Abstract
Obstructive sleep apnea (OSA) has emerged as a new and important risk factor for cardiovascular disease (CVD). Over the last decade, epidemiological and clinical research has consistently supported the association of OSA with increased CV morbidity and mortality. Such evidence prompted the American Heart Association to issue a Scientific Statement describing the need to recognize OSA as an important target for therapy in reducing CV risk. Emerging facts suggest that marked racial differences exist in the association of OSA with CVD. While both conditions are more prevalent in blacks, almost all NIH-funded research projects evaluating the relationship between OSA and CV risk have been conducted in predominantly non-black populations. There is an urgent need for research studies investigating the CV impact of OSA among high-risk minorities, especially blacks. This article first examines the evidence supporting the association between OSA and CVD and reviews the influence of ethnic/racial differences on this association. Public health implications of OSA and future directions, especially regarding minority populations, are discussed.
Keywords: Sleep apnea, Cardiovascular disease, Blacks
Introduction
Obstructive sleep apnea (OSA), the most common type of sleep disordered-breathing (SDB), is a major public health problem in the U.S.,1 particularly among blacks.2,3 It is often characterized by loud snoring, breathing interruptions, awakenings, gasping or choking, and daytime sleepiness as a result of upper-airway collapse and intermittent impairment of ventilation during sleep. Although OSA was clinically recognized as a disease approximately three decades ago, awareness of the condition outside the specialized field of sleep medicine has been slow to develop, and the majority of those affected, particularly blacks, remain undiagnosed.2,4,5 In general, OSA is common in adults, with men, older individuals, and the obese being at higher risk.4
Studies suggest that OSA is associated with hypertension14–16, cardiac arrhythmia17–20, coronary heart disease (CHD) 21,22, heart failure (HF)22,23, pulmonary hypertension24, stroke25–27, traffic accidents28,29 and increased mortality11. Cardiovascular disease (CVD), despite recent decline, remains the leading cause of death in both blacks and whites in the U.S. However, the decline is less steep for blacks who carry a substantially higher cardiac mortality burden with a higher sudden cardiac death rate and significantly lower life expectancy.12,13 Convincing evidence has unequivocally demonstrated disproportional burden of CVD in blacks with a marked racial disparities in care and outcome by race in the U.S.12,30,31
Despite these observations, large studies evaluating OSA and the CV connection have not focused on black participants. Moreover, inclusion of blacks in the majority of longitudinal sleep studies is limited2 or non-existent4 with the exception of the Jackson Heart Study32 (Table 1). In this paper, we review findings supporting associations between OSA and CVD, and we examine the influence of ethnic/racial differences on these associations. The public health implications of CV risk attributed to OSA, especially as it applies to minority populations, are also discussed. Finally, proposals for future directions are offered.
Table 1.
Population sample of major longitudinal studies on obstructive sleep apnea in the U.S.
| Major Longitudinal Studies on Sleep Apnea in the US | |||||
|---|---|---|---|---|---|
| Author | Study | Year of enrollment | Diagnostic technique for OSA | Cohort size | Population |
| Young et al. 1993.4 | Wisconsin Sleep Cohort | 1988 | In-lab polysomnography | 3,513 | White employees in Wisconsin. No record of black sample. |
| Redline et al. 1997. 2 | Cleveland Family Study | 1990 | Home polysomnography | 847 | 27% Black 73% Whites |
| O’Conor et al. 2003.35 | Sleep Heart Health Study | 1995 | Home polysomnography | 13,194 | 5% Black 77% White |
| Vgontzas et al. 2010.78 | Penn State Sleep Cohort | 1996 | In-lab polysomnography | 1,741 | <15% Black 85% White |
| Fülöp T et al. 2012. 32 | Jackson Heart Study | 2000 | None. Symptoms based | 5301 | All black |
Method
We searched MEDLINE using the index terms “sleep,” “sleep apnea,” “race,” “ethnicity,” “hypertension,” “coronary heart disease,” “heart failure,” cardiac arrhythmia,” stroke,” intermittent hypoxia,” sympathetic activity,” inflammation,” and “continuous positive airway pressure” for articles published between January 1980 and September 2012. Articles were examined and selected based on their relevance. Additional data were extracted from the bibliographies of selected articles. Priority was given to large prospective cohort studies, randomized controlled trials and experimental studies. This research was supported by funding from the NIH (R25HL105444, R01HL095799, and R01MD004113). The authors are solely responsible for the design, drafting, writing, and editing of this paper and its final contents.
