Abstract
Introduction
The aim of the study was to identify the association of systolic blood pressure (SBP) levels with cardiovascular events, all-cause mortality, and falls among elderly persons taking antihypertensive medication.
Methods
US adults ≥45 years of age taking antihypertensive medication enrolled in the REGARDS study were categorized into 3 age groups: 55–64, 65–74 and ≥75 years old and baseline on-treatment SBP levels. Our primary analyses focused on incident cardiovascular disease (CVD) (n=9,787) and all-cause mortality (n=13,948).
Results
During follow-up, 530 (5.4%) participants had CVD events and 2095 (15%) participants died. After multivariable adjustment among participants ≥75, the incidence of CVD per 1,000 person-years (95% confidence interval) was 16.9 (11.1–25.7), 13.4 (9.2–19.7), 11.6 (7.6–17.7), 17.8 (11.2–27.5) and 36.7 (26.6–50.8) at SBP levels of <120, 120–129, 130–139, 140–149, and ≥150mmHg, respectively. For the same SBP categories, the adjusted CVD incidence rates were 9.3 (7.2–12.0), 10.0 (8.1–12.3), 9.4 (7.5–11.8), 14.0 (11.0–17.8), and 16.4 (12.5–21.4), respectively, among participants 55–64 years, and 16.5 (13.6–21.5), 17.4 (14.8–20.6), 19.2 (16.4–22.5), 22.3 (18.6–26.9), and 27.6 (22.7–33.4), respectively, for participants 65–74 years. Among participants aged 55–64 and 65–74 years, a linear association was present between higher SBP categories and all-cause mortality risk (each p-trend<0.001). In contrast, for participants ≥75 years no association was present between SBP and all-cause mortality (p-trend=0.319). No association was observed between SBP and falls among participants in all age groups.
Conclusions
Among adults aged ≥55 taking antihypertensive medication, SBP between 120–139mmHg was significantly associated with a reduced risk for cardiovascular and all-cause mortality outcomes.
Keywords: blood pressure, elderly, hypertension, treatment, mortality, stroke, coronary heart disease
INTRODUCTION
Over the past few decades, a number of randomized trials and meta-analyses have supported the benefits of antihypertensive medication on reducing the incidence of cardiovascular disease (CVD) among the elderly. However, these studies were not designed to identify the appropriate target blood pressure (BP) in this population.1–7 The American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) 2011 Expert Consensus Document on Hypertension in the Elderly guidelines suggested that a target of <140/90 mmHg in persons aged 65–79 years and a systolic BP (SBP) of 140–145 mmHg, if tolerated, in persons aged 80 years and older.5 Additionally, the 2013 European Society of Hypertension (ESH)/European Society of Cardiology (ESC) guidelines for the management of hypertension8 emphasized that the available randomized trials of antihypertensive treatment in the elderly showed a reduction in cardiovascular (CV) events through lowering of BP; however the average achieved SBP in trials never attained values <140 mmHg. Therefore the ESH/ESC guidelines suggest that in elderly hypertensives, both less than and over 80 years old with SBP ≥160 mmHg, it is recommended to reduce SBP to between 150 and 140 mmHg.8 However, in fit persons <80 years old SBP <140 mmHg may be considered, whereas in the fragile elderly population SBP goals should be adapted to individual tolerability.8 Experts have also emphasized that very limited data exist to make definitive recommendations on targeted BP levels in the elderly.5–12 These suggestions were confirmed in the recent the Eighth Joint National Committee (JNC8) guidelines13, which are still based on limitted data from available randomized controlled trials, and therefore are usually expert based opinions, especially concerning the lower BP goals (<140 mmHg) in the general population aged ≥60 years (Expert Opinion – Grade E)13.
Randomized controlled trials are needed to provide definitive evidence on the appropriate BP target for elderly patients taking antihypertensive medication. In absence of these data, our aim was to investigate the SBP associated with the lowest CVD risk in elderly persons taking antihypertensive medication on the basis of data from a national observational cohort study in the United States, the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
METHODS
Study Participants
The REGARDS study is a population-based investigation of stroke incidence among US adults ≥45 years of age.14 The study was designed to oversample blacks and individuals living in the “stroke belt” and “stroke buckle” regions of the United States. The “stroke buckle” was defined as the coastal plain region of North Carolina, South Carolina, and Georgia and the “stroke belt” as the remainder of North Carolina, South Carolina, and Georgia as well as Alabama, Mississippi, Tennessee, Arkansas and Louisiana. Between January 1st, 2003 and October 31st, 2007, 30,239 participants were enrolled. Additional information on the design and conduct of the REGARDS study has been described.14
The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers. All participants provided informed consent.
Data Collection
Baseline data were collected through a telephone interview, self-administered questionnaires and an in-home examination. Trained staff conducted computer-assisted telephone interviews to obtain information on demographics, education, household income, cigarette smoking status, use of antihypertensive medication, and self-report of prior diagnoses of co-morbid conditions including diabetes, myocardial infarction, stroke and atrial fibrillation. Following the interview, trained technicians conducted an in-home visit that included 2 BP measurements, the collection of blood and urine samples, and an electrocardiogram (ECG). Blood collected during the in-home study visit was processed and shipped, overnight to the REGARDS central laboratory at the University of Vermont.
Atrial fibrillation was defined by its presence on the study ECG or a self-report of a prior diagnosis. Diabetes was defined as a serum glucose ≥126 mg/dL for participants who had fasted ≥8 h prior to their blood draw, serum glucose ≥200 mg/dL for those who had not fasted, or current use of insulin or oral hypoglycemic medications14. Total and high-density lipoprotein (HDL) cholesterol were measured using colorimetric reflectance spectrophotometry. Low-density lipoprotein (LDL) was calculated using the Friedewald equation. Dyslipidemia was defined as total cholesterol ≥240, LDL-C ≥160, HDL-C <40 mg/dL or self-reported use of pharmacologic lipid-lowering therapy.14 History of CHD at baseline was defined by a self-reported history or ECG evidence of myocardial infarction or a self-reported history of a revascularization procedure. History of stroke was defined on the basis of self-report. Participants with a history of CHD or stroke were considered to have a history of CVD.14
BP measurement
BP was measured 2 times during the in-home examination using aneroid sphygmomanometers following a standardized protocol by a trained examiner. BP quality control was monitored by central examination of digit preference and retraining of technicians took place as necessary. Participants were asked to sit for 5 min with both feet on the floor prior to the first measurement. A 30 sec rest occurred between measurements. Whenever possible, BP was measured in the left arm and a large size cuff was used if the arm circumference was greater than 13 in (33 cm). Both the cuff bladder width and pulse obliteration level were recorded. The cuff was inflated to 20 mmHg above the pulse obliteration level and deflated at approximately 2 mmHg per sec. The 2 BP measurements were averaged for analyses.
