Abstract
Ischemic heart disease (IHD) is the greatest single cause of mortality and loss of disability-adjusted life years (DALYs) worldwide, and a substantial portion of this burden falls on low- and middle-income countries (LMICs). Deaths from IHD and acute coronary syndrome (ACS) occur, on average, at younger ages in LMICs than in high-income countries, often at economically productive ages, and likewise frequently affect the poor within LMICs. While data regarding ACS in LMICs are limited, there is a growing literature in this area and the research gaps are being steadily filled. In high-income countries, decades of investigation into the risk factors for ACS and development of behavioral programs, medications, interventional procedures, and guidelines have provided us with the tools to prevent and treat events. Although similar tools can be, and in fact have been, implemented in many LMICs, challenges remain in the development and implementation of cardiovascular health promotion activities across the entire life course, as well as in access to treatment for ACS and IHD. Intersectoral policy initiatives and global coordination are critical elements of ACS and IHD control strategies. Addressing the hurdles and scaling successful health promotion, clinical and policy efforts in LMICs are necessary in order to adequately address the global burden of ACS and IHD.
Keywords: Acute coronary syndrome, epidemiology, global health, low- and middle-income countries, ischemic heart disease
Introduction
Ischemic heart disease (IHD) is the greatest single cause of mortality and loss of disability-adjusted life years (DALYs) worldwide, accounting for roughly seven million deaths and 129 million DALYs annually.1,2 Cardiovascular disease exerts a significant economic toll, accounting for one-third of a projected $47 trillion in economic losses to non-communicable diseases (NCDs) over the next 20 years.3 While high-income countries (HICs) continue to deal with significant IHD mortality, nearly two-thirds of all IHD DALYs and over half of deaths occur in LMICs. Many of these countries have experienced transformational economic growth and lifestyle changes over the past several decades that have increased the prevalence of IHD risk factors and rates of mortality.4-8 Understanding this change, how it compares with past experience in HICs, and available measures to stem the global tide of IHD mortality make up the research and action frontier regarding acute coronary syndrome (ACS) and IHD in LMICs.
The epidemiologic transition provides a useful framework for understanding the rise of IHD in LMICs (Figure 1).9,10 The epidemiologic transition posits that populations initially start with low life expectancies with mortality primarily driven by infections, under-nutrition, and illness and injury related to childbirth and early childhood (“age of pestilence and famine”). As sanitation and agriculture improve, these causes of death gradually recede (“age of receding pandemics”) until NCDs, particularly IHD and cancers, dominate the causes of death (“age of degenerative and man-made diseases”). Still later, as cancers and IHD become preventable or controllable, the burden of these diseases shift to older ages (“age of delayed degenerative diseases”).11 A “fifth stage” has also been proposed, in light of recent adverse trends in physical activity and diet—an age of “obesity and inactivity”.12
Figure 1.
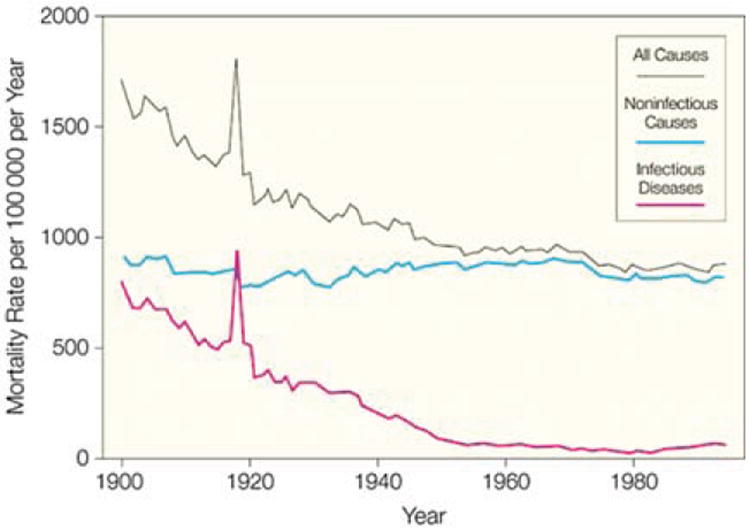
The epidemiologic transition in the United States, which was already well underway by 1900. From: JAMA. 1999;281(1):61-66.205
Rapid urbanization, mechanization of transport, and increasingly sedentary jobs in LMICs have led to an acceleration and overlap between the stages of the epidemiologic transition.13-16 While infections, under-nutrition, and maternal/child mortality are still important, they are no longer dominant causes of death in many LMICs: IHD is now one of the top five causes of death in all regions of the world except sub-Saharan Africa. Even in sub-Saharan Africa, cardiovascular disease is the leading cause of death among individuals over age 30.17 Overall, the numbers of deaths and DALYs attributable to IHD have risen since 1990.18,19 This acceleration in the rise of NCDs without a similar reduction of infectious disease burden, has led to a challenging “double burden of disease” in many countries.13,20,21 In addition, the age-standardized mortality rates from IHD are higher in many LMICs than in HICs, thus indicating more individuals are dying at a younger age from IHD in LMICs (Figure 2).19 While much of the IHD burden in LMICs is occurring as those regions and individuals enter higher economic strata, there remains a substantial health and economic burden on the poorer segments of LMIC societies resulting from IHD and related NCDs.22-24 In addition, given population growth in LMICs, the absolute numbers of individuals with premature IHD is substantial even as global, age-standardized IHD mortality rates have declined.18,19
Figure 2.
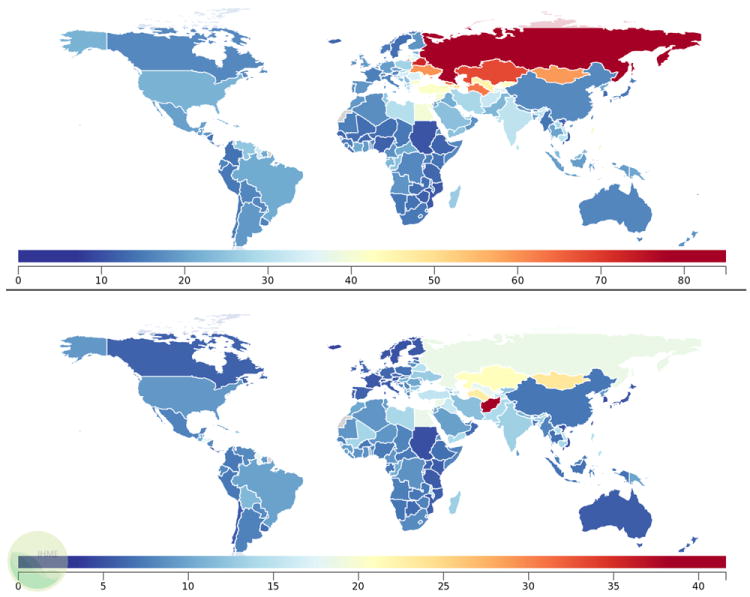
Mortality rates from IHD per 100,000 in 2010 by country, ages 15-49, males (top) and females (bottom). Data from 2012 Global Burden of Disease.206
There has also been a dramatic rise in several IHD risk factors. Obesity and overweight prevalence has been increasing in many LMICs,6,25-27 more than tripling between 1975 and 1997 among children in Brazil from 4.1% to 13.9%.25 The age-standardized prevalence of obesity and overweight increased from 30.8% in 1980 to 46.4% in 2008, with half of the increase occurring after 2000.27 Globally, mean body mass index has been increasing in nearly every region of the world (Figure 3). Other biological risk factors have demonstrated geographic and temporal variability. Comprehensive analyses of systolic blood pressure have revealed increases in sub-Saharan Africa and South/Southeast Asia, relatively little change in Latin America, and substantial decreases in HICs (Figure 4).4 In contrast, mean serum cholesterol levels have tended to decline in several regions of the world, although at varying rates5: while HICs and the former Soviet Union have experienced notable declines, South Asia has seen much more modest declines, while Latin America and the Middle East did not change and levels actually rose in East Asia (Figure 5).
Figure 3.
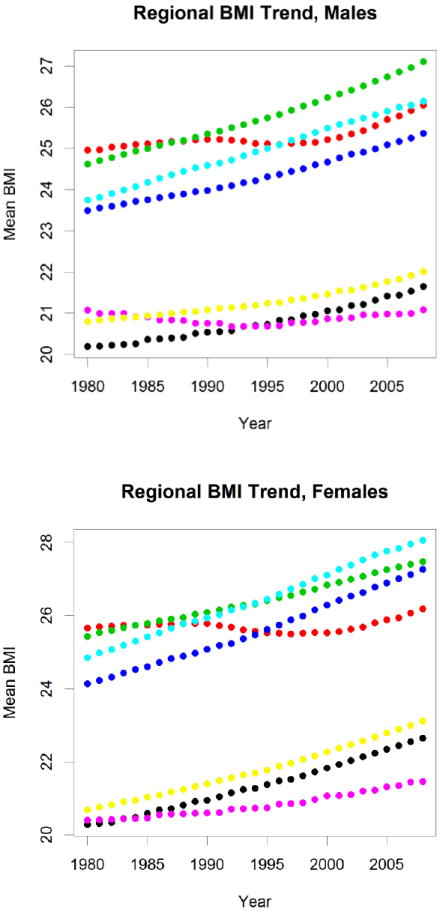
Age-standardized mean body-mass index (BMI) by sex and region. Green: high-income countries, Black: East Asia/Pacific, Red: Eastern Europe/Central Asia, Dark Blue: Latin America/Caribbean, Light Blue: Middle East/North Africa, Purple: South Asia, Yellow: Sub-Saharan Africa.
Figure 4.
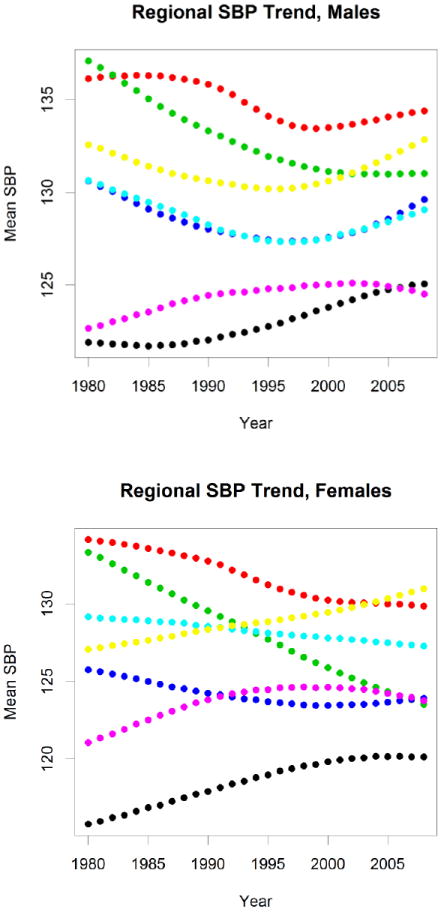
Age-standardized mean systolic blood pressure (SBP) by sex and region. Color coding as in Figure 3.
Figure 5.