Ethnic/racial differences in awareness and prevalence of OSA
The prevalence of OSA appears to vary with the severity of the disease. Using the Apnea-Hypopnea Index (AHI), AHI (5–15 per hour) indicates mild disease and AHI (16–30 per hour) is moderate disease, while AHI (>30 per hour) represents severe disease. In the general middle-aged population with daytime sleepiness as a complaint, 2–4% have at least mild SDB, with higher incidence occurring in the older group of this segment.4 These estimates appear to be similar among North Americans, European whites, and Asians, with rates between 2% and 7%.6 However, some studies have suggested that approximately 20% of adults have at least mild OSA and that 6.6% of adults have at least moderate OSA.33
Blacks, in contrast, have a higher prevalence of OSA after controlling for confounding variables.2,3,7 Specifically, blacks younger than 25 years2 and older than 65 years3 have a higher prevalence of OSA than those of other racial groups. In the Cleveland Family Study, blacks presented with OSA at a younger age than their white counterparts.2 In addition, Ancoli-Israel et al. found that blacks over age 65 were 2.5 times more likely than whites to have severe OSA.3 This finding is in accord with that of another population-based study of adult Americans ages 40–60 years, suggesting that OSA rates were much higher among members of minority groups compared to non-Hispanic whites.7 However, the multicenter Sleep Heart Health Study of over 6000 subjects failed to show a higher prevalence of OSA in blacks compared with whites after adjustment for age, sex, and BMI34.
In 1995, Ancoli-Israel et al. found a higher prevalence of symptoms associated with OSA in blacks compared to whites (Figure 1).3 This is also supported by relatively newer evidence indicating that blacks have a higher rate of daytime sleepiness and snoring (predominant symptoms of OSA) compared to whites.35,36 In the Sleep Heart Health Study involving 77% white and only 5% black participants, black men and women had significantly higher Epworth Sleepiness Scores (reflecting greater daytime sleepiness) than their non-Hispanic white counterparts.35 A prospective study of 523 subjects (55% black) showed that 33% of blacks considered snoring to be normal, compared to 20% of whites.36 In that study, bed partners of individuals of the black race/ethnicity were more likely to accept loud snoring as being normal.36 Awareness of the predominant symptom of OSA and knowledge of its clinical significance appear to be low among blacks.
Figure 1.
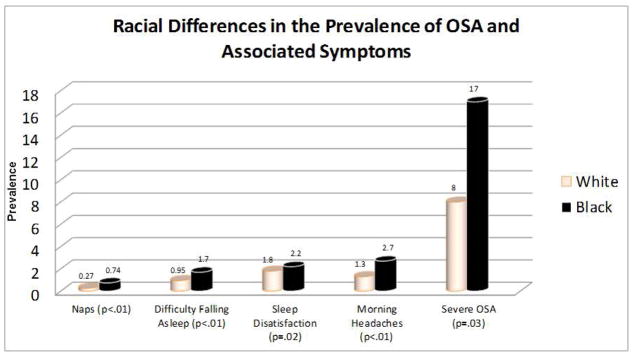
Differences in the prevalence of obstructive severe apnea (OSA) and associated symptoms between blacks and whites. Based on the average number of naps per day; number of times per week of having difficulty falling asleep; sleep satisfaction ranked as 1=best and 4=worst; number of morning headaches per month; percentage of study sample with severe OSA. Adapted from Ancoli- Israel et al.3
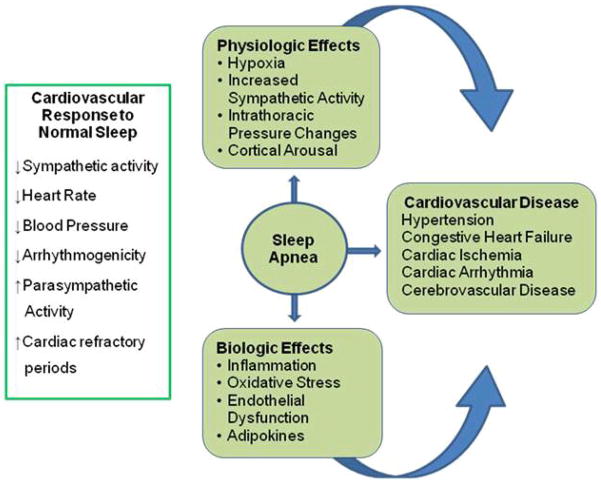
Proposed mechanisms for the link between OSA and cardiovascular disease
As depicted in Figure 2, the pathophysiological mechanisms linking OSA to CVD are complex, involving interactions among the respiratory, central nervous and cardiovascular systems. These mechanisms include increased sympathetic tone,37 changes in intrathoracic pressure, oxidative stress, and vascular inflammation resulting from the nocturnal hypoxia and reoxygenation cycles.38 Hypoxemia appears to drive most of these important pathophysiological pathways.39
Figure 2.
