Abstract
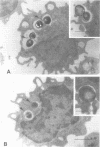
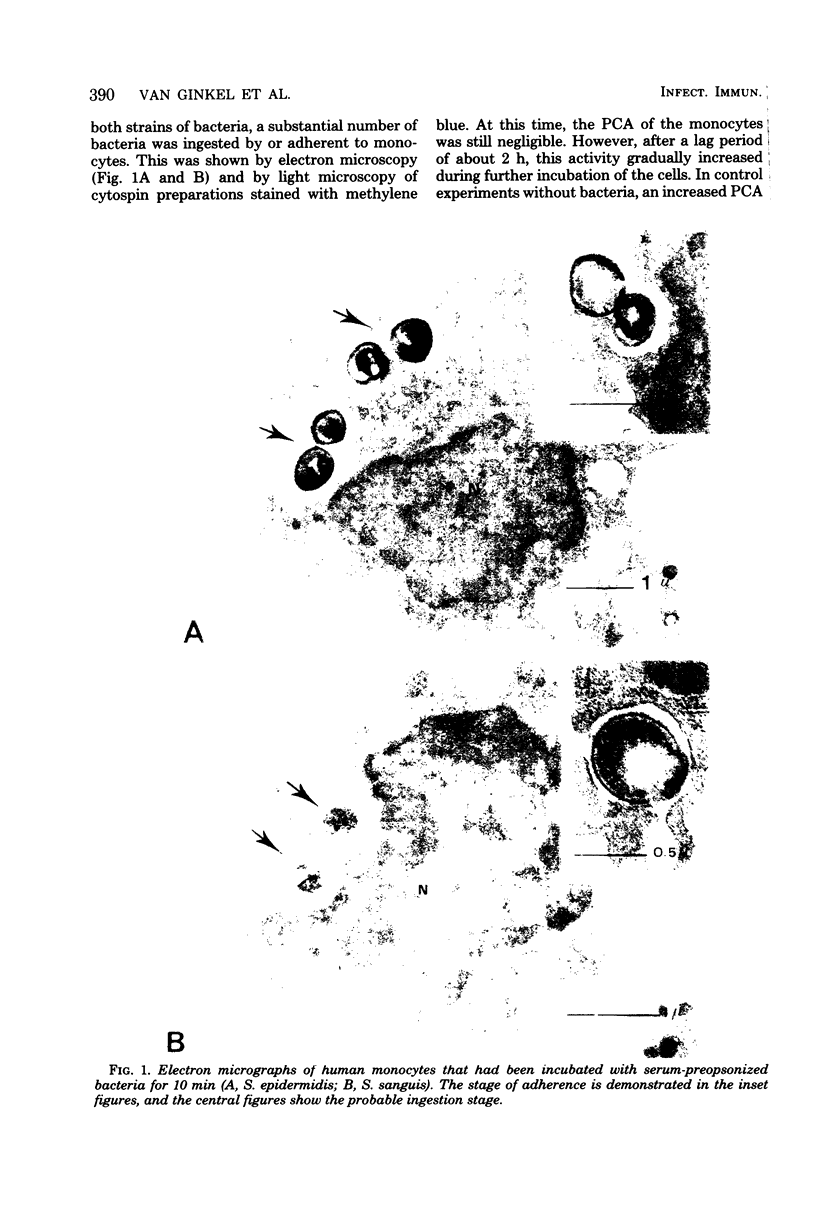
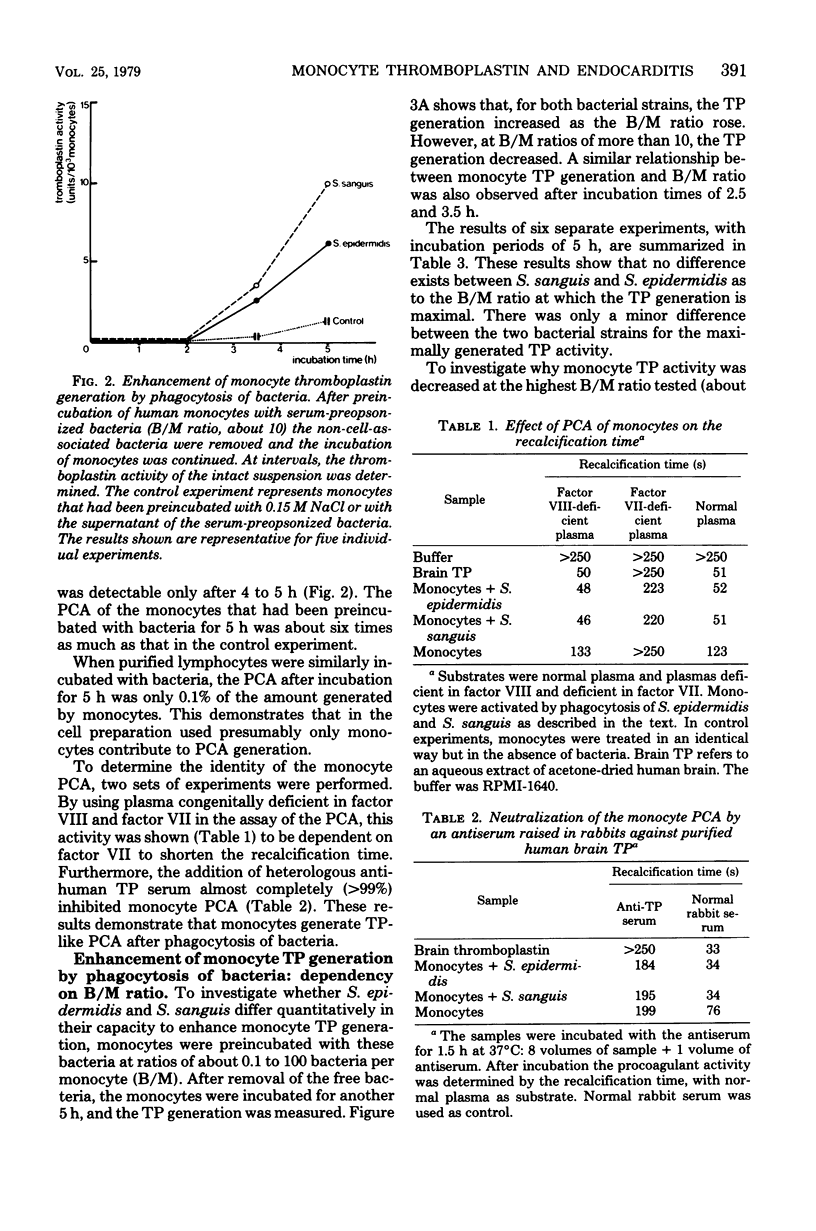
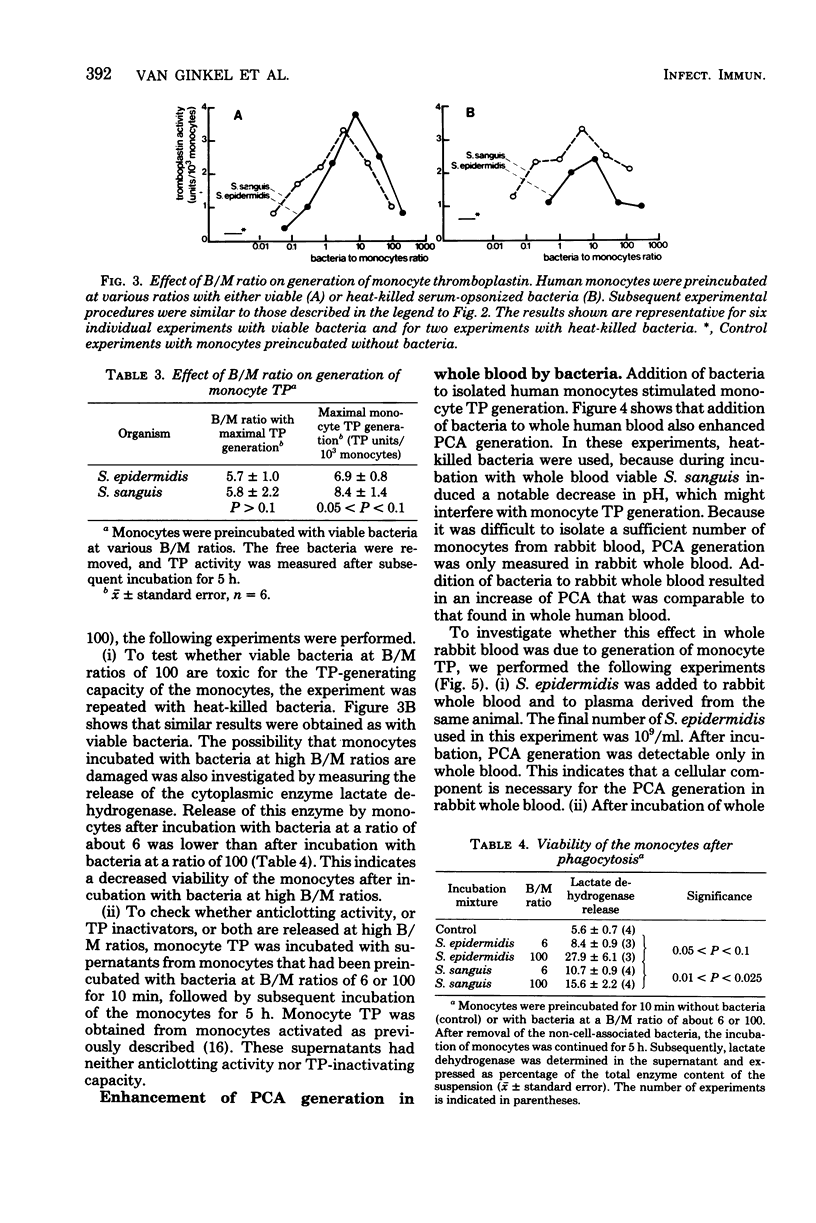
The deposition of fibrin on infected vegetations and the presence of mononuclear phagocytes that have phagocytized bacteria are remarkabe features in experimental bacterial endocarditis. In a study in vitro, we show that phagocytosis of bacteria by human monocytes enhances thromboplastin generation by these cells. Maximal enhancement of the generation of thromboplastin by monocytes was about six times compared with that in the control experiment without bacteria, and it was obtained by preincubation of the monocytes with 5 to 10 bacteria per monocyte. No quantitative difference was observed between Staphylococcus epidermidis