Study Outcomes
REGARDS participants or their proxies were contacted by telephone every 6 months following baseline to assess stroke and CHD events and all-cause mortality. Medical records were retrieved for suspected stroke and CHD-related hospitalizations and deaths; adjudication of these records was performed by physician panels. We studied 5 outcomes in the current analysis: CVD, CHD, stroke, all-cause mortality, and recurrent falls. For each outcome, time-to-event analysis was performed; participants were followed until their first event and recurrent events were not studied.
CHD
The occurrence of CHD events was defined as nonfatal MI or CHD death. Medical records were used by trained physicians to adjudicate hospitalizations or deaths that could potentially be related to CHD, following published guidelines.15,16 Specifically, medical records were examined for the presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac troponin or creatine kinase-MB over 6 or more hours with a peak value greater than or equal to twice the upper limit of normal (diagnostic cardiac enzymes); and ECG changes consistent with ischemia or MI, guided by the Minnesota Code and classified as evolving diagnostic, positive, nonspecific or not consistent with ischemia.17,18 Additionally, following published guidelines, medical records in the last year of life, death certificates and autopsy reports were collected and reviewed to determine if the death was a CHD death.15,16 Incident CHD events through December 31st, 2009 were available for the current analyses.
Stroke
The investigators obtained medical records when participants self-reported a possible stroke/transient ischemic attack (TIA), reported a stroke symptom on the Questionnaire for Verifying Stroke-Free Status resulting in hospitalization or when a participant died with a potential cause of stroke.19 When potential events were reported, hospital charts were retrieved for adjudication and events were confirmed by a panel of physicians, according to the World Health Organization (WHO) definition.20 Events not meeting the WHO definition but characterized by symptoms lasting <24 h with neuroimaging consistent with acute infarct or hemorrhage were classified as clinical strokes. Additionally, medical records in the last year of life, death certificates, and autopsy reports were collected and reviewed to determine if the death was stroke-related. This analysis included WHO defined and clinical stroke cases.21 Data on incident stroke was available through August 17th, 2011.
CVD
CVD events included the first occurrence of a CHD or stroke event. As CHD events were only available through 2009, strokes that occurred after January 1st, 2010 were not included in the analysis of CVD.
All-Cause Mortality
Participant deaths were detected by report of next-of-kin, online sources (e.g. the Social Security Death Index), and the National Death Index. Proxies or next-of-kin were interviewed to obtain information surrounding the circumstances of participant death. Additionally, death certificates and autopsy reports were collected. Deaths occurring through April 1st, 2012 were included in the current analysis.
Recurrent Falls
Recurrent falls were assessed by telephone at the first follow-up interview (i.e., 6 months following baseline). Using a modified version of the Study of Osteoporotic Fractures22 falls ascertainment question, participants were asked “Since the last time we contacted you, have you fallen and landed on the ground or floor or fallen and hit an object like a table or chair?”. Those responding in the affirmative were subsequently asked, “How many times have you fallen in the last 6 months?”. Recurrent falls was defined as 2 or more falls. This definition is emphasized by the American Geriatrics Society Fall clinical practice guideline for prevention of falls.23
Statistical Analyses
Among participants free of CVD at baseline, characteristics were calculated by age (55–64, 65–74 and 75 years and older). Also, within age group, characteristics of participants free of CVD were calculated by SBP (<120, 120–129, 130–139, 140–149 and ≥150 mmHg). ANOVA and chi-square tests were used for the comparison across the age groups and, within age group, across SBP categories. For each age group, incidence rates for CVD, CHD, stroke, and all-cause mortality were calculated by SBP. Cox proportional hazards models were used to calculate the hazard ratio (HR) for CVD, CHD, stroke, and all-cause mortality associated with SBP categories with <120 mmHg serving as the referent. Initially, incidence rates and hazard ratios were unadjusted (Model 1) and adjusted for age, race, gender, region of residence, household income and education (Model 2). Full covariate adjustment (Model 3) included all of the variables in Model 2, and dyslipidemia, diabetes, current smoking, atrial fibrillation, diastolic BP, history of stroke (for CHD outcome), history of CHD (for stroke outcome), and history of stroke and CHD for all-cause mortality. Next, we used restricted quadratic splines to calculate the multivariable adjusted HR for each outcome associated with SBP modeled as continuous variables. Finally, within each age group, the percentage of participants with recurrent falls and, using logistic regression, odds ratios for recurrent falls were calculated by SBP category. Three levels of adjustment, identical to the CVD and all-cause mortality outcomes, were performed. The p-values for trends were calculated by assigning the median SBP value to all participants within each SBP category and modeling this as a continuous variable. Deviations from linearity were tested by adding a quadratic term for SBP to the regression model. We used a two-sided p-value with a threshold set at 0.05. All analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The current analysis was limited to participants 55 years of age and older (n=26,396). We excluded participants without valid systolic and diastolic BP measurements at baseline (n=78) and missing follow-up data (n=414). An additional 11,956 participants who were not taking antihypertensive medications at baseline were excluded. After these exclusion criteria were applied, 13,948 participants formed the base population and were included in the analysis of all-cause mortality. Participants with a history of CHD (n=3,310) were excluded from analyses of incident CHD (leaving n=10,638 individuals). Also, participants with a history of stroke (n=1,333) and CVD (n=4,161) were excluded from analyses of these outcomes, leaving n=12,615 and n=9787 individuals for the analysis of incident stroke and incident CVD, respectively.
Participant characteristics are presented by age group in Table 1 and by SBP within each age group separately in eTables 1–3. Participants 55–64 and 65–74 years of age were more likely to be black and less likely to have atrial fibrillation when compared with their counterparts ≥75 years of age. The percentage of participants with less than a high school education and household income <$20,000 increased at older age. Current smoking and diabetes were less common at older age.
Table 1.