Diagrammatic representation of the pathophysiological link between obstructive sleep apnea and cardiovascular disease.
The repetitive desaturations cause activation of the sympathetic nervous system37–39 and produce sustained hypertension.39 A similar mechanism might explain the association between OSA and tachyarrhythmia.17 Intermittent hypoxemia and reoxygenation have also been implicated in the pathogenesis of systemic inflammation due to OSA.40,41 Animal and cellular experiments suggest that repetitive reoxygenation promotes oxidative stress through the formation of reactive oxygen species.40 This process has been implicated in the selective activation of inflammation-promoting NF-κB.41 Investigators have also shown that reactive oxygen species could activate the transcriptional activator, hypoxia-inducible factor 1 (HIF-1), which mediates the cellular effects of hypoxia, particularly during the reoxygenation period.42 These physiological pathways contribute to free-radical production, increased production of adhesion molecules, diminished vasodilator production, and endothelial injury.43
Patients with OSA have characteristically higher levels of endothelin and lower levels of nitric oxide than healthy sleepers.38,44 This elevated endothelin concentration increases peripheral vascular resistance and, consequently, blood pressure (BP). Notably, levels of endothelin and circulating nitric oxide invariably return to normal following the treatment of OSA with Continuous Positive Airway Pressure (CPAP).44 In addition, this activation pathway also affects inflammatory and immune responses by promoting the activation of endothelial cells, leukocytes, and platelets.38 Once activated, these cells express adhesion molecules and proinflammatory cytokines that may lead to endothelial injury and dysfunction, 38,43 which inevitably leads to development of atherosclerosis.
Observing this chain of events, investigators postulate that endothelial dysfunction43 starts soon after the onset of OSA, and may be the mechanism by which OSA mediates the genesis or worsening of hypertension, arrhythmia, CHD, stroke, and, ultimately, HF.
Ethnic/racial differences in cardiovascular risk associated with OSA
Several longitudinal studies such as the Sleep Heart Health Study, Wisconsin Sleep Cohort, Pennsylvania Sleep Cohort, Cleveland Family Study, and recently the Jackson Heart Study have demonstrated to some degree that OSA is an independent risk factor for adverse CV outcome.9,14,32,45
OSA and hypertension
Epidemiological and clinical studies suggest that between 35% and 91% of patients with hypertension have OSA.15,46 Emerging evidence also indicates that the presence of OSA in hypertensive patients is associated with treatment resistance.16 In a cross-sectional analysis of the Sleep Heart Health Study, individuals with severe OSA (AHI > 30 per hour) had a higher risk of hypertension compared to those without OSA (AHI < 1.5 per hour).45 Among subjects who were followed for four years in the Wisconsin Sleep Cohort, the risk of hypertension increased with increasing baseline AHI.14 In the cohort with SDB followed for an average of approximately 7 years, a dose-response increase in the risks of incident nocturnal nondipping of systolic BP was observed.47
A strong racial disparity exists in the prevalence48 and treatment49 of hypertension, and its relationship to OSA. Among hypertensive blacks, we previously reported a 91% prevalence of SDB.15 It is notable that hypertensive blacks had higher baseline BP, a greater number of oxygen desaturations, and higher AHI than their white counterparts.15 OSA may, in fact, be partly responsible for the higher prevalence of hypertension and treatment resistance in this group. Whether this link is mediated fully or in part via the strong association of obesity with OSA remains unclear. Of note, analysis of data from 2,470 participants of prospective cohort of Sleep Heart Health Study50 revealed that much of the relationship between AHI and risk of incident hypertension in people with SDB was accounted for by obesity. In that study, SDB was not an independent risk factor for hypertension after adjusting for the effect of body mass index. 50 However, in a recent study, weight gain over a decade did not appear to diminish the protective effect of CPAP therapy against development of new-onset hypertension in OSA. 51
