Abstract
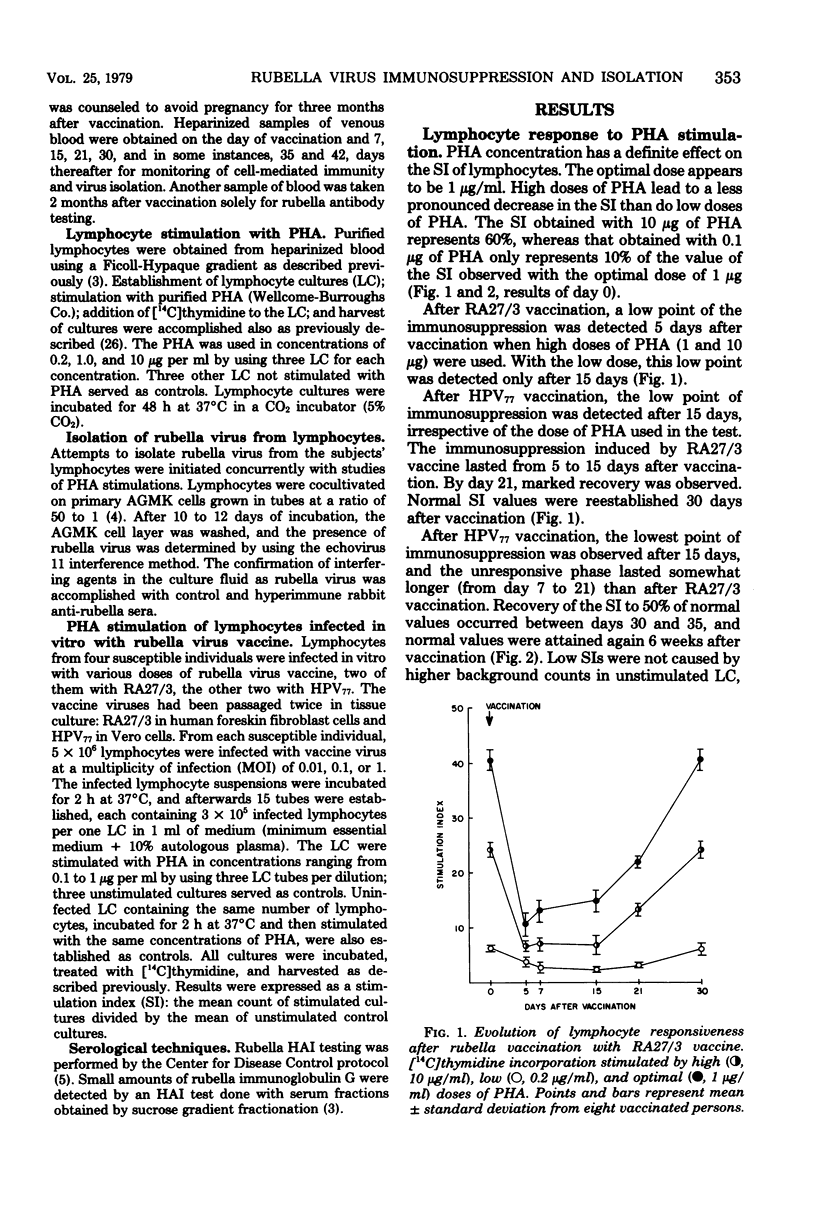
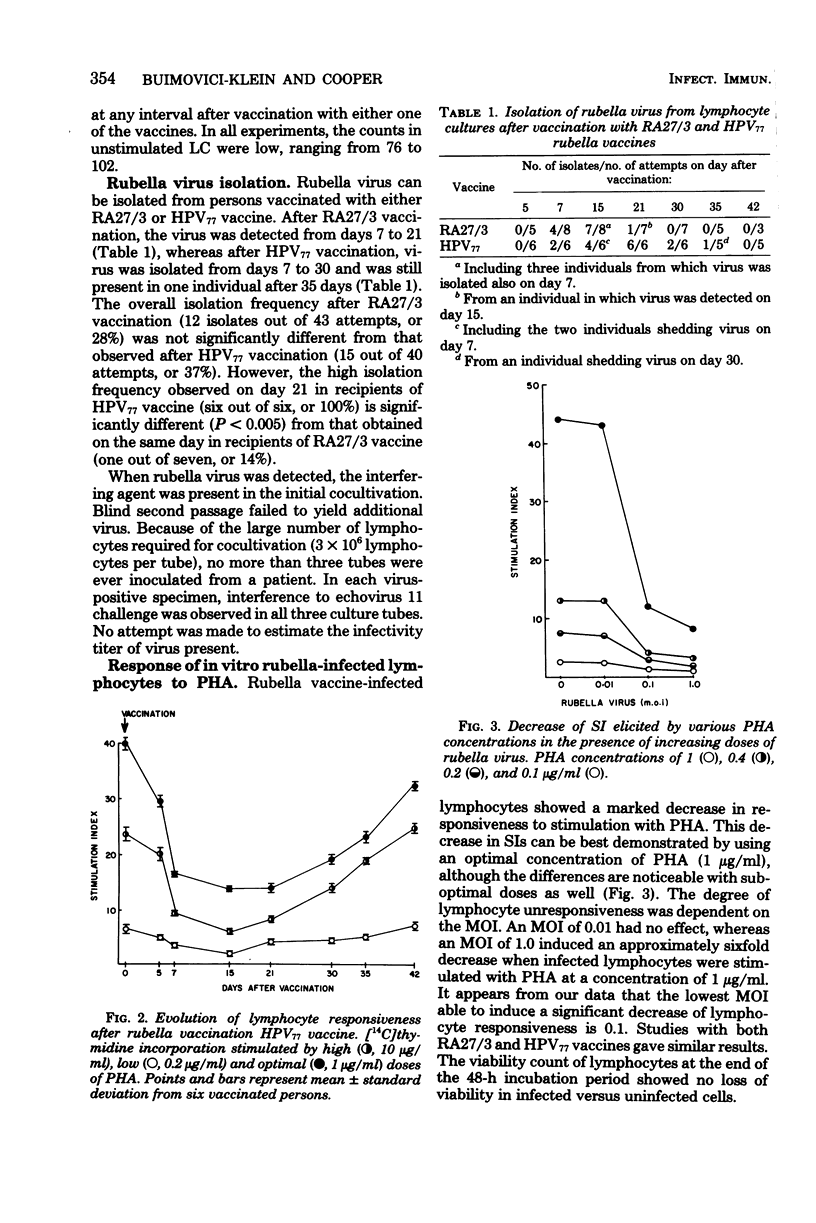
Two groups of young rubella-susceptible women were vaccinated with two rubella vaccines. Heparinized blood samples were taken from all individuals the day of vaccination and 5, 7, 15, 21, 30, 35, and 42 days later. Purified lymphocytes from these samples were cocultivated with AGMK cells for rubella virus isolation. Parallel samples of lymphocytes were stimulated with phytohemagglutinin, and the rate of [14C]thymidine incorporation was determined. Rubella virus was isolated from lymphocytes collected on days 7, 15, and 21 after RA27/3 vaccination in contrast to days 7 to 35 after HPV77 vaccination. The lymphocyte response to phytohemagglutinin was markedly suppressed from day 5 to 15. Normal lymphocyte responses were restored within 1 month after vaccination with RA27/3, but even later (1 week) after HPV77 vaccine. Lymphocytes from rubella-susceptible persons infected invitro with rubella virus vaccines and stimulated with phytohemagglutin displayed a decrease in their responsiveness to the mitogen similar to that observed with lymphocytes from vaccinees. The transient immunosuppression observed in vaccinees is probably due to virus-induced functional damage of the lymphocytes since no direct cytocidal effect of rubella vaccine has been demonstrated on human lymphocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buimovici-Klein E., Hite R. L., Byrne T., Cooper L. Z. Isolation of rubella virus in milk after postpartum immunization. J Pediatr. 1977 Dec;91(6):939–941. doi: 10.1016/s0022-3476(77)80894-9. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E., Vesikari T., Santangelo C. F., Cooper L. Z. Study of the lymphocyte in vitro response to rubella antigen and phytohemagglutinin by a whole blood method. Arch Virol. 1976;52(4):323–331. doi: 10.1007/BF01315621. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E., Weiss K. E., Cooper L. Z. Interferon production in lymphocyte cultures after rubella infection in humans. J Infect Dis. 1977 Mar;135(3):380–385. doi: 10.1093/infdis/135.3.380. [DOI] [PubMed] [Google Scholar]
- Cappel R. Cell mediated immunity in experimental rubella infections. Arch Virol. 1975;47(4):375–379. doi: 10.1007/BF01347979. [DOI] [PubMed] [Google Scholar]