Baseline characteristics of the Reasons for Geographic and Racial Difference in Stroke (REGARDS) study population without a history of cardiovascular disease taking antihypertensive medication by age.
| Age (years) | ||||
|---|---|---|---|---|
| 55–64 (n = 4181) | 65–74 (n = 3767) | ≥ 75 (n = 1839) | p-value | |
| Age, y (sd) | 59.7 (2.8) | 69.0 (2.8) | 79.3 (3.7) | |
| Men, no. (%) | 1590 (38.0) | 1481 (39.3) | 679 (36.9) | 0.580 |
| Black, no. (%) | 2351 (56.2) | 1882 (50.0) | 835 (45.4) | <0.001 |
| Region of Residence | ||||
| Stroke belt, no. (%)** | 1543 (36.9) | 1279 (34.0) | 584 (31.8) | <0.001 |
| Stroke buckle, no. (%)** | 915 (21.9) | 795 (21.1) | 347 (18.9) | |
| Non-belt, no. (%) | 2638 (63.1) | 2488 (66.1) | 1255 (68.2) | |
| Less than a high school education, no. (%) | 452 (10.8) | 580 (15.4) | 383 (20.9) | <0.001 |
| Household Income < $20,000, no. (%) | 713 (19.1) | 793 (24.0) | 461 (30.4) | <0.001 |
| Current smoker, no. (%) | 667 (16.0) | 362 (9.7) | 95 (5.2) | <0.001 |
| Diabetes Mellitus, no/(%) | 1172 (28.9) | 1037 (28.4) | 435 (24.7) | 0.005 |
| Isolated Systolic Hypertension, no. (%) | 610 (14.6) | 789 (21.0) | 473 (25.7) | <0.001 |
| HDL-cholesterol (mg/dL), mean (sd) | 51.2 (15.5) | 52.2 (16.4) | 53.9 (16.7) | <0.001 |
| LDL-cholesterol (mg/dL), mean (sd) | 114.7 (34.0) | 110.2 (33.7) | 109.9 (31.9) | <0.001 |
| Total cholesterol (mg/dL), mean (sd) | 193.2 (37.8) | 189.3 (39.2) | 188.7 (37.8) | <0.001 |
| Atrial Fibrillation, no. (%) | 310 (7.6) | 275 (7.4) | 197 (11.0) | <0.001 |
| Dyslipidemia, no. (%) | 2478 (59.3) | 2303 (61.1) | 1040 (56.6) | 0.216 |
| Follow-up*** | ||||
| Cardiovascular disease events**** | 154 (3.6%) | 217 (5.7%) | 159 (8.5%) | <0.001 |
| Coronary heart disease events† | 112 (2.4%) | 145 (3.5%) | 106 (5.1%) | <0.001 |
| Stroke†† | 101 (1.9%) | 188 (3.8%) | 136 (5.2%) | <0.001 |
| All-cause mortality‡ | 460 (8.2%) | 775 (14.1%) | 860 (28.4%) | <0.001 |
ABBREVIATIONS: HDL, high density lipoprotein; LDL, low density lipoprotein; sd, standard deviation.
The “stroke buckle” was defined as the coastal plain region of North Carolina, South Carolina, and Georgia and the “stroke belt” as the remainder of North Carolina, South Carolina, and Georgia as well as Alabama, Mississippi, Tennessee, Arkansas and Louisiana. The non-belt region represents the remainder of the contiguous United States.
Median follow-up for cardiovascular disease and coronary heart disease was 4.5 years (maximum 7 years), for stroke was 5.7 years (maximum 8.5 years) and for all-cause mortality was 6.0 years (maximum 9.1 years).
After the exclusion criteria were applied, 9,787 individuals were included in the analyses of CVD.
The analysis of CHD included 10,638 participants and only excluded participants with a history of heart disease.
The analysis of stroke included 12,615 participants and only excluded participants with a history of stroke.
The analysis of all-cause mortality included 13,948 participants (i.e., including those with a history of CVD, CHD, or stroke).
Median follow-up for CVD and CHD was 4.5 years (25–75th percentiles: 3.1–5.5 years, maximum 7 years), for stroke was 5.7 years (25–75th percentiles: 4.1–6.9 years, maximum 8.5 years) and for all-cause mortality was 6.0 years (25–75th percentiles: 4.4–7.1 years, maximum 9.1 years). During follow-up, there were 530 (5.4%), 363 (3.4%), and 425 (3.4%) cases of CVD, CHD and stroke, respectively, and 2095 (15%) deaths. Of the 425 strokes that occurred during follow-up, 38 (8.9%) were hemorrhagic, 359 (84.5%) were ischemic and the type was undetermined for 28 (6.6%) events.
The incidence of CVD increased at higher levels of SBP for participants aged 55–64 and 65–74. In contrast, for participants 75 years and older, the incidence of CVD was highest at SBP <120 and ≥140 mmHg (Table 2). After multivariable adjustment and compared with SBP <120 mmHg, SBP ≥150 mmHg was associated with an increased risk of CVD among those 65 to 74 (HR 2.33; 95%CI: 1.37–3.97) and ≥75 years of age (HR 2.18, 95%CI: 1.27–3.76). For those <65 years of age, the multivariable adjusted HR for CVD comparing SBP ≥150 to <120 mmHg was 1.56 (95%CI: 0.79–3.09). SBP 130–139 mmHg was characterized by the numerically lowest CVD risk for participants at ≥75 (adjusted HR 0.69; 95%CI: 0.39–1.24). For participants ≥75 years of age the association between SBP and CVD incidence was not linear but rather J-shaped as noted by the significant association for the quadratic term (SBP squared - p = 0.002) in the full multivariable adjusted model (Table 2). When modeled using splines, the risk for CVD began to increase at SBP levels ≥130 to 150 mmHg, depending on age (Figure 1). SBP <120 mmHg was not associated with an increased CVD risk for participants 55–64 and 65–74 years of age; however CVD risk appeared to be increased at SBP <120 mm Hg for participants ≥75 years of age.
Table 2.