OSA and coronary heart disease
The evidence linking OSA to CHD is rapidly increasing. A high prevalence (30%) of OSA was found among 223 patients with angiographically proven CHD.21 In addition, OSA of moderate severity (AHI > 20) was independently associated with myocardial infarction.21 Data from the Sleep Heart Health Study also revealed a higher risk of self-reported CHD for individuals with high AHI,10 but subsequent longitudinal analyses of the study data indicate that the risk of incident CHD occurred primarily in men younger than 70 years.22 Following percutaneous coronary intervention, the presence of OSA was associated with increased vessel remodeling and restenosis52 and increased incidence of major adverse cardiac events, such as revascularizations and cardiac mortality.53 Studies evaluating the impact of race on the association of CHD with OSA are lacking.
OSA and arrhythmia
A wide range of cardiac arrhythmias, including atrial fibrillation, non-sustained ventricular tachycardia, and complex ventricular ectopies, have been described in persons with SDB.17–19,54 In a study of patients with OSA, Guilleminault et al. found that 48% had cardiac arrhythmias, including 2% with ventricular tachycardia, 11% with sinus arrest, 8% with second-degree atrioventricular block, and 19% with premature ventricular contractions.18 Subsequent tracheostomy in selected patients cured them of OSA and abolished their arrhythmias. Building on these earlier findings, Mehra et al19 reports that individuals with severe SDB have up to 4-fold higher odds of complex arrhythmias than those without SDB. Further detailing this SDB-arrhythmia risk, Monahan et al54 recent study showed a nearly 18-fold increase in the risk of nocturnal arrhythmia after the occurrence of apneas and hypopneas.
Among patients referred to a general cardiology practice, OSA was found in 49% of patients with atrial fibrillation, compared to 32% of those without atrial fibrillation 20 The risk of incident atrial fibrillation was higher in younger patients (< 65 years) and those with severe nocturnal hypoxemia.17 A recent meta-analysis showed that patients with OSA have a 25% greater risk of atrial fibrillation recurrence after catheter ablation than those without OSA.55 The inadequate inclusion of blacks in most of these studies limits the generalizability of the findings. Particularly worrisome is the lack of OSA-related arrhythmia evidence in blacks in light of their higher sudden cardiac death (SCD) rate and the possible operative role of the OSA-arrhythmia-SCD56 connection.
OSA and stroke
Stroke, one of the most debilitating diseases, especially in blacks, has a significant association with OSA.10,25,26 In patients with first-time strokes or transient ischemic attacks, SDB was frequently observed.25 An analysis of prospective data from the Sleep Heart Health Study indicates that severe SDB is an independent risk factor for stroke only in men.27 Similar findings were reported in the Wisconsin Cohort Study, although the fully adjusted odds ratio failed to reach statistical significance, likely due to inadequate study power.57
The impact of race/ethnicity on the association of OSA with stroke is largely unknown. Mortality resulting from stroke is greater in blacks compared to whites.30 This finding, along with the aforementioned relationship between OSA and stroke, provides further compelling evidence of the need for more research in this area among blacks.