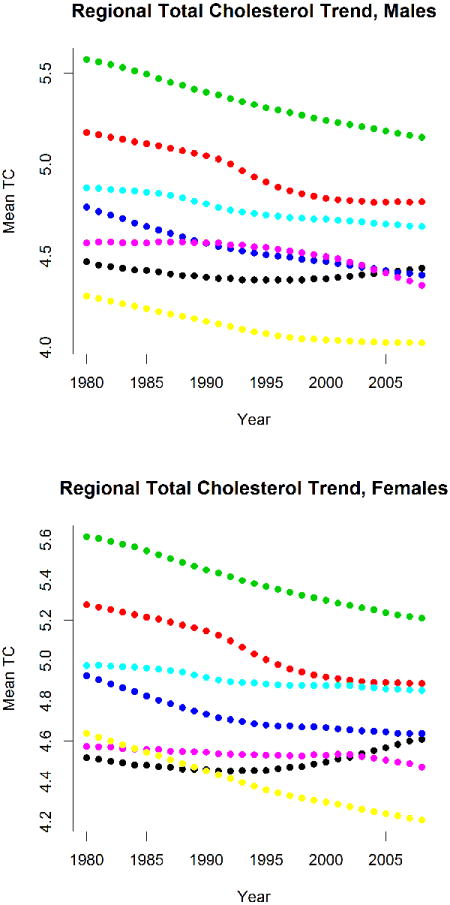
Age-standardized mean total cholesterol by sex and region. Color coding as in Figure 3.
Underpinning the rises in biological risk factors have been rises in behavioral risk factors. Though recent reports suggest that global smoking prevalence has declined since 1980, the total number of smokers has increased to nearly one billion people in 201228 and remains common in many LMICs29 despite some notable successes.30-32 There is also significant geographic variability in smoking rates, with certain areas (Russia, Eastern Europe, Central Asia, China, Southeast Asia, North Africa, and parts of South America) characterized by high age-standardized prevalence of daily smoking (Figure 6). Consumption of other unhealthy products, such as sugary beverages, processed foods, and alcohol have increased.33 Likewise, large numbers of adults around the world have low levels of physical activity; although there are significant regional variations, several LMICs have physical inactivity levels that rival those of HICs (Figure 7).34 From the most recent Global Burden of Disease estimates, the top 10 risk factors contributing to mortality and DALYs in LMICs were all behavioral or biological risks for NCDs (Figure 8).35
Figure 6.
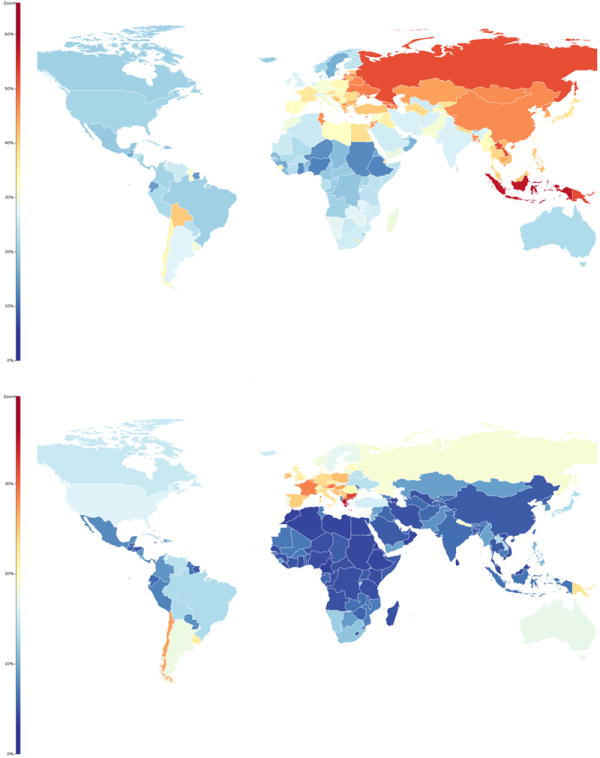
Age-standardized prevalence of smoking as percent of population, males (top), females (bottom). From JAMA. 2014;311(2):183-192.28
Figure 7.
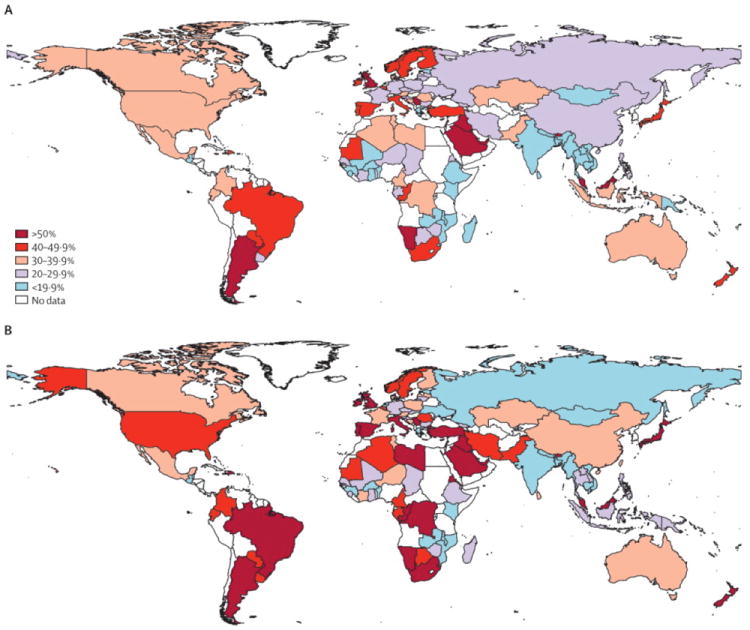
Age-adjusted percent of adults who are physically inactive, males (A) and females (B). From Lancet 2012, 380(9838):247-257.34
Figure 8.
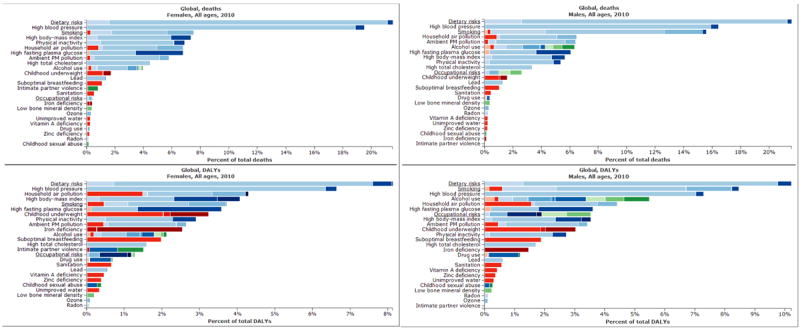
Percentage of total deaths (top) and DALYs (bottom) in LMICs for males (left) and females (right) of all ages attributable to different categories of risk factors. Data from 2012 Global Burden of Disease.206
In this review, we aim to describe the global perspective regarding ACS. However, given the limited, though growing, body of data regarding ACS outside of HICs, we use IHD as a surrogate for ACS when data for ACS are not available. Thus, we examine in detail the trends of IHD burden in HICs, compare and contrast the recent experience in LMICs, discuss the history of ACS treatment and prevention in HICs, and outline steps for addressing ACS in LMICs.
Rise and Fall of IHD Mortality in HICs
Current HICs struggled with growing rates of ACS and associated mortality during the mid-twentieth century.36-38 However, HICs have experienced significant declines mortality rates from all cardiovascular conditions since the 1960s.39-41 Both treatment and prevention have contributed to the observed reductions in IHD mortality in HICs (Table 1).42-44 Treatment includes improved care for ACS, as well as chronic medical management of IHD. Preventive efforts include both behavioral and pharmacological initiatives.
Table 1.
Declines in coronary heart disease mortality in selected high- and middle-income countries, with attributable portions due to risk factor changes (prevention) and treatment.
| Region | Years | Age Range | Initial CHD Mortality Rate+ | Final CHD Mortality Rate+ | Percent Change | Percent Attributable to Prevention | Percent Attributable to Treatment |
|---|---|---|---|---|---|---|---|
| England & Wales207 | 1981-2000 | 25-84 | Males: ~530 | Males: ~250 | -53 | 58 | 42 |
| Females: ~180 | Females: ~90 | -50 | |||||
| Finland208 | 1982-1997 | 35-64 | Males: 420 | Males: 150 | -64.3 | 53-72 | 23 |
| Females: 70 | Females: 20 | -71 | |||||
| Ireland209 | 1985-2000 | 25-84 | 8681* | 4918# | -47 | 48.1 | 43.6 |
| Italy210 | 1980-2000 | 25-84 | Males: 267.1 | Males: 141.3 | -47.1 | 55 | 40 |
| Females: 161.3 | Females: 78.8 | -51.1 | |||||
| Auckland, New Zealand211 | 1982-1993 | All Ages | 2366* | 1808# | -23.6 | 54 | 46 |
| Scotland212 | 1975-1994 | 21438* | 15234# | -28.9 | 51 | 40 | |
| Sweden213 | 1986-2002 | 25-84 | Males: 544.1 | Males: 253.4 | -53.4 | 55 | 36 |
| Females: 291.5 | Females: 140.0 | -52.0 | |||||
| USA214 | 1980-2000 | 25-84 | Males: 542.9 | Males: 266.8 | -50.9 | 44 | 47 |
| Females: 263.3 | Females: 134.4 | -49.0 |
Rates given per 100,000 population
Expected number of deaths in final year with age-specific rates of initial year
Observed number of deaths in final year
CHD = coronary heart disease
Advances in the acute management of ACS include many celebrated achievements in intensive care-related and interventional approaches to cardiovascular medicine: the creation of the coronary care unit45; the introduction of streptokinase46 and later thrombolytic drugs;47 and the development of coronary artery catheterization, balloon angioplasty,48 and surgical revascularization.41,49 These advances made it possible, rather than to simply observe the natural history of ACS complications, to intervene and attempt to modify the natural course of illness. Use of emergency medical systems, initially established for trauma care and to transport patients with suspected ACS, has also helped reduce the time between symptom onset and intervention, despite underutilization by many individuals who have acute events.50-52 These interventions have changed acute management from passivity and impotence to activity and intervention, with the potential to avert premature death and disability.
Further success in IHD treatment is reflected in the multi-dimensional approach to medical management of IHD and secondary prevention of further events. This foundation of optimal medical therapy includes a combination of medications that is started acutely and maintained post-ACS: aspirin, beta blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers, and statins. The evidence for their use has been established over several decades, including the ISIS series of trials for beta blockers,53 aspirin (alongside streptokinase),54 and ACE inhibitors.55 Longer-term evidence for these medications has been built up in meta-analyses of numerous trials.56-59 Evidence for statins began to emerge with low-dose therapies in the 4S and CARE trials,60,61 while later trials showed the increased benefits of more intensive lipid-lowering.62-64
Improvements in primordial and primary prevention were driven first by an understanding of the underlying risks of cardiovascular disease, particularly through the Framingham Heart Study65,66 and the Seven Countries Study.67 These and related studies68 helped define the roles of tobacco use, blood pressure, and cholesterol as risk factors for IHD, challenging beliefs about the benign nature of some risk factors and making what had seemed an inevitable consequence of aging become something that could potentially be prevented.69,70
Building on the evidence of risk, intervention studies changed the practice of prevention. Pharmacological intervention was pioneered by the VA Cooperative Studies,71 showing reductions in morbidity and mortality in individuals treated for hypertension. For cholesterol, the Lipid Research Clinics trial provided early evidence that cholesterol reduction using medication could reduce incidence of IHD.72 Likewise, dietary and behavior change were first validated with the Oslo Study Group trial,73 while the North Karelia Project and later the Five Community Study provided evidence of the effects of public health approaches.74,75 The modern-day guidelines for cholesterol, blood pressure, and lifestyle modification are the result of decades-long efforts to accommodate the latest evidence from rigorous clinical trials into best practice recommendations.76-80 These efforts, in combination with regulatory initiatives to ban trans fats81,82 and global tobacco control,83 have made substantial impact, with corresponding decreases in blood pressure, lipid levels, and tobacco use over time.84-88
However, there are still major gaps in the midst of these overall improvements, and also concerning trends with respect to overweight, obesity, and diabetes. Body mass index has increased worldwide virtually without exception.6 Within the United States, the past decade has seen a rise in the prevalence of obesity among adult men as well as women belonging to racial or ethnic minorities.89 There have also been declines in physical activity and increases in caloric intake.90,91 In terms of overall cardiovascular health, less than 1% of adults in the United States were found to have “ideal cardiovascular health”, with high prevalence noted for poor diet (>90%) and high body-mass index (>50%).92 Projections of these trends suggest that, without intervention, poor health behaviors will continue to be highly prevalent with increases in diabetes prevalence and subsequent cardiovascular complications.93
ACS and IHD Burden in LMICs
In some respects, the ACS and IHD situation in LMICs today is more similar to that of HICs in decades past. In particular, the burden of ACS is not solely on the rich nor on the elderly, but also on the poor and working-age94. According to the most recent Global Burden of Disease study, the median age of death from IHD among males was a decade younger in LMICs than in HICs in 2010 (Figure 9). This may be due to earlier onset of ACS and IHD, as well as shorter survival after ACS. The available results from the OASIS-1 and -2 registries95 and epidemiologic studies in India96-98 suggest that earlier age for first ACS in LMICs is a major contributing factor. Registry data from many other LMICs also support the assertion that ACS often occur at younger ages than in HICs:99-109 Strikingly, a registry from the United Arab Emirates reported a mean age of 50.8 years.110 Earlier age for first ACS is likely due to earlier acquisition of adverse health behaviors and IHD risk factors10,97,111,112 in the current context of economic development and globalization. One notable exception to this overall trend is the experience of an ACS registry in Thailand.113,114
Figure 9.
