin Cancer Res 20,782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A.M., Miller N., Wall D., Martyn L.M., Ball G., Sweeney K.J., and Kerin M.J. (2014). Identification and validation of oncologic miRNA biomarkers for luminal A-like breast cancer. PLoS One 9,e87032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P.G., Nicotra M.R., Bigotti A., Venturo I., Slamon D.J., Fendly B.M., and Ullrich A. (1990). Expression of the p185 encoded by HER2 oncogene in normal and transformed human tissues. Int J Cancer 45,457–461 [DOI] [PubMed] [Google Scholar]
- Pecot C.V., Rupaimoole R., Yang D., Akbani R., Ivan C., Lu C., Wu S., Han H.D., Shah M.Y., Rodriguez-Aguayo C., Bottsford-Miller J., Liu Y., Kim S.B., et al. (2013). Tumor angiogenesis regulation by the miR-200 family. Nat Commun 4,2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou C.M., Jeffrey S.S., van de Rijn M., Rees C.A., Eisen M.B., Ross D.T., Pergamenschikov A., Williams C.F., Zhu S.X., Lee J.C., Lashkari D., Shalon D., Brown P.O., and Bolstein D. (1999). Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci U S A 96,9212–9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou C.M., Sorlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., Fluge O., Pergamenschikov A., Williams C., Zhu S.X., Lonning P.E., Borrensen-Dale A.L., Brown P.O., and Bolstein D. (2000). Molecular portraits of human breast tumors. Nature 406,747–752 [DOI] [PubMed] [Google Scholar]
- Peurala H., Greco D., Heikkinen T., Kaur S., Bartkova J., Jamshidi M., Aittomaki K., Heikkila P., Bartek J., Blomqvist C., Butzow R., and Nevanlinna H. (2011). MiR-34a expression has an effect for lower risk of metastasis and associates with expression patterns predicting clinical outcome in breast cancer. PLoS One 6,e26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell S., Newman B., Boyd I., and King M.C. (1994). Inherited predisposition to breast and ovarian cancer. Am J Hum Genet 55,861–865 [PMC free article] [PubMed] [Google Scholar]
- Saber A. (2010). Ancient Egyptian surgical heritage. J Invest Surg 23,327–334 [DOI] [PubMed] [Google Scholar]
- Schinzinger A. (1889). Über carcinoma mammae. Vehr Dtsch Ges Chir 18,28–29 [Google Scholar]