OSA and heart failure
With regard to HF, a disease affecting almost 6 million people,12,58 report from the Sleep Heart Health Study revealed a two-fold increase in the risk of HF among subjects with OSA.10 A prospective study of patients with systolic HF revealed a high prevalence (49%) of OSA.23 Some studies evaluating the relationship between HF and SDB have focused on central sleep apnea (CSA) – a less common type of SDB characterized by intermittent sleep disruptions due to impaired control of breathing by the brain that is more commonly encountered in HF patients. Research has shown that CSA with Cheyne-Stokes respiration is associated with increased incidence of cardiac arrhythmia59 and higher mortality in HF patients.60 However, preliminary analyses of data from the Sleep Heart Health Study indicate that men have an increased risk of incident HF as a consequence of SDB, even after exclusion of subjects with CSA.22
OSA is common among patients with CVD (Figure 3). Studies investigating the role of OSA on CVD in the minority population are limited, and there are no adequately powered specific studies on the interaction of race on the association of OSA with increased CV risk or adverse outcomes.
Figure 3.
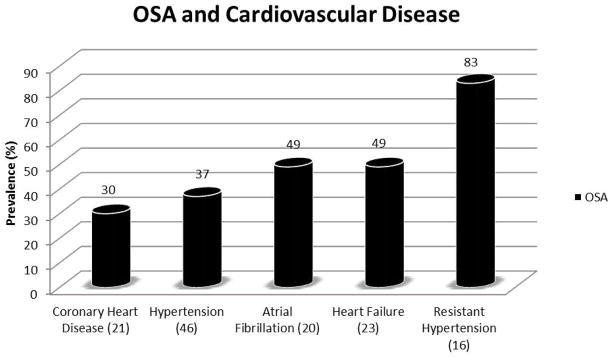
Prevalence of obstructive sleep apnea in various cardiovascular diseases.
OSA interventions and the CVD impact
The association of CVD with OSA is further corroborated by evidence suggesting that treatment of OSA decreases CV morbidity and mortality.61,62,63 Buchner et al reported that treatment of OSA is associated with a 64% reduction in CV risk.63 In the study by Milleron et al., significantly lower combined endpoints of CV, acute coronary syndrome, hospitalization for HF, or a need for coronary revascularization were observed in persons treated for OSA compared to those with OSA who declined therapy (hazard ratio 0.24).62 Following corrective surgery and the use of mandibular adjustment devices in patients with OSA, a significant reduction in BP was observed.64 Similarly, some studies have demonstrated improved response to antihypertensive therapy with the use of CPAP in patients with daytime sleepiness.65 These reductions in systolic BP have been observed in the range of 2–5 mm Hg in a meta-analysis.66 A smaller study demonstrated even more impressive findings in both systolic and diastolic BP, with a decrease of 11.0 ± 4.4 mm Hg in 24-hour systolic BP after two months of CPAP therapy.67 Nocturnal diastolic BP was reduced by 7.8 ± 3.0 mm Hg.
Among patients with HF and OSA, both left ventricular ejection fraction (LVEF) and quality of life improve with CPAP therapy.68 However, the effectiveness of CPAP therapy in patients with CSA and HF remains unclear. In the Canadian Continuous Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) Trial, CPAP therapy improved six-minute walk distance, nocturnal oxygenation, and LVEF, but not CSA.69 Whether advanced methods of applying airway pressure in HF patients will be more efficacious remains to be determined. Small clinical studies have indicated that both adaptive servo ventilation and bi-level positive airway pressure may provide some benefits to patients with HF and CSA.70
The electrical instability and risk of arrhythmia in patients with OSA decrease with CPAP treatment.71 Also, the recurrence rate of atrial fibrillation in OSA patients after elective cardioversion was lower in those treated with CPAP therapy.72 Among 23 patients with moderate to severe OSA who were monitored for arrhythmia over a 14-month period using a subcutaneously implanted loop recorder, the occurrence of severe arrhythmia decreased significantly after CPAP therapy.73
The treatment of OSA has also been shown to improve survival in patients with CHD and stroke.61,74 Following percutaneous coronary intervention, the treatment of OSA was associated with a reduction in the number of cardiac deaths.61 Although CPAP is not well-tolerated in post-stroke patients and compliance is low, long-term survival post-stroke appears to be improved among those patients who are compliant with CPAP therapy.74 Although, blacks are at greater risk of OSA-related morbidity, the existing literature does not provide any specific findings addressing possible race/ethnic-based differences in treatment response. Currently, there is no reason to suspect that blacks would be more or less responsive to treatment.