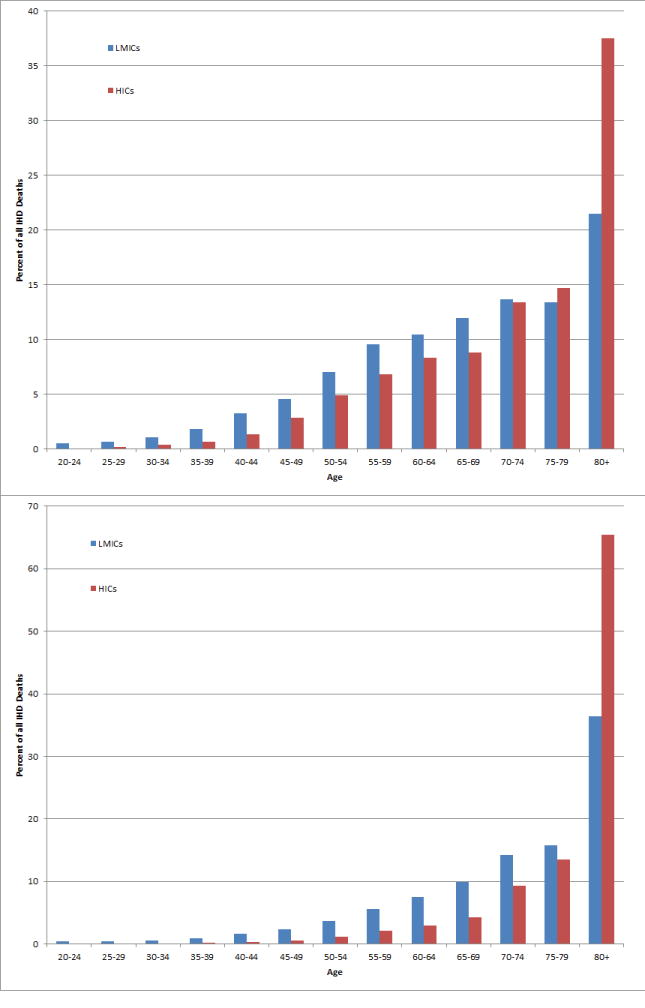
Age distribution of male (top) and female (bottom) IHD (ischemic heart disease) deaths in 2010 among countries classified as high-income versus low- and middle-income. Data from 2012 Global Burden of Disease.1
The treatment and outcomes of ACS in LMICs are variable but often suboptimal (Table 2).115 Observational studies do suggest that, to a large extent, the in-hospital treatment of ACS in LMICs includes the use of aspirin, ACE inhibitors, and beta blockers.95,98,116-118 The ACCESS Study, a prospective observational registry of patients hospitalized for ACS between 2007 and 2008 in 19 LMICs, found that aspirin and lipid-lowering therapies were each given to more than 90% of patients, while uptake of beta-blockers and ACE inhibitors were at 78% and 68%, respectively.116 However, comparison of countries participating in the OASIS registries found lower use of heparin in LMICs than in HICs, while the ACCESS investigators found that only 39% of patients presenting with ST-elevation myocardial infarction received fibrinolysis. In addition, there also exist substantive within-country differences in the management of ACS, as is the case in India.115
Table 2.
Summary of published literature regarding acute coronary syndrome in low- and middle-income countries.
| Study | Region | Study Design | N | Mean Age | In-Hospital | Discharge | F/U | %Morality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASA | ACE-I/ARB | Statin | BB | Reperfusion | ASA | ACE-I/ARB | Statin | BB | |||||||
| ACCEPT (106) | Brazil | Registry | 2,485 | 63 | 0.97 | 0.68 | 0.91 | 0.80 | 0.88* | NR | NR | NR | NR | 30-Day | 1.6 |
| ACCESS (116) | Algeria, Argentina, Brazil, Colombia, Dominican Republic, Ecuador, Egypt, Guatemala, Iran, Jordan, Kuwait, Lebanon, Mexico, Morocco, Saudi Arabia, South Africa, Tunisia, United Arab Emirates, Venezuela | Registry | 12,068 | 59.2 | 0.93 | 0.78 | 0.94 | 0.78 | 0.28 | 0.90 | 0.75 | 0.89 | 0.76 | 12 months | 7.3 |
| Aga Khan University Hospital, Nairobi (101) | Nairobi, Kenya | Registry | 111 | 64 | NR | NR | NR | NR | 0.38 | NR | NR | NR | NR | In-Hospital | 8.1 |
| Brazilian Registry of Acute Coronary Syndromes (RBSCA) (100) | Brazil | Registry | 2,693 | 63 | 0.95 | 0.70 | 0.77 | 0.77 | 0.643
|
NR | NR | NR | NR | In-Hospital | 5.5 |
| BRIDGE-ACS (177)◆) | Brazil | Randomized Trial | 548 | 62 | 0.958 | NR | 0.729 | NR | NR | 0.928 | 0.761 | 0.86 | 0.817 | 30-Day | 8.4 |
| CRACE (108) | China | Registry | 1,301 | 63 | 0.98 | 0.82 | 0.74 | 0.98 | 0.64 | NR | NR | NR | NR | In-Hospital | 4 |
| CREATE (130) | India | Registry | 20,937 | 58 | 0.979** | 0.568 | 0.52 | 0.59 | 0.104
|
NR | NR | NR | NR | 30-Day | 6.7 |
| DEMAT (118) | India | Registry | 1,565 | 58 | 0.96 | NR | 0.93 | 0.79 | 0.67 | 0.94 | 0.66 | 0.88 | 0.79 | 30-Day | 2.1 |
| Euro Heart Survey 2009 AMI Snapshot (107) ♓ | Armenia, Bosnia-Herzegovinia, Bulgaria, Croatia, Czech Republic, Georgia, Hungary, Kazakhstan, Kosovo, Macedonia, Moldova, Poland, Romania, Russian Federation, Serbia and Montenegro, Slovakia, Slovenia, Ukraine | Cross-Sectional Survey | 1,329 | 64 | 0.96 | 0.86 | 0.92 | 0.84 | 0.62 | 0.92 | 0.85 | 0.92 | 0.84 | In-Hospital | 9 |
| Gulf RACE-2 (109) | Bahrain, Saudi Arabiam Qatar, Oman, United Arab Emirates, Yemen | Registry | 7,930 | 57 | 0.98 | 0.76 | 0.95 | 0.74 | 0.18 | 0.93 | 0.78 | 0.91 | 0.79 | 12 months | 12 |
| Kerala ACS Registry (98) | Kerala, India | Registry | 25,748 | 60 | 0.93 | 0.28 | 0.70 | 0.66 | 0.33 | 0.76 | 0.26 | 0.70 | 0.63 | In-Hospital | 3.9 |
| OASIS-2 (95) | Bangladesh, China, India, Lithuania, Russia, Maldives, Slovenia, Ukraine | Registry | 4,615 | 60.7 | 0.95 | NR | NR | 0.67 | NR | NR | NR | NR | NR | 24 months | 9.5 |
| PURE (119,120) ❖ | Argentina, Bangladesh, Brazil, Canada, Chile, China, Colombia, India, Iran, Malaysia, Poland, Pakistan, South Africa, Sweden, Turkey, United Arab Emirates, Zimbabwe | Prospective Cohort | 153,966 | 57 | NA | NA | NA | NA | NA | 0.21 | 0.16 | 0.09 | 0.17 | NR | NR |
| RENASICA II (99) | Mexico | Registry | 8,600 | 62 | 0.88 | 0.71 | 0.14 | 0.51
|
0.51 | NR | NR | NR | NR | 30-Day | 10 |
| TACSR (113) | Thailand | Registry | 9,373 | NR | 0.95 | 0.63 | 0.80 | 0.62 | 0.37 | NR | NR | NR | NR | In-Hospital | 12.6 |
| TRACS (114) | Thailand | Registry | 2,007 | 64 | 0.99 | 0.71 | 0.94 | 0.62 | 0.48 | NR | NR | NR | NR | 12-Months | 17.7 |
| UAE-ACS Registry (110) | United Arab Emirates | Registry | 1,842 | 51 | 0.95 | 0.70 | 0.93 | 0.81 | 0.814*** | 0.92 | 0.68 | 0.90 | 0.82 | In-Hospital | 1.7 |
| WHO-PREMISE (121) | Brazil, Egypt, India, Indonesia, Iran, Pakistan, Russia, Sri Lanka, Tunisia, Turkey | Cross-Sectional Survey | 10,000 | 59 | NA | NA | NA | NA | NA | 0.821 | 0.398 | 0.298 | 0.481 | NA | NA |
| WHO-SAGE (128) | China, Ghana, India, Mexico, Russia, South Africa | Prospective Cohort | 47,443 | 44 | NA | NA | NA | NA | NA | NR | NR | NR | NR | NR | NR |
BRIDGE-ACS (177) data from control arm only.
Euro Heart Study data from Central and Eastern regions only
PURE study ages for CHD only, proportions for CHD in LMICs only.
STEMI patients only;
![]()
PCI + CABG;
Any antiplatelet agents;
![]()
![]()
Fibrinolytics & CABG & deferred PCI;
STEMI with LBBB patients only
NA = not applicable; NT = not reported
Further, continuation of these medications following discharge is poor: investigators from the PURE study found that among individuals with previous cardiovascular events, nearly 70% of those in lower-middle-income countries and 80% of those in low-income countries were on no medication for secondary prevention.119 They also found lower uptake of lifestyle changes following cardiovascular events in LMICs than in HICs, with 75% of individuals in HICs quitting smoking versus 42% in lower-middle- and 38% in low-income countries.120 Likewise, the WHO PREMISE survey of coronary artery disease patients in LMICs found that while aspirin was in widespread use and that over 75% of respondents were aware of behavioral risk factors for cardiovascular disease, a majority engaged in less than 30 minutes of physical activity weekly and less than a third were taking statins.121 Administrative data among IHD patients from Andhra Pradesh were similarly discouraging, with only 15.6% receiving aspirin and 6.0% on cholesterol-lowering medications.105 Evidence for the quality of clinical outcomes is conflicting. The OASIS registries found comparable mortality rates across participating countries after age-adjustment. By contrast, a review of randomized trials of ST-elevation myocardial infarction treatments found that trial sites in LMICs had higher mortality rates than their counterparts in HICs, which the authors explained by increased numbers of high-risk patients.122 Further clarification of the quality of ACS outcomes in LMICs is needed.