Cardiovascular disease incidence rates and hazard ratios (HRs) by systolic blood pressure and age.
| Systolic blood pressure category in mmHg | |||||||
|---|---|---|---|---|---|---|---|
| Age 55 to 64 years | <120 (n = 949) | 120–129 (n = 1189) | 130–139 (n = 1006) | 140–149 (n = 592) | ≥150 (n = 445) | p-linear | p-quadratic |
| Number of events | 27 | 46 | 31 | 21 | 29 | ||
| Person-years | 3973.7 | 4974.0 | 4369.0 | 2594.3 | 1952.9 | ||
| Incidence† (95% CI) | |||||||
| Model 1 | 6.8 (4.7–9.9) | 9.2 (6.9–12.3) | 7.1 (5.0–10.1) | 8.1 (5.3–12.4) | 14.8 (10.3–21.4) | 0.017 | 0.200 |
| Model 2 | 6.7 (4.6–9.8) | 8.9 (6.7–12.0) | 6.5 (4.5–9.3) | 7.3 (4.7–11.3) | 12.8 (8.8–18.7) | 0.070 | 0.181 |
| Model 3 | 6.4 (4.2–9.9) | 8.8 (6.5–12.0) | 5.7 (3.9–8.3) | 6.3 (3.9–10.1) | 10.1 (6.3–16.1) | 0.474 | 0.236 |
| Hazard ratio (95% CI) | |||||||
| Model 1 | 1 (ref) | 1.36 (0.85–2.19) | 1.04 (0.62–1.73) | 1.19 (0.67–2.10) | 2.18 (1.29–3.68) | 0.018 | 0.193 |
| Model 2 | 1 (ref) | 1.33 (0.83–2.14) | 0.96 (0.57–1.61) | 1.09 (0.61–1.93) | 1.91 (1.12–3.25) | 0.070 | 0.176 |
| Model 3 | 1 (ref) | 1.37 (0.82–2.28) | 0.88 (0.50–1.57) | 0.98 (0.51–1.88) | 1.56 (0.79–3.09) | 0.481 | 0.237 |
| Systolic blood pressure category in mmHg | |||||||
|---|---|---|---|---|---|---|---|
| Age 65 to 74 years | <120 (n = 772) | 120–129 (n =1018) | 130–139 (n = 923) | 140–149 (n = 602) | ≥150 (n = 452) | p-linear | p-quadratic |
| Number of events | 33 | 48 | 55 | 35 | 46 | ||
| Person-years | 3214.6 | 4259.6 | 4004.5 | 2574.2 | 1955.0 | ||
| Incidence† (95% CI) | |||||||
| Model 1 | 10.3 (7.3–14.4) | 11.3 (8.5–15.0) | 13.7 (10.6–17.9) | 13.6 (9.8–18.9) | 23.5 (17.6–31.4) | 0.009 | 0.327 |
| Model 2 | 9.7 (6.9 – 13.7) | 10.7 (8.0–14.3) | 12.2 (9.3–16.1) | 11.8 (8.4–16.6) | 19.6 (14.4–26.5) | 0.002 | 0.340 |
| Model 3 | 8.7 (6.0–12.6) | 9.9 (7.3–13.4) | 11.7 (8.8–15.6) | 12.5 (8.8–17.8) | 20.2 (14.3–28.6) | 0.001 | 0.452 |
| Hazard ratio (95% CI) | |||||||
| Model 1 | 1 (ref) | 1.10 (0.70–1.71) | 1.33 (0.86–2.04) | 1.31 (0.82–2.11) | 2.27 (1.45–3.55) | <0.001 | 0.317 |
| Model 2 | 1 (ref) | 1.10 (0.71–1.72) | 1.25 (0.81–1.93) | 1.21 (0.75–1.95) | 2.00 (1.28–3.14) | 0.002 | 0.334 |
| Model 3 | 1 (ref) | 1.14 (0.72–1.80) | 1.34 (0.85–2.14) | 1.44 (0.86–2.41) | 2.33 (1.37–3.97) | 0.001 | 0.443 |
| Systolic blood pressure category in mmHg | |||||||
|---|---|---|---|---|---|---|---|
| Age ≥ 75 years | <120 (n = 343) | 120–129 (n = 479) | 130–139 (n = 448) | 140–149 (n = 278) | ≥150 (n = 291) | p-linear | p-quadratic |
| Number of events | 27 | 32 | 27 | 24 | 49 | ||
| Person-years | 1365.5 | 1890.6 | 1847.8 | 1099.6 | 1156.9 | ||
| Incidence† (95% CI) | |||||||
| Model 1 | 19.8 (13.4–28.8) | 16.9 (12.0–23.9) | 14.6 (10.0–21.3) | 21.8 (14.6–32.6) | 42.4 (32.0–56.0) | <0.001 | 0.003 |
| Model 2 | 19.3 (13.2–28.2) | 15.6 (11.0–22.2) | 13.2 (8.9–19.4) | 20.7 (13.8–31.1) | 36.9 (27.5–49.6) | <0.001 | 0.003 |
| Model 3 | 16.9 (11.1–25.7) | 13.4 (9.2–19.7) | 11.6 (7.6–17.7) | 17.8 (11.5–27.5) | 36.7 (26.6–50.8) | <0.001 | 0.002 |
| Hazard ratio (95% CI) | |||||||
| Model 1 | 1 (ref) | 0.86 (0.51–1.43) | 0.74 (0.44–1.26) | 1.11 (0.64–1.92) | 2.15 (1.34–3.43) | <0.001 | 0.003 |
| Model 2 | 1 (ref) | 0.81 (0.48–1.35) | 0.68 (0.40–1.18) | 1.07 (0.62–1.86) | 1.91 (1.19–3.08) | <0.001 | 0.003 |
| Model 3 | 1 (ref) | 0.80 (0.46–1.38) | 0.69 (0.39–1.24) | 1.06 (0.58–1.93) | 2.18 (1.27–3.76) | <0.001 | 0.002 |
Incidence per 1,000 person-years. Model 1 is unadjusted. Model 2 is adjusted for age, race, gender, region of residence, income and education. Model 3 is adjusted for variables in Model 2 + dyslipidemia, diabetes, current smoking, atrial fibrillation and diastolic blood pressure.
Figure 1.
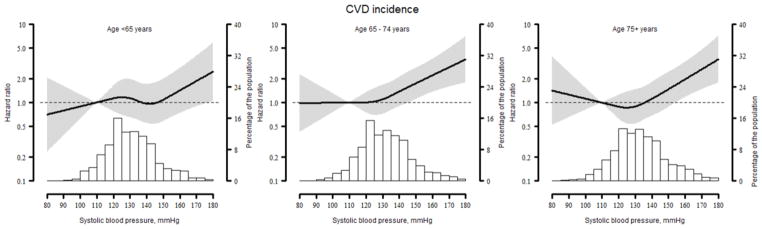
Restricted quadratic splines showing the association between systolic blood pressure and cardiovascular disease among REGARDS participants by age group.
Line represents the hazard ratio with the reference set at a systolic blood pressure of 110 mmHg. Grey area represents the 95% confidence interval. Histograms represent the distribution of systolic blood pressure in the population analyzed. The association is adjusted for age, race, gender, region of residence, income education, dyslipidaemia, diabetes, current smoking, atrial fibrillation and diastolic blood pressure.