The public health implication of cardiovascular risk associated with OSA among blacks
Potential costs attributable to OSA have been estimated to be in the billions of dollars.1 Since the disorder has detrimental multi-organ consequences, substantial increase in medical costs can be anticipated. The annual cost of treating the medical consequences of OSA was estimated at $3.4 billion in the United States.75 Research has also shown an approximately two-fold increase in medical costs prior to intervention in patients with clinically suspected OSA relative to a matched control group.76 In 2000, OSA-related motor vehicle collisions were estimated to cost $15.9 billion.28 The amount of indirect nonmedical costs related to days off work and quality of life in patients with OSA remain unknown.77 However, Findley et al. suggested that treating 500 patients with OSA for three years would save over 1 million in property damage, medical expenses, and legal and administrative costs.29
The economic impact of OSA is indeed enormous, and its treatment may prove beneficial in reducing the overall public health burden, especially among blacks, given the higher prevalence of OSA and increased CV risk among this racial group.
Future directions
Many difficult ethical questions loom as we design future randomized controlled interventional trials because of the encouraging treatment benefits previously observed. Nevertheless, a few large randomized clinical trials (RCT) have been started to further determine the impact of treating OSA on CV risk. The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease (SAVE) is a planned multicenter international study slated to enroll 5,000 volunteers to investigate whether CPAP will reduce incident CVD. In the United States, the Heart Biomarker Evaluation in Apnea Treatment (HeartBEAT) study is an ongoing multicenter randomized controlled trial that is enrolling patients with OSA and CHD or CHD risk factors to employ CPAP, low-flow nocturnal oxygen, and health lifestyle instruction to determine whether CPAP or oxygen will change cardiac biomarkers. Also in the United States, the proposed ancillary study to the Multi-Ethnic Study of Atherosclerosis (MESA) is aimed to perform standardized in-home polysomnography and 7-day wrist actigraphy in 2,500 African American, Hispanic, Asian American and Caucasian individuals participating in MESA to derive indices of sleep apnea, quality, duration and timing. The study will address the role of sleep disturbances in the pathogenesis of cardiovascular disease across ethnic groups. In Europe, the Randomized Intervention of Patients with CPAP in CAD and OSA (RICCADSA) trial is aimed at evaluating the impact of CPAP treatment on a composite endpoint of new revascularization, myocardial infarction, stroke, and CV mortality over a three-year period in persons with CHD and OSA.
It is notable that most of these trials are being conducted in predominantly non-black communities. Furthermore, major longitudinal studies in the United States that have evaluated the association between OSA and CVD involved small number of blacks2,4,32,35,78 (Table 1). Studies of HF often underrepresent women, minorities and the elderly.79 One exception is the Jackson Heart Study, a rare all-African American cohort of over 5,000 participants that recently found an association of prevalent hypertension and CVD with higher odds of SDB.32 However the sleep data from the Jackson Heart Study were based on subjective sleep symptoms rather than objective evaluation as in other major longitudinal studies. Nevertheless, data on blacks from such a large study cohort is encouraging.
Perhaps, there are ethical dilemmas in randomizing minority patients to placebo arms of studies if the active treatments have shown benefit in majority studies. We advocate a range of investigational techniques and approaches including RCT, as appropriate, databases and longitudinal registries. One of the Achilles’ heels of addressing OSA is the high rate, across race and ethnicity, of refusal of, or non-compliance with, recommended treatments. Observational studies such as that by Marin et al,11 utilizing the large population of patients who refuse treatment as the “placebo” arm have provided important information in OSA treatment trials. In addition, adequate recruitment of minorities, occasionally voiced as a hurdle to minority inclusion into clinical trials, can be successfully accomplished by culturally competent investigators and minority community-based programs.
There is need for enhanced social awareness, widespread community-anchored programs targeted at minority populations, and establishment of relationship with the community leaders and stakeholders as highlighted in Table 2. Professional societies such as the American College of Physicians, American College of Cardiology, American Heart Association, American Academy of Sleep Medicine and the Association of Black Cardiologists among others should collaborate to support these efforts. Although position statements, guidelines, seminars, community outreach are a few of the educational tools currently being utilized, the efforts are still short of what is required to have the desired public health impact.
Table 2.