In addition, the impact of ACS and IHD on household livelihood is substantial in LMICs. As noted above, in LMICs these events occur at younger ages, often during peak economic productivity. Households experience a double burden both from the great expense of treating ACS and from the loss of income of the affected individual. In general, NCD expenditures increased as a proportion of out-of-pocket health care costs in India from 1995 to 2004, and were found to have greater odds of carrying catastrophic health expenditure than did communicable diseases among hospitalized patients.123 Additional work confirms that individuals with NCDs have higher health expenditures than individuals with communicable diseases24,124 and that households with an individual with an NCD are more likely to face catastrophic health expenditures.124,125 In a multi-country survey of individuals hospitalized for cardiovascular conditions, more than half of the hospitalized individuals reported catastrophic health expenditures or distress financing.23 Survival with disability, such as diminished exercise capacity that may arise from heart failure related to IHD, may further burden households’ stretched finances. Testimonies from individuals living with NCDs are notable for their desire not to be a burden on their families.126
The socio-economic gradient of ACS in LMICs is also quite striking. Even in low-income countries, these are diseases that inflict a large burden on the poor. For instance, in South Africa, poorer districts of Cape Town had higher rates of mortality from NCDs than did wealthier districts.127 In the SAGE surveys of health in six middle-income countries, hypertension prevalence was high, between 30% and 36%, across all income strata.128 A survey of 1600 rural villages in India found higher prevalence of tobacco and alcohol use and lower intake of fruits and vegetables among poorer respondents.129 Likewise, more than 70% of individuals with suspected myocardial infarction in the Indian CREATE registry were classified as “poor” or “lower middle income”.130 Further, higher socio-economic status was found to be protective against risk of myocardial infarction in India.131 While there are likely geographic variations—the poor of sub-Saharan Africa face considerable burden from infectious and nutritional diseases—it is clear that the poor in LMICs do indeed experience a substantial burden of ACS and IHD.
Stemming the Tide: Cardiovascular Health Promotion Throughout the Life Course
Despite the challenges of addressing ACS in LMICs, the time is now to implement interventions aimed at cardiovascular health promotion throughout the life course.132,133 Health promotion activities directed at all ages, access to essential medicines,134 improved quality of health care services to manage risk factors and treat acute events, and intersectoral policy initiatives135 can, in combination, prevent millions of premature deaths in the coming decades. A wide array of interventions are cost-effective and scalable in LMICs136, and an analysis by the World Health Organization (WHO) of intervention packages for specific risk factors suggests that implementation can be done at very low cost per-person.137
There is increasing evidence that IHD risk factors have their origins in early childhood.138-143 Health promotion interventions targeted at young children may have beneficial impacts both on short-term health behaviors, as well as long-term risk factors.144-149 Effective school-based interventions are those that include the family, and that center on realistic intermediate objectives such as changes in attitude, knowledge, dietary patterns or levels of physical activity, as early control measures that improve cardiovascular health.150 Critical to the success of these interventions is community engagement, cultural relevance and appropriateness, optimization of the school environment, and involvement of the family.151-153 Programs that have been implemented successfully in LMICs144,145 can be reproduced in other low-resource settings.154,155
Addressing dietary risk factors can be cost-effective. An analysis of salt-reduction measures estimated conservatively that a 15% reduction in salt intake could save 2.4 million lives over 10 years at a cost of $0.50 per person,156 in line with other findings suggesting that salt reduction at processing stages of food production can be cost-effective.157 Likewise, reductions in saturated and trans-fats by controlling use in food processing can be cost-effective, estimated at $40 per DALY in Latin America when focusing on trans-fats.136,157 By focusing on processing sources, these interventions are readily scalable, costing less than $0.01 per person.137
Physical activity remains a challenge to evaluate; however, the WHO estimates that a public awareness campaign could be implemented at $0.038 per person. One major challenge to physical activity has been urbanization, which has produced mechanized transportation environments that discourage physical activity.158 However, there are clear interventions for addressing lifestyle and the built environment,159 and WHO guidelines on physical activity provide a clear way forward.160 Efforts in Brazil and Colombia to improve physical activity in cities are one example of success in this area,161 although there is substantial room to improve policies promoting healthy diets and physical activity.162
Effective interventions are also well known to reduce tobacco use, many of which are provisions of the Framework Convention on Tobacco Control: restrictions on advertising, packaging, marketing to minors, use in public spaces, and taxes.83 Extensive analysis has suggested that these interventions produce robust results, with particularly strong response to taxation in developing countries.163 Further, these are highly cost-effective, from $3-$42 for a 33% tax rate on tobacco to $55-$761per DALY in LMICs for nicotine replacement therapy.164 The WHO’s “best buys” for global health estimate that a package of interventions including taxation, packaging and advertising restrictions, counter-advertising, and use restrictions could be implemented for approximately $0.11 per person.137 However, there have been several challenges to ratification and implementation of the Framework Convention.165 One major challenge has been the push for voluntary, nation-level approaches rather than a coordinated global treaty.166-170 After passage of the Framework Convention, it has been documented that the tobacco industry has attempted to undermine this treaty by subverting its provisions,171-173 has pursued lawsuits against signatories that have implemented treaty provisions,174 and has been noted by the WHO as actively preventing implementation of treaty provisions that aim to keep the tobacco industry out of public health policy-making.175 Robust and transparent governance is necessary to overcome these challenges.176
Treatment of ACS and IHD
Approaches to treatment can be divided into two categories: interventions for acute events and interventions for primary and secondary prevention. According to the Disease Control Priorities Project,17 streptokinase was the most cost-effective reperfusion therapy at cost $634-$734 per DALY. This strategy, however, became more expensive per DALY saved as time to treatment increased. Alteplase and coronary artery bypass surgery were both over $10,000 per DALY. The use of aspirin, beta blockers, and ACE inhibitors was found to be cost-effective, and in some circumstances cost-saving. To date, there remains a dearth of research on the cost-effectiveness of reperfusion therapies in LMICs.136 In-hospital use of other evidence-based management, such as anticoagulation, antiplatelet medications, and statins, can be improved with the implementation of systems-level approaches such as reminder, checklists, case management, and educational materials.177
For primary and secondary prevention, a multidrug regimen consisting of a beta-blocker or calcium channel-blocker, an ACE-inhibitor, aspirin, and a statin was found to be cost-effective.178 On the basis of the need for a multidrug regimen for pharmacotherapy, efforts have been made at creating a polypill that brings together these various medications and makes adherence simpler.179-182 A meta-analysis of trials found that, compared to placebo, a polypill did reduce blood pressure and serum lipids.183 The polypill strategy is generally thought to be associated with improved adherence related to fewer number of pills to be consumed on a daily basis. However, the data regarding adherence of a cardiovascular polypill are mixed. The meta-analysis concluded that the polypill strategy had lower rates of adherence, with 20% of polypill recipients discontinuing use versus 14% of those on placebo or monotherapy, although the trials included were highly heterogenous.183 On the other hand, the more recent UMPIRE study found much higher rates of adherence for those receiving a polypill versus usual care.184 If the challenges in developing a polypill are overcome, it is possible that this, too, will be a cost-effective and essential tool for prevention.185,186
Medication access remains a significant challenge in LMICs.187,188 Data from India indicate that poor patients are less likely to receive evidence-based in-hospital treatments such as revascularization, thrombolytics, and lipid-lowering drugs.130 Surveys of medication use suggest low levels of uptake of therapy for primary and secondary prevention of IHD.119,121 Affordability is an important reason as to why uptake is so low: IHD medication may cost more than an individual’s daily income.24,136 As these medications must be taken daily for many years, cost is a primary concern in ensuring that the majority of those in need have the ability to access appropriate care. Innovative strategies to optimize the health care workforce to manage IHD and ACS,189 as well as to improve access to essential medicines,134 are required.
Integrated Health Service Delivery, Intersectoral Policy, and Global Coordination
Health care delivery systems in LMICs would benefit from a comprehensive approach that integrates services related to NCDs and communicable diseases such as HIV.190 Beyond the above-described interventions for specifically ACS and IHD, overall cardiovascular care in LMICs would benefit from strengthening health systems, improving quality of care, optimizing human resources for health, establishing secure supply chains of drugs and technology, and promoting equitable access to care. Instead of vertical, disease-specific programs, a “diagonal” approach in which cardiovascular-related health care delivery is integrated into a comprehensive approach to health systems strengthening will likely yield superior results191-195. In order to maximize the effectiveness of health sector-specific interventions, they should be implemented in the context of broader population-level policy changes and community-level programs.
Thus, the remaining intervention necessary – perhaps underlying all of the above interventions – is comprehensive intersectoral policy dedicated to NCDs in general.135,196,197 The roots of IHD risk are not contained solely within the health sector, and the entire spectrum of stakeholders are required to commit to policies and programs that will create the conditions that improve cardiovascular health. This would include, at a minimum, representation from the health, education, infrastructure, transportation, urban planning, trade, and finance sectors. In addition, productive partnerships among government, private sector, and civil society are possible and required.
Finally, although the large global burden of NCDs is well-established, funding from donors does not match this reality. In 2010, although NCDs collectively accounted for 49.8% of DALY burden of disease, they received only 2.3% of development assistance for health.198 Within the WHO’s own budget, only 12% was allocated to NCDs for the 2006-2007 fiscal year, out of line with disease burden.199 Analysis of spending by donors broadly, including private philanthropies such as the Gates Foundation, showed a similarly skewed allocation, with HIV/AIDS accounting for the largest portion of health spending, far in excess of its DALY-measured burden compared to NCDs and to other communicable diseases.200 Another study reported spending of $0.78 per DALY for NCDs versus $23.90 per DALY for HIV, TB, and malaria.201 While funding for NCDs has increased over the past decade,198,201 allocation of funding that is more in-line with actual disease burden may aid the achievement of an array of global health goals,. The prospects for improving this balance of funding are challenging and require greater participation among aid recipient countries and alignment of UN institutions around NCD goals.202 At the country level, health insurance and payment systems ideally should ensure equitable access to both in-hospital treatment of ACS as well as long-term outpatient access to medications and rehabilitative services.203 In addition, public-private partnerships and other innovative financing mechanisms will be required.
Conclusion
Similar to the situation in HICs decades ago, LMICs today are well into a transition toward increasing morbidity and mortality from ACS and IHD. However, there are features that distinguish the patterns of ACS in LMICs from HICs. First, the death toll falls more heavily upon younger, productive ages in LMICs than in HICs, with mortality rates among adults aged 15-49 in some LMICs nearly double that in HICs. Second, the rate of the epidemiologic transition to increased burden from IHD has happened more rapidly in LMICs today than in HICs in the past. In addition, ACS and IHD are not diseases confined to the privileged classes; rather, there is a significant burden that also falls hard upon the lower-income strata in LMICs, who often are unable to afford the costs of medical therapy for primary or secondary prevention of IHD, let alone the expensive treatments for ACS that have helped make acute events more survivable in HICs. Together, these help explain the substantial economic burden that is anticipated from ACS and IHD if no action is taken.