For participants aged 55–64 and 65–74 years of age, the incidence of CHD increased progressively at higher SBP levels (p-value for linear trend = 0.028 and 0.001, respectively) (eTable 4). Among individuals ≥75 years of age, the incidence of CHD was lowest for those with SBP of 120 to 129 mmHg. These patterns remained present after multivariable adjustment (eTable 4). When modeled as a continuous variable, a direct linear association was present between SBP and CHD risk for participants 55 to 64 years of age while an increased CHD risk was present for SBP <120 and ≥130 mmHg for participants 65 to 74 and ≥75 years of age (eFigure 1).
For participants 55–64 and 65–74 years of age no association between SBP and stroke incidence was observed. (eTable 5). For participants ≥75 years of age, stroke incidence increased across the full range of SBP. After multivariable adjustment, SBP ≥150 mmHg was associated with a non-significantly increased hazard ratio of stroke (1.61; 95%CI: 0.84–3.07; p = 0.091) but no increased risk was observed for SBP levels of 120–129, 130–139 and 140–149 mmHg. When SBP was modeled as a continuous variable, higher SBP was associated with an increased risk for stroke among participants ≥75 years of age (eFigure 2).
Within each age group, all-cause mortality was higher at SBP levels ≥140 mm Hg (Table 3). Among participants 55 to 64 and 65 to 74 years of age, after multivariable adjustment and compared to participants with SBP <120 mmHg, SBP levels of 140 to 149 and ≥150 mmHg were associated with an increased hazard ratio for all-cause mortality (1.50; 95%Cl: 1.06–2.14 and 1.77; 95%Cl: 1.20–2.62, respectively, for the 55–64 age group, and 1.35; 95%Cl: 1.03–1.75 and 1.67; 95%Cl: 1.27–2.21, respectively, for the 65–74 age group). For participants ≥75 years of age, no association was present between SBP and all-cause mortality after multivariable adjustment. When modeled as a continuous variable, an increased risk for all-cause mortality was present at higher SBP for participants 55–64 and 65–74 years of age but not those ≥75 years of age. For participants ≥75 years of age, the risk for all-cause mortality was also increased at SBP levels <120 mmHg (Figure 2).
Table 3.
All-cause mortality rates and hazard ratios (HRs) by systolic blood pressure and age.
| Systolic blood pressure category in mmHg | |||||||
|---|---|---|---|---|---|---|---|
| Age 55 to 64 years | < 120 (n=1223) | 120 – 129 (n=1544) | 130 – 139 (n=1325) | 140 – 149 (n=795) | ≥150 (n=626) | p-linear | p-quadratic |
| Number of events | 86 | 112 | 94 | 84 | 84 | ||
| Person-years | 6996.6 | 8796.6 | 7652.7 | 4644.6 | 3511.4 | ||
| Incidence† (95% CI) | |||||||
| Model 1 | 12.3 (10.0–15.2) | 12.7 (10.6–15.3) | 12.3 (10.0–15.0) | 18.1 (14.6–22.4) | 23.9 (19.3–29.6) | <0.001 | 0.064 |
| Model 2 | 11.8 (9.6–14.7) | 11.7 (9.7–14.2) | 10.8 (8.8–13.3) | 15.5 (12.5–13.4) | 19.0 (15.2–23.8) | <0.001 | 0.070 |
| Model 3 | 9.3 (7.2–12.0) | 10.0 (8.1–12.3) | 9.4 (7.5–11.8) | 14.0 (11.0–17.8) | 16.4 (12.5–21.4) | <0.001 | 0.283 |
| Hazard ratio (95% CI) | |||||||
| Model 1 | 1 (ref) | 1.04 (0.78–1.37) | 1.00 (0.74–1.34) | 1.46 (1.08–1.98) | 1.95 (1.44–2.63) | <0.001 | 0.060 |
| Model 2 | 1 (ref) | 0.99 (0.75–1.31) | 0.91 (0.68–1.22) | 1.30 (0.96–1.76) | 1.60 (1.18–2.17) | 0.001 | 0.065 |
| Model 3 | 1 (ref) | 1.07 (0.79–1.47) | 1.02 (0.73–1.42) | 1.50 (1.06–2.14) | 1.77 (1.20–2.62) | 0.001 | 0.272 |
| Systolic blood pressure category in mmHg | |||||||
|---|---|---|---|---|---|---|---|
| Age 65 to 74 years | < 120 (n=1118) | 120 – 129 (n=1437) | 130 – 139 (n=1336) | 140 – 149 (n=844) | ≥150 (n=703) | p-linear | p-quadratic |
| Number of events | 136 | 169 | 183 | 132 | 155 | ||
| Person-years | 6325.5 | 8194.2 | 7633.5 | 4832.4 | 3878.3 | ||
| Incidence† (95% CI) | |||||||
| Model 1 | 21.5 (18.2–25.4) | 20.6 (17.7–24.0) | 24.0 (10.7–27.7) | 27.3 (23.0–32.4) | 40.0 (34.1–49.8) | <0.001 | 0.026 |
| Model 2 | 20.2 (17.1–24.0) | 19.8 (17.0–23.1) | 21.1 (18.2–24.5) | 23.8 20.0–28.3) | 31.9 (27.0–37.6) | <0.001 | 0.055 |
| Model 3 | 16.5 (13.6–21.0) | 17.4 (14.8–20.6) | 19.2 (16.4–22.5) | 22.3 (18.6–26.9) | 27.6 (22.7–33.4) | <0.001 | 0.433 |
| Hazard ratio (95% CI) | |||||||
| Model 1 | 1 (ref) | 0.96 (0.76–1.20) | 1.11 (0.89–1.38) | 1.26 (0.99–1.60) | 1.85 (1.47–2.33) | <0.001 | 0.023 |
| Model 2 | 1 (ref) | 0.98 (0.78–1.23) | 1.04 (0.83–1.30) | 1.16 (0.92–1.48) | 1.57 (1.25–1.98) | <0.001 | 0.047 |
| Model 3 | 1 (ref) | 1.06 (0.83–1.34) | 1.16 (0.91–1.48) | 1.35 (1.03–1.75) | 1.67 (1.27–2.21) | <0.001 | 0.413 |
| Systolic blood pressure category in mmHg | |||||||
|---|---|---|---|---|---|---|---|
| Age ≥ 75 years | < 120 (n=568) | 120 – 129 (n=764) | 130 – 139 (n=724) | 140 – 149 (n=459) | ≥150 (n=482) | p-linear | p-quadratic |
| Number of events | 153 | 202 | 179 | 164 | 162 | ||
| Person-years | 2931.9 | 3935.8 | 3942.9 | 2320.8 | 2515.5 | ||
| Incidence† (95% CI) | |||||||
| Model 1 | 52.2 (44.5–61.1) | 51.3 (44.7–58.9) | 45.4 (39.2–52.6) | 70.7 (60.6–82.4) | 64.4 (55.2–75.1) | 0.005 | 0.317 |
| Model 2 | 49.9 (42.5–58.6) | 48.1 (41.8–55.3) | 42.7 (36.8–49.6) | 65.1 (55.7–76.2) | 57.0 (48.6–66.8) | 0.036 | 0.411 |
| Model 3 | 49.4 (41.2–59.2) | 43.5 (37.2–50.7) | 40.1 (34.2–47.0) | 59.8 (50.5–70.8) | 51.0 (42.7–60.9) | 0.219 | 0.336 |
| Hazard ratio (95% CI) | |||||||
| Model 1 | 1 (ref) | 0.98 (0.80–1.21) | 0.86 (0.69–1.06) | 1.34 (1.08–1.67) | 1.21 (0.97–1.51) | 0.008 | 0.289 |
| Model 2 | 1 (ref) | 0.95 (0.77–1.18) | 0.84 (0.67–1.04) | 1.28 (1.03–1.60) | 1.12 (0.89–1.39) | 0.059 | 0.360 |
| Model 3 | 1 (ref) | 0.86 (0.68–1.08) | 0.78 (0.62–0.99) | 1.18 (0.91–1.51) | 1.00 (0.77–1.29) | 0.319 | 0.245 |
Incidence per 1,000 person-years. Model 1 is unadjusted. Model 2 is adjusted for age, race, gender, region of residence, income and education. Model 3 is adjusted for variables in Model 2 + dyslipidemia, diabetes, current smoking, atrial fibrillation, history of stroke, history of coronary heart disease, and diastolic blood pressure.