- Detels R., Kim K. S., Gale J. L., Grayston J. T. Viral shedding in Chinese children following vaccination with HPV-77 and Cendehill-51 live attenuated rubella vaccines. Am J Epidemiol. 1971 Nov;94(5):473–478. doi: 10.1093/oxfordjournals.aje.a121344. [DOI] [PubMed] [Google Scholar]
- Fuccillo D. A., Steele R. W., Hensen S. A., Vincent M. M., Hardy J. B., Bellanti J. A. Impaired cellular immunity to rubella virus in congenital rubella. Infect Immun. 1974 Jan;9(1):81–84. doi: 10.1128/iai.9.1.81-84.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggie A. D. Pathogenesis of the rubella exanthem: distribution of rubella virus in the skin during rubella with and without rash. J Infect Dis. 1978 Jan;137(1):74–77. doi: 10.1093/infdis/137.1.74. [DOI] [PubMed] [Google Scholar]
- Honeyman M. C., Forrest J. M., Dorman D. C. Cell-mediated immune response following natural rubella and rubella vaccination. Clin Exp Immunol. 1974 Aug;17(4):665–671. [PMC free article] [PubMed] [Google Scholar]
- Kauffman C. A., Phair J. P., Linnemann C. C., Jr, Schiff G. M. Cell-mediated immunity in humans during viral infection. I. Effect of rubella on dermal hypersensitivity, phytohemagglutinin response, and T lymphocyte numbers. Infect Immun. 1974 Jul;10(1):212–215. doi: 10.1128/iai.10.1.212-215.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Sigel M. M. A differential effect of IgM and IgG antibodies on the blastogenic response of lymphocytes to rubella virus. Cell Immunol. 1974 Jul;13(1):22–31. doi: 10.1016/0008-8749(74)90223-8. [DOI] [PubMed] [Google Scholar]
- Maller R., Sörén L. In vitro effects of rubella virus, strain RA 27/3, on human lymphocytes. I. Viral inhibition of mitogen stimulation in relation to Rubella Haemagglutination inhibition antibodies. Acta Pathol Microbiol Scand C. 1977 Feb;85(1):49–56. doi: 10.1111/j.1699-0463.1977.tb03610.x. [DOI] [PubMed] [Google Scholar]
- McFarland H. F. The effect of measles virus infection on T and B lymphocytes in the mouse. I. Suppression of helper cell activity. J Immunol. 1974 Dec;113(6):1978–1983. [PubMed] [Google Scholar]