Despite the immense burden, the global community is aided by new knowledge and discoveries made during the past half century. We continue to evolve more efficient and efficacious treatment strategies for ACS, ranging from rapid transport of patients to hospitals via emergency medical systems, to timely reperfusion, through to long-term cardiac rehabilitation, optimal medical therapy and risk factor control. We understand what the underlying risk factors for ACS are and how to prevent them through cardiovascular health promotion activities throughout the life course, appropriate therapeutic strategies, and intersectoral policy. The scientific challenge is primarily in translating this existing knowledge to new settings. In addition, the growing body of ACS literature from LMICs highlights the importance of local, national, and regional registries as important sources of information about current practices and inspiration for improvement and change.115,204
The critical task is implementation: the tools that we have need to be materialized. This involves engaging in health promotion activities in early childhood, ensuring access to essential medicines to treat and prevent ACS, developing resource- and context-specific clinical guidelines, implementing laws and regulations to protect the public’s health from harmful products, planning the growth of cities to promote healthy behavior, and support from donor countries and international organizations to match the scale of the global burden of ACS, IHD, and other NCDs. We are well-equipped to confront this challenge so long as our will meets it.
Supplementary Material
Acknowledgments
The authors would like to thank Claire Hutchinson for editorial assistance.
Funding: RV receives partial salary support from the Fogarty International Center of the National Institutes of Health under Award Number K01 TW 009218 - 03. BS is supported by the Stanford Interdisciplinary Graduate Fellowship and partly by NIA grant R24AG0393345. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations and Acronyms
- ACE
angiotensin-converting enzyme
- ACS
acute coronary syndrome
- DALY
disability-adjusted life year
- HICs
high-income countries
- IHD
ischemic heart disease
- LMICs
low- and middle-income countries
- NCDs
non-communicable diseases
- WHO
World Health Organization
Footnotes
Disclosures: None
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C, et al. The Global Economic Burden of Non-communicable Diseases. Geneva: 2011. [Google Scholar]
- 4.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–77. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 5.Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek CJ, Singh GM, Lin JK, Stevens GA, Riley LM, Ezzati M. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet. 2011;377:578–86. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 6.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera JA, Barquera S, González-Cossío T, Olaiz G, Sepúlveda J. Nutrition Transition in Mexico and in Other Latin American Countries. Nutr Rev. 2004;62:S149–S157. doi: 10.1111/j.1753-4887.2004.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 8.Shetty PS. Nutrition transition in India. Public Health Nutr. 2002;5:175–82. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- 9.Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49:509–38. [PubMed] [Google Scholar]
- 10.Yusuf S, Reddy S, Ounpuu S, Anand S. Global Burden of Cardiovascular Diseases: Part I: General Considerations, the Epidemiologic Transition, Risk Factors, and Impact of Urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 11.Olshansky SJ, Ault AB. The fourth stage of the epidemiologic transition: the age of delayed degenerative diseases. Milbank Q. 1986;64:355–91. [PubMed] [Google Scholar]
- 12.Gaziano JM. Fifth phase of the epidemiologic transition: the age of obesity and inactivity. JAMA. 2010;303:275–6. doi: 10.1001/jama.2009.2025. [DOI] [PubMed] [Google Scholar]
- 13.Frenk J, Bobadilla JL, Sepúlveda J, Cervantes ML. Health transition in middle-income countries: new challenges for health care. Health Policy Plan. 1989;4:29–39. [Google Scholar]
- 14.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006;100:191–9. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Remais JV, Zeng G, Li G, Tian L, Engelgau MM. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int J Epidemiol. 2013;42:221–7. doi: 10.1093/ije/dys135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bygbjerg IC. Double burden of noncommunicable and infectious diseases in developing countries. Science. 2012;337:1499–501. doi: 10.1126/science.1223466. [DOI] [PubMed] [Google Scholar]
- 17.Gaziano TA, Reddy KS, Paccaud F, Horton S, Chaturvedi V. Cardiovascular Disease. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease Control Priorities in Developing Countries. 2. World Bank; 2006. [Google Scholar]
- 18.Moran AE, Forouzanfar MH, Roth G, Mensah GA, Ezzati M, Flaxman A, Murray CJL, Naghavi M. The Global Burden of Ischemic Heart Disease in 1990 and 2010: The Global Burden of Disease 2010 Study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran AE, Forouzanfar MH, Roth G, Mensah G, Ezzati M, Murray CJL, Naghavi M. Temporal Trends in Ischemic Heart Disease Mortality in 21 World Regions, 1980-2010: The Global Burden of Disease 2010 Study. Circulation. 2014;129:1483–1492. doi: 10.1161/CIRCULATIONAHA.113.004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yach D, Hawkes C, Gould CL, Hofman KJ. The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA. 2004;291:2616–22. doi: 10.1001/jama.291.21.2616. [DOI] [PubMed] [Google Scholar]
- 21.Ali MK, Rabadán-Diehl C, Flanigan J, Blanchard C, Narayan KMV, Engelgau M. Systems and capacity to address noncommunicable diseases in low- and middle-income countries. Sci Transl Med. 2013;5:181cm4. doi: 10.1126/scitranslmed.3005121. [DOI] [PubMed] [Google Scholar]
- 22.Ezzati M, Vander Hoorn S, Lawes CMM, Leach R, James WPT, Lopez AD, Rodgers A, Murray CJL. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. In: Novotny T, editor. PLoS Med. Vol. 2. 2005. p. e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huffman MD, Rao KD, Pichon-Riviere A, et al. A cross-sectional study of the microeconomic impact of cardiovascular disease hospitalization in four low- and middle-income countries. In: Malaga G, editor. PLoS One. Vol. 6. 2011. p. e20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binnendijk E, Koren R, Dror DM. Can the rural poor in India afford to treat non-communicable diseases. Trop Med Int Heal. 2012;17:1376–85. doi: 10.1111/j.1365-3156.2012.03070.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am J Clin Nutr. 2002;75:971–977. doi: 10.1093/ajcn/75.6.971. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Du S, Zhai F, Popkin BM. Trends in the distribution of body mass index among Chinese adults, aged 20-45 years (1989-2000) Int J Obes (Lond) 2007;31:272–8. doi: 10.1038/sj.ijo.0803416. [DOI] [PubMed] [Google Scholar]
- 27.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, Paciorek CJ, Farzadfar F, Riley L, Ezzati M. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJL, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 29.Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, Peto R, Zatonski W, Hsia J, Morton J, Palipudi KM, Asma S. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–79. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro CA, Cavalcante TM, Moura EC, Claro RM, Szwarcwald CL. Population-based evidence of a strong decline in the prevalence of smokers in Brazil (1989-2003) Bull World Health Organ. 2004;85:527–534. doi: 10.2471/BLT.06.039073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peer N, Bradshaw D, Laubscher R, Steyn K. Trends in adult tobacco use from two South African demographic and health surveys conducted in 1998 and 2003. SAMJ South African Med J. 2009;99:744–749. [PubMed] [Google Scholar]
- 32.Jha P, Peto R. Global Effects of Smoking, of Quitting, and of Taxing Tobacco. N Engl J Med. 2014;370:60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 33.Stuckler D, McKee M, Ebrahim S, Basu S. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012;9:e1001235. doi: 10.1371/journal.pmed.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 35.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puffer RR, Verhoestraete LJ. Mortality from cardiovascular diseases in various countries, with special reference to atherosclerotic heart disease: a preliminary analysis. Bull World Health Organ. 1958;19:315–24. [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell M. The Main Cause of Increased Death Rate From Disease of the Heart: 1920 to 1959. BMJ. 1963;2:712–7. doi: 10.1136/bmj.2.5359.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hechter HH, Borhani NO. Mortality and Geographic Distribution of Arteriosclerotic Heart Disease. Public Health Rep. 1965;80:11–24. [PMC free article] [PubMed] [Google Scholar]
- 39.Levi F. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88:119–124. doi: 10.1136/heart.88.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation. 2012;125:1848–57. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nabel EG, Braunwald E. A Tale of Coronary Artery Disease and Myocardial Infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 42.Ford ES, Capewell S. Proportion of the Decline in Cardiovascular Mortality Disease due to Prevention Versus Treatment: Public Health Versus Clinical Care. Annu Rev Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- 43.Tunstall-Pedoe H, Vanuzzo D, Hobbs M, Mähönen M, Cepaitis Z, Kuulasmaa K, Keil U. Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet. 2000;355:688–700. doi: 10.1016/s0140-6736(99)11181-4. [DOI] [PubMed] [Google Scholar]
- 44.Kuulasmaa K, Tunstall-Pedoe H, Dobson A, Fortmann S, Sans S, Tolonen H, Evans A, Ferrario M. Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet. 2000;355:675–687. doi: 10.1016/s0140-6736(99)11180-2. [DOI] [PubMed] [Google Scholar]
- 45.Julian D. The evolution of the coronary care unit. Cardiovasc Res. 2001;51:621–624. doi: 10.1016/s0008-6363(01)00365-0. [DOI] [PubMed] [Google Scholar]
- 46.Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI) Effectiveness of Intravenous Thrombolytic Treatment in Acute Myocardial Infarction. Lancet. 1986;327:397–402. [PubMed] [Google Scholar]
- 47.The International Study Group. In-hospital mortality and clinical course of 20 891 patients with suspected acute myocardial infarction randomised between alteplase and streptokinase with or without heparin. Lancet. 1990;336:71–75. [PubMed] [Google Scholar]
- 48.Grüntzig AR, Senning Å, Siegenthaler WE. Nonoperative Dilatation of Coronary-Artery Stenosis. N Engl J Med. 1979;301:61–68. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- 49.Malach M, Imperato PJ. Acute Myocardial Infarction and Acute Coronary Syndrome: Then and Now (1950-2005) Prev Cardiol. 2006;9:228–234. doi: 10.1111/j.1520-037x.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- 50.So DYF, Ha ACT, Turek MA, Maloney JP, Higginson LA, Davies RF, Ryan SC, Le May MR. Comparison of mortality patterns in patients with ST-elevation myocardial infarction arriving by emergency medical services versus self-transport (from the prospective Ottawa Hospital STEMI Registry) Am J Cardiol. 2006;97:458–61. doi: 10.1016/j.amjcard.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 51.Mathews R, Peterson ED, Li S, Roe MT, Glickman SW, Wiviott SD, Saucedo JF, Antman EM, Jacobs AK, Wang TY. Use of emergency medical service transport among patients with ST-segment-elevation myocardial infarction: findings from the National Cardiovascular Data Registry Acute Coronary Treatment Intervention Outcomes Network Registry-Get With The Guidelines. Circulation. 2011;124:154–63. doi: 10.1161/CIRCULATIONAHA.110.002345. [DOI] [PubMed] [Google Scholar]