Figure 2.
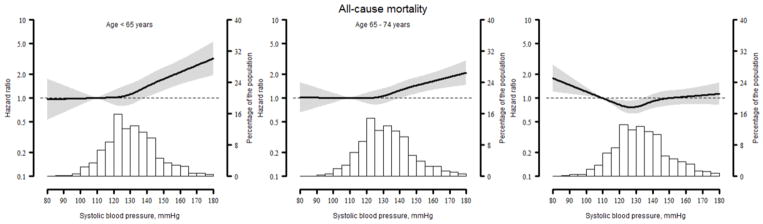
Restricted quadratic splines showing the association between systolic blood pressure and all-cause mortality among REGARDS participants by age group.
Line represents the hazard ratio with the reference set at a systolic blood pressure of 110 mm Hg. Grey area represents the 95% confidence interval. Histograms represent the distribution of systolic blood pressure in the population analyzed. The association is adjusted for age, race, gender, region of residence, income education, dyslipidaemia, diabetes, current smoking, atrial fibrillation and diastolic blood pressure.
Data on recurrent falls were available for 12,054 REGARDS study participants taking antihypertensive medication. During follow-up, 684 (5.7%) participants had a recurrent fall. Among participants age 55–64 years, SBP ≥150 versus <120 mmHg was associated with an increased risk for falls. This association was not present after adjustment for prevalent falls and physical component score. There was no association between SBP and the risk for recurrent falls for individuals aged 65–74. For participants ≥75 years of age, the risk for falls was non-significantly higher for participants with SBP <120 mmHg (eTable 6).
DISCUSSION
In this observational study, we found that for the patients at age 55–64 SBP <140 mmHg was associated with lower incidence of CVD, CHD, stroke and all-cause mortality, with the numerically highest incidence risk at SBP 140–149, and particularly for ≥150 mmHg. SBP values of 130–139 mmHg were characterized by the numerically lowest risk of CVD incidence. The risk of falls was also increased at SBP ≥150 mmHg. For participants aged 65–74 there was a significant increased incidence of CVD and CHD at SBP levels ≥150 mmHg, for stroke at SBP levels ≥130 mmHg and for all-cause mortality for SBP ≥140 mmHg. SBP was not associated with recurrent falls in this age group. Finally for the very elderly individuals (≥75 years of age), an increased risk of CVD, CHD and stroke incidence was observed at SBP values ≥140 mmHg and no association was present between SBP and all-cause mortality. SBP <120 mmHg was associated with an increased incidence of CVD, CHD, all-cause mortality, as well as recurrent falls.
There is considerable debate regarding the appropriate levels of SBP in elderly individuals.4–6 This is a result of limited data from large interventional studies and the fact that despite a reduction in CV events through lowering of BP, no control randomized trial among the elderly has achieved an average SBP <140 mmHg.5,8 Therefore, the current recommendations on the target SBP for elderly individuals are mostly based on expert opinion.5,8 The first trials on antihypertensive treatment in the elderly mainly showed that BP lowering with antihypertensive drugs was effective in reducing the risk of CVD, however the authors did not study BP targets.4,6 Discussion continued after publication of the HYVET trial,7 which was designed to resolve the uncertainty about the relative benefits and risks of antihypertensive therapy in elderly populations. In HYVET 3,845 individuals aged 80 years and older (mean age 83.6 years) with a sustained SBP of 160 mmHg or higher were randomized to indapamide (1.5 mg) or placebo. Perindopril 2 or 4 mg, or matching placebo, was added if needed to achieve the target SBP/DBP of 150/80 mmHg.7 The study was terminated early after a median follow-up of 1.8 years. The authors found that antihypertensive therapy decreased the incidence of the primary endpoint (fatal or nonfatal stroke) by 30% (p = 0.06), and significantly reduced other endpoints – fatal stroke by 39% (p = 0.05), all-cause mortality by 21% (p = 0.02), and HF by 64% (p < 0.001).7 The mean achieved BP in the active-treatment group was 143.5/77.9 mmHg compared with 158.5/83.2 mmHg in the placebo group. However, one should note that the limitations of HYVET. Most notably, HYVET had a relatively healthy sample (e.g., only 12% had a history of CVD) reducing its generalizability to the entire population of elderly hypertensives. Furthermore, HYVET, like all other interventional trials in elderly hypertensives, failed to ascertain whether SBP reduction <140 mmHg would have been beneficial for this particular age group.7 The results obtained from the analysis of the REGARDS cohort study are consistent with the finding that BP lowering might be effective in the very elderly; however, consideration should be given to reducing SBP to levels <140 mmHg to further decrease the risk of CVD, CHD and stroke. In our observational data BP between 120–139 mmHg was not associated with an increased risk for outcomes. However, SBP <120 mmHg was associated with an increased risk of adverse events, especially CVD, CHD incidences and all-cause mortality, and recurrent falls.