Guidelines for implementing a community-based sleep apnea program in minority populations.
| Guidelines for Implementing a Community-based Obstructive Sleep Apnea Program |
|---|
|
Conclusion
Over the past 30 years, a large body of scientific data firmly established the link between OSA and CVD. Blacks have a higher prevalence of OSA and a higher burden of CVD and its risk factors. Despite these facts, blacks are poorly represented in OSA-related clinical trials. We call for a change in this status with heightened awareness and systematic studies addressing OSA and CV risk in blacks.
Acknowledgments
This research was supported by funding from the NIH (R25HL105444, R01HL095799, and R01MD004113).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phillipson EA. Sleep apnea – a major public health problem. N Engl J Med. 1993;328:1271–3. doi: 10.1056/NEJM199304293281712. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Tishler PV, Hans MG, et al. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Klauber MR, Stepnowsky C, et al. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr 29;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 5.Rosen RC, Zozula R, Jahn EG, et al. Low rates of recognition of sleep disorders in primary care: comparison of a community-based versus clinical academic setting. Sleep Med. 2001;2:47–55. doi: 10.1016/s1389-9457(00)00043-5. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kripke DF, Ancoli-Israel S, Klauber MR, et al. Prevalence of sleep disordered breathing in ages 40–64 years: a population-based survey. Sleep. 1997;20:65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012 Sep 18;126(12):1495–510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 9.Chami HA, Resnick HE, Quan SF, et al. Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation. 2011 Mar 29;123(12):1280–6. doi: 10.1161/CIRCULATIONAHA.110.974022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease. Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 11.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005 Mar 19–25;365(9464):1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 12.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation Circulation. 2012 Jan 3;125(1):188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 13.Clark LT. Anatomic substrate differences between black and white victims of sudden cardiac death: hypertension, coronary artery disease, or both? Clin Cardiol. 1989 Dec;12(12 Suppl 4):IV13–7. doi: 10.1002/clc.4960121305. [DOI] [PubMed] [Google Scholar]
- 14.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 15.Jean-Louis G, Zizi F, Casimir G, et al. Sleep-disordered breathing and hypertension among African Americans. J Hum Hypertens. 2005;19(6):485–90. doi: 10.1038/sj.jhh.1001855. [DOI] [PubMed] [Google Scholar]
- 16.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug resistant hypertension. J Hypertens. 2001;19(12):2271–7. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007 Feb 6;49(5):565–71. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 18.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 19.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gami AS, Pressman G, Caples SM. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;27:367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 21.Schäfer H, Koehler U, Ewig S, et al. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92(2):79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006 Jan 4;106(1):21–8. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 24.Bady E, Achkar A, Pascal S, et al. Pulmonary arterial hypertension in patients with sleep apnoea syndrome. Thorax. 2000 Nov;55(11):934–9. doi: 10.1136/thorax.55.11.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):375–80. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 26.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 27.Redline S, Diener-West M, Geraghty E, et al. Obstructive sleep apnea hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sassani A, Findley LJ, Kryger M, et al. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 29.Findley LJ, Suratt PM. Serious motor vehicle crashes: the cost of untreated sleep apnoea. Thorax. 2001;56:505. doi: 10.1136/thorax.56.7.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mensah GA, Mokdad AH, Ford ES, et al. State of disparities in cardiovascular health in the United States. Circulation. 2005 Mar 15;111(10):1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell JE, Hellkamp AS, Mark DB, et al. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Am Heart J. 2008 Mar;155(3):501–6. doi: 10.1016/j.ahj.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fülöp T, Hickson DA, Wyatt SB, et al. Sleep-disordered breathing symptoms among African-Americans in the Jackson Heart Study. Sleep Med. 2012 Sep;13(8):1039–49. doi: 10.1016/j.sleep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002 May 1;165(9):1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 34.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor GT, Lind BK, Lee ET, et al. Sleep Heart Health Study Investigators. b Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003 Feb 1;26(1):74–9. [PubMed] [Google Scholar]