- 52.Tan L-L, Wong H-B, Poh C-L, Chan MY, Seow S-C, Yeo T-C, Teo S-G, Ooi SBS, Tan H-C, Lee C-H. Utilisation of emergency medical service among Singapore patients presenting with ST-segment elevation myocardial infarction: prevalence and impact on ischaemic time. Intern Med J. 2011;41:809–14. doi: 10.1111/j.1445-5994.2010.02278.x. [DOI] [PubMed] [Google Scholar]
- 53.First International Study of Infarct Survival Collaborative Group. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. Lancet. 1986;2:57–66. [PubMed] [Google Scholar]
- 54.Baigent C, Collins R, Appleby P, Parish S, Sleight P, Peto R. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ. 1998;316:1337–43. doi: 10.1136/bmj.316.7141.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ISIS-4 Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative. Lancet. 1995;345:669–85. [PubMed] [Google Scholar]
- 56.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 57.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE Inhibitors in the Early Treatment of Acute Myocardial Infarction : Systematic Overview of Individual Data From 100 000 Patients in Randomized Trials. Circulation. 1998;97:2202–2212. doi: 10.1161/01.cir.97.22.2202. [DOI] [PubMed] [Google Scholar]
- 60.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 61.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun C-C, Davis BR, Braunwald E. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz GG. Effects of Atorvastatin on Early Recurrent Ischemic Events in Acute Coronary Syndromes. JAMA. 2001;285:1711. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 63.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 64.Pedersen TR, Faergeman O, Kastelein JJP, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–45. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 65.Kannel WB, Schwartz MJ, McNamara PM. Blood pressure and risk of coronary heart disease: the Framingham study. Dis Chest. 1969;56:43–52. doi: 10.1378/chest.56.1.43. [DOI] [PubMed] [Google Scholar]
- 66.Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum Cholesterol, Lipoproteins, and the Risk of Coronary Heart DiseaseThe Framingham Study. Ann Intern Med. 1971;74:1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- 67.Farchi G, Capocaccia R, Verdecchia A, Menotti A, Keys A. Risk Factors Changes and Coronary Heart Disease in an Observational Study. Int J Epidemiol. 1981;10:31–40. doi: 10.1093/ije/10.1.31. [DOI] [PubMed] [Google Scholar]
- 68.Kromhout D. Prevention of Coronary Heart Disease by Diet and Lifestyle: Evidence From Prospective Cross-Cultural, Cohort, and Intervention Studies. Circulation. 2002;105:893–898. doi: 10.1161/hc0702.103728. [DOI] [PubMed] [Google Scholar]
- 69.Kotchen TA. Historical trends and milestones in hypertension research: a model of the process of translational research. Hypertension. 2011;58:522–38. doi: 10.1161/HYPERTENSIONAHA.111.177766. [DOI] [PubMed] [Google Scholar]
- 70.Moser M. Historical Perspectives on the Management of Hypertension. J Clin Hypertens. 2006;8:15–20. doi: 10.1111/j.1524-6175.2006.05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veterans Administration Cooperative Study Group on Anti-hypertensive Agents. Effects of Treatment on Morbidity in Hypertension: Results in Patients With Diastolic Blood Pressures Averaging 115 Through 129 mm Hg. JAMA J Am Med Assoc. 1967;202:1028. [PubMed] [Google Scholar]
- 72.Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial Results. JAMA. 1984;251:351. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 73.Hjermann I, Holme I, Byre KV, Leren P. Effect of Diet and Smoking Iinterention On the Incidence of Coronary Heart Disease. Lancet. 1981;318:1303–1310. doi: 10.1016/s0140-6736(81)91338-6. [DOI] [PubMed] [Google Scholar]
- 74.Puska P, Nissinen A, Tuomilehto J, Salonen JT, Koskela K, McAlister A, Kottke TE, Maccoby N, Farquhar JW. The community-based strategy to prevent coronary heart disease: conclusions from the ten years of the North Karelia project. Annu Rev Public Health. 1985;6:147–93. doi: 10.1146/annurev.pu.06.050185.001051. [DOI] [PubMed] [Google Scholar]
- 75.Farquhar JW. Effects of Communitywide Education on Cardiovascular Disease Risk Factors. JAMA. 1990;264:359. [PubMed] [Google Scholar]
- 76.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2013;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 77.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 78.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 79.National Institute for Health and Care Excellence. CG67 Lipid Modification. 2010 [Google Scholar]
- 80.National Institute for Health and Care Excellence. CG127 Hypertension. 2011 [Google Scholar]
- 81.McGreevy P. State bans trans fats. The Los Angeles Times. http://articles.latimes.com/2008/jul/26/local/me-transfat26. Published July 26, 2008.
- 82.FOOD PREPARATION AND FOOD ESTABLISHMENTS Section 81.08 Foods containing artificial trans fat. New York City: [Google Scholar]
- 83.Conference of the Parties to the WHO FCTC. WHO Framework Convention on Tobacco Control. 2003 [Google Scholar]
- 84.Evans A, Tolonen H, Hense H-W, Ferrario M, Sans S, Kuulasmaa K. Trends in coronary risk factors in the WHO MONICA Project. Int J Epidemiol. 2001;30:S35–S40. doi: 10.1093/ije/30.suppl_1.s35. [DOI] [PubMed] [Google Scholar]
- 85.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KMV, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–74. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 86.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 87.Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, Jacobson TA. 30-year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999-2006. Am J Cardiol. 2010;106:969–75. doi: 10.1016/j.amjcard.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 88.Garrett BE, Dube SR, Trosclair A, Caraballo RS, Pechacek TF. Cigarette Smoking — United States, 1965–2008. MMWR. 2011;60:109–113. [PubMed] [Google Scholar]
- 89.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 90.King DE, Mainous AG, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988-2006. Am J Med. 2009;122:528–34. doi: 10.1016/j.amjmed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 91.Ford ES, Dietz WH. Trends in energy intake among adults in the United States: findings from NHANES. Am J Clin Nutr. 2013;97:848–53. doi: 10.3945/ajcn.112.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003-2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988-2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–602. doi: 10.1161/CIRCULATIONAHA.111.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leeder S, Raymond S, Greenberg H, Liu H, Esson K. A Race Against Time: The Challenge of Cardiovascular Disease in Developing Economies. New York: 2004. [Google Scholar]
- 95.Prabhakaran D, Yusuf S, Mehta S, et al. Two-year outcomes in patients admitted with non-ST elevation acute coronary syndrome: results of the OASIS registry 1 and 2. Indian Heart J. 2005;57:217–25. [PubMed] [Google Scholar]
- 96.Srivastava A, Mohanty SK. Age and sex pattern of cardiovascular mortality, hospitalisation and associated cost in India. PLoS One. 2013;8:e62134. doi: 10.1371/journal.pone.0062134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joshi P, Islam S, Pais P, Reddy S, Dorairaj P, Kazmi K, Pandey MR, Haque S, Mendis S, Rangarajan S, Yusuf S. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA. 2007;297:286–94. doi: 10.1001/jama.297.3.286. [DOI] [PubMed] [Google Scholar]
- 98.Mohanan PP, Mathew R, Harikrishnan S, Krishnan MN, Zachariah G, Joseph J, Eapen K, Abraham M, Menon J, Thomas M, Jacob S, Huffman MD, Prabhakaran D. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34:121–9. doi: 10.1093/eurheartj/ehs219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Juárez-Herrera Ú, Jerjes-Sánchez C. Risk factors, therapeutic approaches, and in-hospital outcomes in Mexicans with ST-elevation acute myocardial infarction: the RENASICA II multicenter registry. Clin Cardiol. 2013;36:241–8. doi: 10.1002/clc.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piegas LS, Avezum A, Guimarães HP, Muniz AJ, Reis HJL, dos Santos ES, Knobel M, de Souza R. Acute coronary syndrome behavior: results of a Brazilian registry. Arq Bras Cardiol. 2013;100:502–10. doi: 10.5935/abc.20130101. [DOI] [PubMed] [Google Scholar]
- 101.Shavadia J, Yonga G, Otieno H. A prospective review of acute coronary syndromes in an urban hospital in sub-Saharan Africa. Cardiovasc J Afr. 2012;23:318–21. doi: 10.5830/CVJA-2012-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Demirkan B, Ege MR, Doğan P, İpek EG, Güray U, Güray Y. Factors influencing the use of ambulance among patients with acute coronary syndrome: results of two centers in Turkey. Anadolu Kardiyol Derg. 2013;13:516–22. doi: 10.5152/akd.2013.171. [DOI] [PubMed] [Google Scholar]
- 103.Gao R, Patel A, Gao W, Hu D, Huang D, Kong L, Qi W, Wu Y, Yang Y, Harris P, Algert C, Groenestein P, Turnbull F. Prospective observational study of acute coronary syndromes in China: practice patterns and outcomes. Heart. 2008;94:554–60. doi: 10.1136/hrt.2007.119750. [DOI] [PubMed] [Google Scholar]
- 104.Saidi O, Ben Mansour N, O’Flaherty M, Capewell S, Critchley JA, Ben Romdhane H. Analyzing recent coronary heart disease mortality trends in Tunisia between 1997 and 2009. PLoS One. 2013;8:e63202. doi: 10.1371/journal.pone.0063202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joshi R, Chow CK, Raju PK, Raju R, Reddy KS, Macmahon S, Lopez AD, Neal B. Fatal and nonfatal cardiovascular disease and the use of therapies for secondary prevention in a rural region of India. Circulation. 2009;119:1950–5. doi: 10.1161/CIRCULATIONAHA.108.819201. [DOI] [PubMed] [Google Scholar]
- 106.Mattos L, Alberto Pe, Berwanger O, dos Santos ES, Reis HJL, Romano ER, Petriz JLF, Sousa ACS, Neuenschwander FC, Guimarães JI, de Andrade JP. Desfechos clínicos aos 30 dias do registro brasileiro das síndromes coronárias agudas (ACCEPT) Arq Bras Cardiol. 2013;100:6–13. [Google Scholar]
- 107.Puymirat E, Battler A, Birkhead J, et al. Euro Heart Survey 2009 Snapshot: regional variations in presentation and management of patients with AMI in 47 countries. Eur Hear journal Acute Cardiovasc care. 2013;2:359–70. doi: 10.1177/2048872613497341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song X-T, Chen Y-D, Pan W-Q, Lü S-Z. Gender based differences in patients with acute coronary syndrome: findings from Chinese Registry of Acute Coronary Events (CRACE) Chin Med J (Engl) 2007;120:1063–7. [PubMed] [Google Scholar]
- 109.Ahmed E, Alhabib KF, El-Menyar A, Asaad N, Sulaiman K, Hersi A, Almahmeed W, Alsheikh-Ali AA, Amin H, Al-Motarreb A, Al Saif S, Singh R, Al-Lawati J, Al Suwaidi J. Age and clinical outcomes in patients presenting with acute coronary syndromes. J Cardiovasc Dis Res. 2013;4:134–9. doi: 10.1016/j.jcdr.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yusufali AM, AlMahmeed W, Tabatabai S, Rao K, Binbrek A. Acute coronary syndrome registry from four large centres in United Arab Emirates (UAE-ACS Registry) Heart Asia. 2010;2:118–121. doi: 10.1136/ha.2009.001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reddy KS. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2006;5:231–237. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 112.Critchley J, Liu J, Zhao D, Wei W, Capewell S. Explaining the increase in coronary heart disease mortality in Beijing between 1984 and 1999. Circulation. 2004;110:1236–44. doi: 10.1161/01.CIR.0000140668.91896.AE. [DOI] [PubMed] [Google Scholar]