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)9 compared the effect of moderate intensity SBP reduction (<140 mmHg) with less intense SBP reduction (140–160 mmHg) in 4418 elderly (age 65–85 years) hypertensive patients treated with the baseline drug - efonidipine hydrochloride. SBP achieved in these groups differed by about 10 mmHg (135.9/74.8 vs. 145.6/78.1 mmHg, respectively) after a 2-year follow-up.9 The authors did not observe a significant difference in the primary endpoint (a composite of CVD, cardiac and vascular disease and renal failure) or secondary endpoints (total deaths), or any added benefits from more aggressive BP reduction. Similar results were obtained in the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study10 that aimed to establish whether strict BP control (<140 mmHg) was superior to moderate control (≥140 to <150 mmHg) in reducing CV mortality and morbidity in 3260 elderly patients (age 70–84 years at baseline) with isolated systolic hypertension10. At 3 years, BP reached 136.6/74.8 and 142.0/76.5 mmHg, respectively (p < 0.001 for both). The overall rate of the primary composite end point (composite of CV events) was 10.6 per 1000 patient-years in the strict control group and 12.0 per 1000 patient-years in the moderate one (HR 0.89; p = 0.38). Both JATOS and VALISH were underpowered to definitively answer whether strict control was superior to less stringent BP targets, and did not allow the analysis of benefits and risks involved with excess BP reduction.9,10 The results of our study suggest slightly different conclusions. For participants aged 65–74, increased incidence risk was observed for CHD and all-cause mortality, when comparing SBP values 130–139 vs. 140–149 mmHg (with almost no difference for CVD and stroke). For persons ≥75 years of age, increased risk was observed for CVD, CHD, and all-cause mortality when comparing SBP of 140–149 vs. 130–139 mm Hg.
In the Studio Italiano Sugli Cardiovascolari del Controllo della Pressione Arteriosa Sistolica (CARDIO-SIS) trial,24 in 1111 nondiabetic patients (mean age 67 years) with baseline SBP ≥150 mmHg, the authors reported a significant reduction in the primary outcome (the rate of electrocardiographic left ventricular hypertrophy) at 2 years median follow-up in patients randomized to SBP goal <130 vs. <140 mmHg using open-label drugs (11.4 vs. 17%, odds ratio [OR] 0.63; 95%CI: 0.43–0.91; p = 0.013).24 In addition, a composite CV endpoint occurred in 9.4% in the usual control group vs. 4.8% in the tight control group (HR 0.50; 95%CI: 0.31–0.79; p = 0.003). Although the age-treatment group interaction was not significant, the greatest reduction in the primary outcome was seen in the subgroup >70 years of age.24 Analyzing the group of individuals at age 65–74 years old in our study, and comparing SBP values 120–129 and 130–139 mmHg we noticed an increase incidence risk only for stroke (HR 0.92 vs. 1.28).
In the Practitioner’s Trial on the Efficacy of Antihypertensive Treatment in Elderly Patients with Hypertension II (PATE-hypertension II study)25 Ogihara et al. investigated whether intensive BP lowering with candesartan might be harmful in this group of patients. They included 1500 elderly Japanese patients >60 years old (mean age 71.1 ± 6.9). At 3 years they showed that patients with SBP <120 mmHg had a higher incidence of CV events compared with those with SBP of 120–139 mmHg.25 In our study a possible J-curve relationship (an increase in CV risk with SBP <120 mmHg) was observed for CVD (by assessing a quadratic SBP term in individuals ≥75 years), CHD and all-cause mortality (when modeled as a continuous variable) especially in persons ≥65 years old.
There are still a limited number of trials evaluating BP targets in the elderly, and many of the available ones are post hoc analyses of previously conducted studies comparing different achieved BP levels. What is more, these studies essentially varied, not only in their target BPs, but also in patient selection, drug choices and duration of treatment. Furthermore, they were all based primarily on SBP and generally did not attempt to reach the aggressive BP goals used in more contemporary hypertension trials in younger cohorts.4,5,26 Forthcoming studies, including the Systolic Blood Pressure Intervention Trial (SPRINT)27 and the Stroke in Hypertension Optimal Treatment trial of the European Society of Hypertension and the Chinese Hypertension League (ESH-CHL-SHOT)28 are aimed at providing the necessary data to establish clear guidelines on the safe target BP level for these patients.
Our study suggests a hypothesis that we should consider a SBP target <140 mmHg for persons ≥55 years old, including those at age ≥75 years, for whom SBP values <140 mmHg were associated with a reduced risk of CVD, CHD, stroke and all-cause mortality. Additionally, SBP values 130–139 mmHg were associated with the numerically lowest risk of CVD and all-cause mortality in these individuals. Similar results were observed in the subanalysis of the Felodipine Event Reduction (FEVER) trial.11; 9711 Chinese hypertensives were included, in whom CV outcomes were significantly reduced by achieving a mean SBP of 138 mmHg compared with less-intense therapy achieving a mean SBP of 142 mmHg. Significant reductions in stroke events were found in uncomplicated hypertensives, in hypertensives with randomization SBP <153 mmHg, and in elderly hypertensives (at mean age 69.5) (-44%, p < 0.001), when their SBP was lowered by more intense treatment.11 Significant reductions were also found in all CV events and all deaths. Achieving mean SBP values <140 mmHg, by adding a small dose of a generic drug, prevented 5.2 CV events for every 100 patients treated for 3.3 years.11 Finally, in the recently published the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, the authors investigated the effects of different BP targets on the rate of recurrent stroke in 3020 patients at mean age 63±11 years with recent lacunar stroke. Their results support the hypothesis that in patients with recent lacunar stroke, a target SBP <130 mmHg is likely to be beneficial.29 The results of these trials, and the observations of our study strengthen the recent recommendations of the 2013 ESH-ESC Guidelines 8 that, at least in fit elderly individuals, including persons ≥75 years old, SBP targets <140 mmHg should be considered.