- McMorrow L. E., Vesikari T., Wolman S. R., Giles J. P., Cooper L. Z. Suppression of the response of lymphocytes to phytohemagglutinin in rubella. J Infect Dis. 1974 Nov;130(5):464–469. doi: 10.1093/infdis/130.5.464. [DOI] [PubMed] [Google Scholar]
- Montgomery J. R., South M. A., Rawls W. E., Melnick J. L., Olson G. B., Dent P. B., Good R. A. Viral inhibition of lymphocyte response to phytohemagglutinin. Science. 1967 Sep 1;157(3792):1068–1070. doi: 10.1126/science.157.3792.1068. [DOI] [PubMed] [Google Scholar]
- Morag A., Morag B., Bernstein J. M., Beutner K., Ogra P. L. In vitro correlates of cell-mediated immunity in human tonsils after natural or induced Rubella virus infection. J Infect Dis. 1975 Apr;131(4):409–416. doi: 10.1093/infdis/131.4.409. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Herd J. K. Arthritis associated with induced rubella infection. J Immunol. 1971 Sep;107(3):810–813. [PubMed] [Google Scholar]
- Olson G. B., South M. A., Good R. A. Phytohaemagglutinin unresponsiveness of lymphocytes from babies with congenital rubella. Nature. 1967 May 13;214(5089):695–696. doi: 10.1038/214695a0. [DOI] [PubMed] [Google Scholar]
- Osunkoya B. O., Adeleye G. I., Adejumo T. A., Salimonu L. S. Studies on leukocyte cultures in measles. II. Detection of measles virus antigen in human leucocytes by immunofluorescence. Arch Gesamte Virusforsch. 1974;44(4):323–329. doi: 10.1007/BF01251013. [DOI] [PubMed] [Google Scholar]
- Seppälä M., Vaheri A. Natural rubella infection of the female genital tract. Lancet. 1974 Jan 12;1(7846):46–47. doi: 10.1016/s0140-6736(74)93042-6. [DOI] [PubMed] [Google Scholar]
- Simons M. J., Fitzgerald M. G. Rubella virus and human lymphocytes in culture. Lancet. 1968 Nov 2;2(7575):937–940. doi: 10.1016/s0140-6736(68)91167-7. [DOI] [PubMed] [Google Scholar]
- Squadrini F., Taparelli F., De Rienzo B., Giovannini G., Pagani C. Rubella virus isolation from cerebrospinal fluid in postnatal rubella encephalitis. Br Med J. 1977 Nov 19;2(6098):1329–1330. doi: 10.1136/bmj.2.6098.1329-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdirmarsson H., Agnarsdottir G., Lachmann P. J. Measles virus receptor on human T lymphocytes. Nature. 1975 Jun 12;255(5509):554–556. doi: 10.1038/255554a0. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