- 113.Srimahachota S, Kanjanavanit R, Boonyaratavej S, Boonsom W, Veerakul G, Tresukosol D. Demographic, management practices and in-hospital outcomes of Thai Acute Coronary Syndrome Registry (TACSR): the difference from the Western world. J Med Assoc Thai. 2007;90(Suppl 1):1–11. [PubMed] [Google Scholar]
- 114.Srimahachota S, Boonyaratavej S, Kanjanavanit R, Sritara P, Krittayaphong R, Kunjara-Naayudhya R, Tatsanavivat P. Thai Registry in Acute Coronary Syndrome (TRACS)--an extension of Thai Acute Coronary Syndrome registry (TACS) group: lower in-hospital but still high mortality at one-year. J Med Assoc Thail. 2012;95:508–18. [PubMed] [Google Scholar]
- 115.Karthikeyan G, Xavier D, Prabhakaran D, Pais P. Perspectives on the management of coronary artery disease in India. Heart. 2007;93:1334–8. doi: 10.1136/hrt.2007.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.ACCESS Investigators. Management of acute coronary syndromes in developing countries: Acute Coronary Events—a multinational Survey of current management Strategies. Am Heart J. 2011;162:852–859.e22. doi: 10.1016/j.ahj.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 117.Huffman MD, Prabhakaran D, Abraham AK, Krishnan MN, Nambiar AC, Mohanan PP. Optimal in-hospital and discharge medical therapy in acute coronary syndromes in Kerala: results from the Kerala acute coronary syndrome registry. Circ Cardiovasc Qual Outcomes. 2013;6:436–43. doi: 10.1161/CIRCOUTCOMES.113.000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pagidipati NJ, Huffman MD, Jeemon P, et al. Association between gender, process of care measures, and outcomes in ACS in India: results from the detection and management of coronary heart disease (DEMAT) registry. In: Schnabel RB, editor. PLoS One. Vol. 8. 2013. p. e62061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yusuf S, Islam S, Chow CK, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE Study): a prospective epidemiological survey. Lancet. 2011;378:1231–43. doi: 10.1016/S0140-6736(11)61215-4. [DOI] [PubMed] [Google Scholar]
- 120.Teo K, Lear S, Islam S, et al. Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: The Prospective Urban Rural Epidemiology (PURE) study. JAMA. 2013;309:1613–21. doi: 10.1001/jama.2013.3519. [DOI] [PubMed] [Google Scholar]
- 121.Mendis S, Abegunde D, Yusuf S, Ebrahim S, Shaper G, Ghannem H, Shengelia B. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE) Bull World Health Organ. 2005;83:820–829. [PMC free article] [PubMed] [Google Scholar]
- 122.Orlandini A, Díaz R, Wojdyla D, Pieper K, Van de Werf F, Granger CB, Harrington RA, Boersma E, Califf RM, Armstrong P, White H, Simes J, Paolasso E. Outcomes of patients in clinical trials with ST-segment elevation myocardial infarction among countries with different gross national incomes. Eur Heart J. 2006;27:527–33. doi: 10.1093/eurheartj/ehi701. [DOI] [PubMed] [Google Scholar]
- 123.Engelgau MM, Karan A, Mahal A. The Economic impact of Non-communicable Diseases on households in India. Global Health. 2012;8:9. doi: 10.1186/1744-8603-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kankeu HT, Saksena P, Xu K, Evans DB. The financial burden from non-communicable diseases in low- and middle-income countries: a literature review. Health Res Policy Syst. 2013;11:31. doi: 10.1186/1478-4505-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rahman MM, Gilmour S, Saito E, Sultana P, Shibuya K. Health-related financial catastrophe, inequality and chronic illness in Bangladesh. In: van Baal PHM, editor. PLoS One. Vol. 8. 2013. p. e56873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.World Health Organization. Preventing Chronic Diseases: A Vital Investment. Geneva: World Health Organization; 2005. [Google Scholar]
- 127.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374:934–47. doi: 10.1016/S0140-6736(09)61087-4. [DOI] [PubMed] [Google Scholar]
- 128.Basu S, Millett C. Social epidemiology of hypertension in middle-income countries: determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension. 2013;62:18–26. doi: 10.1161/HYPERTENSIONAHA.113.01374. [DOI] [PubMed] [Google Scholar]
- 129.Kinra S, Bowen LJ, Lyngdoh T, Prabhakaran D, Reddy KS, Ramakrishnan L, Gupta R, Bharathi AV, Vaz M, Kurpad AV, Smith GD, Ben-Shlomo Y, Ebrahim S. Sociodemographic patterning of non-communicable disease risk factors in rural India: a cross sectional study. BMJ. 2010;341:c4974. doi: 10.1136/bmj.c4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xavier D, Pais P, Devereaux PJ, et al. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–42. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 131.Pais P, Pogue J, Gerstein H, Zachariah E, Savitha D, Jayprakash S, Nayak P, Yusuf S. Risk factors for acute myocardial infarction in Indians: a case-control study. Lancet. 1996;348:358–363. doi: 10.1016/s0140-6736(96)02507-x. [DOI] [PubMed] [Google Scholar]
- 132.Fuster V, Bridget B. Promoting Cardiovascular Health in the Developing World:A Critical Challenge to Achieve Global Health. The National Academies Press; 2010. Kelly EC on P the GE of CDM the C in DCI of M. [PubMed] [Google Scholar]
- 133.Fuster V, Kelly BB, Vedanthan R. Promoting global cardiovascular health: moving forward. Circulation. 2011;123:1671–8. doi: 10.1161/CIRCULATIONAHA.110.009522. [DOI] [PubMed] [Google Scholar]
- 134.Kishore SP, Vedanthan R, Fuster V. Promoting global cardiovascular health ensuring access to essential cardiovascular medicines in low- and middle-income countries. J Am Coll Cardiol. 2011;57:1980–7. doi: 10.1016/j.jacc.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 135.Fuster V, Kelly BB, Vedanthan R. Global cardiovascular health: urgent need for an intersectoral approach. J Am Coll Cardiol. 2011;58:1208–10. doi: 10.1016/j.jacc.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 136.Gaziano TA, Pagidipati N. Scaling up chronic disease prevention interventions in lower- and middle-income countries. Annu Rev Public Health. 2013;34:317–35. doi: 10.1146/annurev-publhealth-031912-114402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.World Health Organization. Scaling up action against noncommunicable diseases: how much will it cost? 2011 [Google Scholar]
- 138.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004;145:20–5. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 140.Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168:30–7. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 141.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115:22–7. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- 142.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–9. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 143.Cunningham SA, Kramer MR, Narayan KMV. Incidence of Childhood Obesity in the United States. N Engl J Med. 2014;370:403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Céspedes J, Briceño G, Farkouh ME, Vedanthan R, Baxter J, Leal M, Boffetta P, Woodward M, Hunn M, Dennis R, Fuster V. Targeting preschool children to promote cardiovascular health: cluster randomized trial. Am J Med. 2013;126:27–35.e3. doi: 10.1016/j.amjmed.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Céspedes J, Briceño G, Farkouh ME, Vedanthan R, Baxter J, Leal M, Boffetta P, Hunn M, Dennis R, Fuster V. Promotion of cardiovascular health in preschool children: 36-month cohort follow-up. Am J Med. 2013;126:1122–6. doi: 10.1016/j.amjmed.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Draper CE, de Villiers A, Lambert EV, Fourie J, Hill J, Dalais L, Abrahams Z, Steyn NP. HealthKick: a nutrition and physical activity intervention for primary schools in low-income settings. BMC Public Health. 2010;10:398. doi: 10.1186/1471-2458-10-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Foster GD, Linder B, Baranowski T, Cooper DM, Goldberg L, Harrell JS, Kaufman F, Marcus MD, Treviño RP, Hirst K. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363:443–53. doi: 10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Galvez MP, Hong L, Choi E, Liao L, Godbold J, Brenner B. Childhood obesity and neighborhood food-store availability in an inner-city community. Acad Pediatr. 2009;9:339–43. doi: 10.1016/j.acap.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ritchie LD, Sharma S, Ikeda JP, Mitchell RA, Raman A, Green BS, Hudes ML, Fleming SE. Taking Action Together: a YMCA-based protocol to prevent type-2 diabetes in high-BMI inner-city African American children. Trials. 2010;11:60. doi: 10.1186/1745-6215-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Birch LL, Ventura AK. Preventing childhood obesity: what works? Int J Obes. 2009;33(Suppl 1):S74–81. doi: 10.1038/ijo.2009.22. [DOI] [PubMed] [Google Scholar]
- 151.Progress in Preventing Childhood Obesity: How Do We Measure Up? The National Academies Press; 2007. on Progress in Preventing Childhood Obesity C. [Google Scholar]
- 152.US Department of Health and Human Services. Strategic Plan for NIH obesity research: a report of the NIH Obesity Task Force. Bethesda: 2004. [Google Scholar]
- 153.Katz DL, O’Connell M, Njike VY, Yeh M-C, Nawaz H. Strategies for the prevention and control of obesity in the school setting: systematic review and meta-analysis. Int J Obes. 2008;32:1780–9. doi: 10.1038/ijo.2008.158. [DOI] [PubMed] [Google Scholar]
- 154.Peñalvo JL, Sotos-Prieto M, Santos-Beneit G, Pocock S, Redondo J, Fuster V. The Program SI! intervention for enhancing a healthy lifestyle in preschoolers: first results from a cluster randomized trial. BMC Public Health. 2013;13:1208. doi: 10.1186/1471-2458-13-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Peñalvo JL, Santos-Beneit G, Sotos-Prieto M, Martínez R, Rodríguez C, Franco M, López-Romero P, Pocock S, Redondo J, Fuster V. A cluster randomized trial to evaluate the efficacy of a school-based behavioral intervention for health promotion among children aged 3 to 5. BMC Public Health. 2013;13:656. doi: 10.1186/1471-2458-13-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–53. doi: 10.1016/S0140-6736(07)61698-5. [DOI] [PubMed] [Google Scholar]
- 157.Willett WC, Koplan JP, Nugent R, Dusenbury C, Puska P, Gaziano TA. Prevention of Chronic Disease by Means of Diet and Lifestyle Changes. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease Control Priorities in Developing Countries. 2. World Bank; 2006. [PubMed] [Google Scholar]
- 158.Smit W, Hancock T, Kumaresen J, Santos-Burgoa C, Sánchez-Kobashi Meneses R, Friel S. Toward a research and action agenda on urban planning/design and health equity in cities in low and middle-income countries. J Urban Health. 2011;88:875–85. doi: 10.1007/s11524-011-9605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trowbridge MJ, Schmid TL. Built environment and physical activity promotion: place-based obesity prevention strategies. J Law Med Ethics. 2013;41(Suppl 2):46–51. doi: 10.1111/jlme.12109. [DOI] [PubMed] [Google Scholar]
- 160.World Health Organization. Global recommendations on physical activity for health. 2010 [PubMed] [Google Scholar]
- 161.Díaz Del Castillo A, Sarmiento OL, Reis RS, Brownson RC. Translating evidence to policy: urban interventions and physical activity promotion in Bogotá, Colombia and Curitiba, Brazil. Transl Behav Med. 2011;1:350–60. doi: 10.1007/s13142-011-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lachat C, Otchere S, Roberfroid D, Abdulai A, Seret FMA, Milesevic J, Xuereb G, Candeias V, Kolsteren P. Diet and physical activity for the prevention of noncommunicable diseases in low- and middle-income countries: a systematic policy review. In: Cobiac LJ, editor. PLoS Med. Vol. 10. 2013. p. e1001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jha P, Chaloupka FJ, editors. Tobacco Control in Developing Countries. New York: Oxford University Press; 2000. [Google Scholar]