The results of the current study need to be considered in the context of potential and known limitations. BP was measured 2 times on a single occasion which may have led to exposure misclassification for some study participants. The study is also observational and the causality of the BP levels studied for reducing CVD outcomes cannot be determined. Reverse causality (i.e., the association between low SBP and outcomes may be the result of people with very low SBP being sick and thus having a higher outcome risk) in subjects with lower BP values is possible. However, we think this is unlikely in the current study because everyone was receiving antihypertensive medication. It needs to be emphasized that these data are hypothesis generating and future trials addressing the appropriate SBP goal for people taking antihypertensive medications should take into account an individual’s frailty status. We also chose to not examine the association of DBP and pulse pressure on CV and mortality outcomes. Vascular stiffness and function measures were not collected during the REGARDS baseline visit. Therefore, we were not able to examine the mechanisms underlying the SBP-CVD association in the current analysis. Another limitation is a relatively low number of stroke and CHD cases in some of the subgroups. Also, the REGARDS study does not have a surveillance component and therefore there may be under-reporting of events. One should also notice that the REGARDS study over-sampled participants in the stroke belt of the US a region with a high percentage of African-Americans. However these variables were adjusted for the final results.
This study also has several strengths. These include its recruitment of a population-based sample from across the United States with oversampling of African-Americans, a population with a high risk for stroke. The REGARDS study included a stringent quality control process, standardized criteria and protocols for assessing BP which should limit potential bias. Additionally, both suspected coronary and stroke cases were adjudicated by physician panels following a standardized process.
In conclusion, among adults at age ≥55 taking antihypertensive medication, SBP in the range of 120–139 mmHg compared with higher or lower ranges was not significantly associated with an increased risk for CVD and all-cause mortality outcomes, as well as recurrent falls. Intensive hypertension treatment (BP <120 mmHg) should be a matter of further investigations. The findings from the current study may inform future research to establish appropriate treatment targets in elderly individuals.
Supplementary Material
HIGHLIGHTS.
The study concerns the subject of SBP goals in the older (≥55) hypertensive adults
SBP 120–139 mmHg didn’t increase the risk of CVD and mortality outcomes.
SBP 120–139 mmHg was not also associated with increased risk of recurrent falls.
Intensive hypertension treatment should be a matter of further investigations.
Acknowledgments
The REGARDS research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. Additional funding was provided by R01 HL080477 from the National Heart Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org/.
The founding sources had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: The authors report no relationships that could be construed as a conflict of interest.
The study was presented at the European Society of Cardiology (ESC) Annual Congress 2013 in Amsterdam during the Clinical Trial Update Hot Line III Session: Updates on Risk and Outcome (4th September 2013).
DECLARATION OF INTEREST:
The paper was written independently; no company or institution supported it financially. Some of the authors have given talks, attended conferences and participated in trials and advisory boards sponsored by various pharmaceutical companies. No professional writer was involved in the preparation of this analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielecka-Dabrowa A, Aronow WS, Rysz J, Banach M. The Rise and Fall of Hypertension: Lessons Learned from Eastern Europe. Curr Cardiovasc Risk Rep. 2011;5(2):174–179. doi: 10.1007/s12170-010-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banach M, Aronow WS. Hypertension therapy in the elderly – do we know the answers to all the questions? The status after publication of the ACCF/AHA 2011 Expert Consensus Document on Hypertension in the Elderly. J Hum Hypertens. 2012;26(11):641–3. doi: 10.1038/jhh.2012.3. [DOI] [PubMed] [Google Scholar]
- 5.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 Expert Consensus Document on Hypertension in the Elderly: A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123(21):2434–506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 6.Fleg JL, Aronow WS, Frishman WH. Cardiovascular drug therapy in the elderly: benefits and challenges. Nat Rev Cardiol. 2011;8(1):13–28. doi: 10.1038/nrcardio.2010.162. [DOI] [PubMed] [Google Scholar]
- 7.Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Eng J Med. 2008;358(18):1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 9.JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS) Hypertens Res. 2008;31(12):2115–2127. doi: 10.1291/hypres.31.2115. [DOI] [PubMed] [Google Scholar]
- 10.Ogihara T, Saruta T, Rakugi H, et al. Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension Study. Hypertension. 2010;56(2):196–202. doi: 10.1161/HYPERTENSIONAHA.109.146035. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang X, Liu L, Zanchetti A. Is a systolic blood pressure target <140mmHg indicated in all hypertensives? Subgroup analyses of findings from the randomized FEVER trial. Eur Heart J. 2011;32(12):1500–1508. doi: 10.1093/eurheartj/ehr039. [DOI] [PubMed] [Google Scholar]
- 12.Banach M, Michalska M, Kjeldsen SE, Malyszko J, Mikhailidis DP, Rysz J. What should be the optimal levels of blood pressure: does the J-curve phenomenon really exist? Expert Opin Pharmacother. 2011;12(12):1835–44. doi: 10.1517/14656566.2011.579106. [DOI] [PubMed] [Google Scholar]
- 13.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–53. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 16.Luepker RV, Apple FS, Christenson RH, et al. AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–9. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 17.Prineas R, Crow RBH. The Minnesota code manual of electrocardiographic findings: Standards and procedures for measurement and classification. Boston, MA: Wright-OSG; 1982. [Google Scholar]
- 18.Prineas R, Crow RZZ-M. Minnesota code Manual of Electrocardiographich Findings. 2. London: Springer-Verlag; 2010. [Google Scholar]
- 19.Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31(5):1076–80. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 20.Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20(10):1407–31. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 21.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619–27. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz AV, Nevitt MC, Brown BW, Jr, Kelsey JL. Increased falling as a risk factor for fracture among older women: the study of osteoporotic fractures. Am J Epidemiol. 2005;161(2):180–185. doi: 10.1093/aje/kwi023. [DOI] [PubMed] [Google Scholar]
- 23.Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 24.Staessen JA, Angeli F, et al. Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374(9689):525–33. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 25.Ogihara T, Matsuoka H, Rakugi H. Practitioner’s trial on the efficacy of antihypertensive treatment in elderly patients with hypertension II (PATE-hypertension II study) in Japan. Geriatr Gerontol Int. 2011;11(4):414–21. doi: 10.1111/j.1447-0594.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- 26.Barylski M, Mayszko J, Rysz J, My liwiec M, Banach M. Lipids, blood pressure, kidney - what was new in 2011? Arch Med Sci. 2011;7(6):1055–66. doi: 10.5114/aoms.2011.26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Systolic Blood Pressure Intervention Trial (SPRINT) ClinicalTrials.gov Identifier: NCT01206062. [Google Scholar]
- 28.Zanchetti A, Liu L, Mancia G, et al. Stroke in Hypertension Optimal Treatment (SHOT) Trial: Protocol and Organization. J Hypertens. 2013;31(e-SupplA):e255. [Google Scholar]
- 29.SPS3 Study Group. Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–15. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