- 36.Friedman M, Bliznikas D, Klein M, et al. Comparison of the incidences of obstructive sleep apnea-hypopnea syndrome in African-Americans versus Caucasian-Americans. Otolaryngol Head Neck Surg. 2006 Apr;134(4):545–50. doi: 10.1016/j.otohns.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90(4):1600–5. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 40.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 41.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways b by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 42.Nanduri J, Nanduri RP. Cellular mechanisms associated with intermittent hypoxia. Essays Biochem. 2007;43:91–104. doi: 10.1042/BSE0430091. [DOI] [PubMed] [Google Scholar]
- 43.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3(4):409–15. [PMC free article] [PubMed] [Google Scholar]
- 44.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 45.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study JAMA. 2000;283(14):1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 46.Sjöström C, Lindberg E, Elmasry A, et al. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002 Jul;57(7):602–7. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hla KM, Young T, Finn L, et al. Longitudinal association of sleep disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lackland DT, Lin Y, Tilley BC, et al. An assessment of racial differences in clinical practices for hypertension at primary care sites for medically underserved patients. J Clin Hypertens (Greenwich) 2004;6:26–31. doi: 10.1111/j.1524-6175.2004.03089.x. [DOI] [PubMed] [Google Scholar]
- 49.Cushman WC, Ford CE, Einhorn PT, et al. ALLHAT Collaborative Research Group. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens (Greenwich) 2008 Oct;10(10):751–60. doi: 10.1111/j.1751-7176.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Connor GT, Caffo B, Newman AB, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009 Jun 15;179(12):1159–64. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012 May 23;307(20):2169–76. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steiner S, Schueller PO, Hennersdorf MG, et al. Impact of obstructive sleep apnea on the occurrence of restenosis after elective percutaneous coronary intervention in ischemic heart disease. Respir Res. 2008;9:50. doi: 10.1186/1465-9921-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yumino D, Tsurumi Y, Takagi A, et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99(1):26–30. doi: 10.1016/j.amjcard.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 54.Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009 Nov 3;54(19):1797–804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng CY, Liu T, Shehata M, et al. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol. 2011 Jul 1;108(1):47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 56.Gami AS, Somers VK. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J Cardiovasc Electrophysiol. 2008 Sep;19(9):997–1003. doi: 10.1111/j.1540-8167.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 57.Arzt M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 59.Lanfranchi PA, Somers VK, Braghiroli A, et al. Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107(5):727–32. doi: 10.1161/01.cir.0000049641.11675.ee. [DOI] [PubMed] [Google Scholar]
- 60.Javaheri S, Shukla R, Zeigler H, et al. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49(20):2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 61.Cassar A, Morgenthaler TI, Lennon RJ, et al. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(14):1310–4. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 62.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Buchner NJ, Sanner BM, Borgel J, et al. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;176:1274–1280. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]
- 64.Shibata N, Nishimura T, Hasegawa K, et al. Influence of sleep respiratory disturbance on nocturnal blood pressure. Acta Otolaryngol Suppl. 2003;(550):32–5. doi: 10.1080/0365523031000056. [DOI] [PubMed] [Google Scholar]
- 65.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 66.Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(1):CD001106. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnoea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21(2):241–7. doi: 10.1183/09031936.03.00035402. [DOI] [PubMed] [Google Scholar]
- 68.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 69.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 70.Naughton MT, Lorenzi-Filho G. Sleep in heart failure. Prog Cardiovasc Dis. 2009;51(4):339–49. doi: 10.1016/j.pcad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101(4):392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 72.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 73.Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25(12):1070–6. doi: 10.1016/j.ehj.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180(1):36–41. doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 75.Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22(6):749–55. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 76.Kapur VK, Alfonso-Cristancho R. Just a good deal or truly a steal? Medical cost savings and the impact on the cost-effectiveness of treating sleep apnea. Sleep. 2009;32(2):135–6. doi: 10.1093/sleep/32.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wittmann V, Rodenstein DO. Health care costs and the sleep apnea syndrome. Sleep Med Rev. 2004 Aug;8(4):269–79. doi: 10.1016/j.smrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State Cohort. Sleep. 2010 Sep;33(9):1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchell JE, Ferdinand KC, Watson KE, et al. Treatment of Heart Failure in African Americans – A Call to Action. J Natl Med Ass. 2011 Feb;:103. doi: 10.1016/s0027-9684(15)30257-1. [DOI] [PubMed] [Google Scholar]