- 164.Jha P, Chaloupka FJ, Moore J, Gajalakshmi V, Gupta PC, Peck R, Asma S, Witold Z. Tobacco Addiction. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease Control Priorities in Developing Countries. 2. World Bank; 2006. [Google Scholar]
- 165.Piné-Abata H, McNeill A, Raw M, Bitton A, Rigotti N, Murray R. A survey of tobacco dependence treatment guidelines in 121 countries. Addiction. 2013;108:1470–5. doi: 10.1111/add.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mamudu HM, Hammond R, Glantz SA. Project Cerberus: tobacco industry strategy to create an alternative to the Framework Convention on Tobacco Control. Am J Public Health. 2008;98:1630–42. doi: 10.2105/AJPH.2007.129478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gonzalez M, Green LW, Glantz SA. Through tobacco industry eyes: civil society and the FCTC process from Philip Morris and British American Tobacco’s perspectives. Tob Control. 2012;21:e1. doi: 10.1136/tc.2010.041657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bitton A, Green C, Colbert J. Improving the delivery of global tobacco control. Mt Sinai J Med. 2011;78:382–93. doi: 10.1002/msj.20252. [DOI] [PubMed] [Google Scholar]
- 169.Bitton A, Raw M, Richards A, McNeill A, Rigotti NA. A comparison of four international surveys of tobacco dependence treatment provision: implications for monitoring the Framework Convention on Tobacco Control. Addiction. 2010;105:2184–91. doi: 10.1111/j.1360-0443.2010.03060.x. [DOI] [PubMed] [Google Scholar]
- 170.Weishaar H, Collin J, Smith K, Grüning T, Mandal S, Gilmore A. Global health governance and the commercial sector: a documentary analysis of tobacco company strategies to influence the WHO framework convention on tobacco control. In: Novotny TE, editor. PLoS Med. Vol. 9. 2012. p. e1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Crosbie E, Sebrié EM, Glantz SA. Tobacco industry success in Costa Rica: the importance of FCTC article 5.3. Salud Publica Mex. 2012;54:28–38. [PMC free article] [PubMed] [Google Scholar]
- 172.Nakkash R, Lee K. The tobacco industry’s thwarting of marketing restrictions and health warnings in Lebanon. Tob Control. 2009;18:310–6. doi: 10.1136/tc.2008.029405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee S, Ling PM, Glantz SA. The vector of the tobacco epidemic: tobacco industry practices in low and middle-income countries. Cancer Causes Control. 2012;23(Suppl 1):117–29. doi: 10.1007/s10552-012-9914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lencucha R. Philip Morris versus Uruguay: health governance challenged. Lancet. 2010;376:852–3. doi: 10.1016/S0140-6736(10)61256-1. [DOI] [PubMed] [Google Scholar]
- 175.WHO Framework Convention on Tobacco Control Convention Secretariat. 2012 Global Progress Report on Implementation of the WHO Framework Convention on Tobacco Control. 2012 [Google Scholar]
- 176.Smith KE, Gilmore AB, Fooks G, Collin J, Weishaar H. Tobacco industry attempts to undermine Article 5.3 and the “good governance” trap. Tob Control. 2009;18:509–11. doi: 10.1136/tc.2009.032300. [DOI] [PubMed] [Google Scholar]
- 177.Berwanger O, Guimarães HP, Laranjeira LN, et al. Effect of a multifaceted intervention on use of evidence-based therapies in patients with acute coronary syndromes in Brazil: the BRIDGE-ACS randomized trial. JAMA. 2012;307:2041–9. doi: 10.1001/jama.2012.413. [DOI] [PubMed] [Google Scholar]
- 178.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet. 2006;368:679–86. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation. 2010;122:2078–88. doi: 10.1161/CIRCULATIONAHA.109.873232. [DOI] [PubMed] [Google Scholar]
- 180.Sanz G, Fuster V. Prevention: Polypills for cardiovascular prevention: a step forward? Nat Rev Cardiol. 2013;10:683–4. doi: 10.1038/nrcardio.2013.157. [DOI] [PubMed] [Google Scholar]
- 181.Sanz G, Fuster V, Guzmán L, Guglietta A, Arnáiz JA, Martínez F, Sarria A, Roncaglioni MC, Taubert K. The fixed-dose combination drug for secondary cardiovascular prevention project: improving equitable access and adherence to secondary cardiovascular prevention with a fixed-dose combination drug. Study design and objectives. Am Heart J. 2011;162:811–817.e1. doi: 10.1016/j.ahj.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 182.Sanz G, Fuster V. Fixed-dose combination therapy and secondary cardiovascular prevention: rationale, selection of drugs and target population. Nat Clin Pract Cardiovasc Med. 2009;6:101–10. doi: 10.1038/ncpcardio1419. [DOI] [PubMed] [Google Scholar]
- 183.Elley CR, Gupta AK, Webster R, Selak V, Jun M, Patel A, Rodgers A, Thom S. The efficacy and tolerability of “polypills”: meta-analysis of randomised controlled trials. In: Wright JM, editor. PLoS One. Vol. 7. 2012. p. e52145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thom S, Poulter N, Field J, Patel A, Prabhakaran D, Stanton A, Grobbee DE, Bots ML, Reddy KS, Cidambi R, Bompoint S, Billot L, Rodgers A. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310:918–29. doi: 10.1001/jama.2013.277064. [DOI] [PubMed] [Google Scholar]
- 185.Bautista LE, Vera-Cala LM, Ferrante D, et al. A “polypill” aimed at preventing cardiovascular disease could prove highly cost-effective for use in Latin America. Health Aff. 2013;32:155–64. doi: 10.1377/hlthaff.2011.0948. [DOI] [PubMed] [Google Scholar]
- 186.Huffman MD, Yusuf S. Polypills: Essential Medicines for Cardiovascular Disease Secondary Prevention? J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2013.08.1665. [DOI] [PubMed] [Google Scholar]
- 187.Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373:240–9. doi: 10.1016/S0140-6736(08)61762-6. [DOI] [PubMed] [Google Scholar]
- 188.Mendis S, Fukino K, Cameron A, Laing R, Filipe A, Jr, Khatib O, Leowski J, Ewen M. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ. 2007;85:279–288. doi: 10.2471/BLT.06.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vedanthan R, Fuster V. Urgent need for human resources to promote global cardiovascular health. Nat Rev Cardiol. 2011;8:114–7. doi: 10.1038/nrcardio.2010.178. [DOI] [PubMed] [Google Scholar]
- 190.Van Olmen J, Schellevis F, Van Damme W, Kegels G, Rasschaert F. Management of Chronic Diseases in Sub-Saharan Africa: Cross-Fertilisation between HIV/AIDS and Diabetes Care. J Trop Med. 2012;2012 doi: 10.1155/2012/349312. 349312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Frenk J. The global health system: strengthening national health systems as the next step for global progress. PLoS Med. 2010;7:e1000089. doi: 10.1371/journal.pmed.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ooms G, Van Damme W, Baker BK, Zeitz P, Schrecker T. The “diagonal” approach to Global Fund financing: a cure for the broader malaise of health systems? Global Health. 2008;4:6. doi: 10.1186/1744-8603-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Samb B, Evans T, Dybul M, Atun R, Moatti J-P, Nishtar S, Wright A, Celletti F, Hsu J, Kim JY, Brugha R, Russell A, Etienne C. An assessment of interactions between global health initiatives and country health systems. Lancet. 2009;373:2137–69. doi: 10.1016/S0140-6736(09)60919-3. [DOI] [PubMed] [Google Scholar]
- 194.Drobac PC, Basinga P, Condo J, et al. Comprehensive and integrated district health systems strengthening: the Rwanda Population Health Implementation and Training (PHIT) Partnership. BMC Health Serv Res. 2013;13(Suppl 2):S5. doi: 10.1186/1472-6963-13-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vasan A, Ellner A, Lawn SD, Gove S, Anatole M, Gupta N, Drobac P, Nicholson T, Seung K, Mabey DC, Farmer PE. Integrated care as a means to improve primary care delivery for adults and adolescents in the developing world: a critical analysis of Integrated Management of Adolescent and Adult Illness (IMAI) BMC Med. 2014;12:6. doi: 10.1186/1741-7015-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mendis S, Fuster V. National policies and strategies for noncommunicable diseases. Nat Rev Cardiol. 2009;6:723–7. doi: 10.1038/nrcardio.2009.171. [DOI] [PubMed] [Google Scholar]
- 197.Narayan KMV, Ali MK, Koplan JP. Global Noncommunicable Diseases — Where Worlds Meet. N Engl J Med. 2010;363:1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- 198.Dieleman JL, Graves CM, Templin T, Johnson E, Baral R, Leach-Kemon K, Haakenstad AM, Murray CJL. Global Health Development Assistance Remained Steady In 2013 But Did Not Align With Recipients’ Disease Burden. Health Aff (Millwood) 2014 doi: 10.1377/hlthaff.2013.1432. hlthaff.2013.1432. [DOI] [PubMed] [Google Scholar]
- 199.Stuckler D, King L, Robinson H, McKee M. WHO’s budgetary allocations and burden of disease: a comparative analysis. Lancet. 2008;372:1563–9. doi: 10.1016/S0140-6736(08)61656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sridhar D, Batniji R. Misfinancing global health: a case for transparency in disbursements and decision making. Lancet. 2008;372:1185–91. doi: 10.1016/S0140-6736(08)61485-3. [DOI] [PubMed] [Google Scholar]
- 201.Nugent R, Feigl AB. Where Have All the Donors Gone? Scarce Donor Funding for Non-Communicable Diseases. 2010 [Google Scholar]
- 202.Sridhar D, Brolan CE, Durrani S, Edge J, Gostin LO, Hill P, McKee M. Recent shifts in global governance: implications for the response to non-communicable diseases. PLoS Med. 2013;10:e1001487. doi: 10.1371/journal.pmed.1001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vamadevan AS, Shah BR, Califf RM, Prabhakaran D. Cardiovascular research in India: a perspective. Am Heart J. 2011;161:431–8. doi: 10.1016/j.ahj.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 204.Gurm HS, Eagle KA. Channelling regional registries for optimization of cardiac care: lessons from around the world. Eur Heart J. 2013;34:83–5. doi: 10.1093/eurheartj/ehs328. [DOI] [PubMed] [Google Scholar]
- 205.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 206.Institute for Health Metrics and Evaluation. GBD Compare. 2013 [Google Scholar]
- 207.Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–7. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- 208.Laatikainen T, Critchley J, Vartiainen E, Salomaa V, Ketonen M, Capewell S. Explaining the decline in coronary heart disease mortality in Finland between 1982 and 1997. Am J Epidemiol. 2005;162:764–73. doi: 10.1093/aje/kwi274. [DOI] [PubMed] [Google Scholar]
- 209.Bennett K, Kabir Z, Unal B, Shelley E, Critchley J, Perry I, Feely J, Capewell S. Explaining the recent decrease in coronary heart disease mortality rates in Ireland, 1985-2000. J Epidemiol Community Health. 2006;60:322–7. doi: 10.1136/jech.2005.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Palmieri L, Bennett K, Giampaoli S, Capewell S. Explaining the decrease in coronary heart disease mortality in Italy between 1980 and 2000. Am J Public Health. 2010;100:684–92. doi: 10.2105/AJPH.2008.147173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Capewell S, Beaglehole R, Seddon M, McMurray J. Explanation for the Decline in Coronary Heart Disease Mortality Rates in Auckland, New Zealand, Between 1982 and 1993. Circulation. 2000;102:1511–1516. doi: 10.1161/01.cir.102.13.1511. [DOI] [PubMed] [Google Scholar]
- 212.Capewell S, Morrison CE, McMurray JJ. Contribution of modern cardiovascular treatment and risk factor changes to the decline in coronary heart disease mortality in Scotland between 1975 and 1994. Heart. 1999;81:380–386. doi: 10.1136/hrt.81.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Björck L, Rosengren A, Bennett K, Lappas G, Capewell S. Modelling the decreasing coronary heart disease mortality in Sweden between 1986 and 2002. Eur Heart J. 2009;30:1046–56. doi: 10.1093/eurheartj/ehn554. [DOI] [PubMed] [Google Scholar]
- 214.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the Decrease in U.S. Deaths from Coronary Disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