and Streptococcus sanguis as to the enhancement of the monocyte thromboplastin generation. An enhancement of the procoagulant activity generation was also observed after addition of bacteria to human or rabbit whole blood. Probably, this generation was also due to synthesis of thromboplastin by monocytes. It is conceivable that fibrin deposition on infected vegetations during bacterial endocarditis is mediated by thromboplastin synthesis by monocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Sande M. A. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect Immun. 1974 Dec;10(6):1433–1438. doi: 10.1128/iai.10.6.1433-1438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes J. L., Bonney R. J., Pelus L., Dahlgren M. E., Sadowski S. J., Kuehl F. A., Jr, Davies P. Macrophages synthesis and release prostaglandins in response to inflammatory stimuli. Nature. 1977 Sep 8;269(5624):149–151. doi: 10.1038/269149a0. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. G., Goldstein R., Cummings G., Lange K. Stimulation of human leukocyte thromboplastic activity by endotoxin. Proc Soc Exp Biol Med. 1971 Oct;138(1):145–148. doi: 10.3181/00379727-138-35848. [DOI] [PubMed] [Google Scholar]
- Loos H., Blok-Schut B., Kipp B., van Doorn R., Meerhof L. Size distribution, electronic recognition, and counting of human blood monocytes. Blood. 1976 Nov;48(5):743–753. [PubMed] [Google Scholar]
- Prydz H., Allison A. C., Schorlemmer H. U. Further link between complement activation and blood coagulation. Nature. 1977 Nov 10;270(5633):173–174. doi: 10.1038/270173a0. [DOI] [PubMed] [Google Scholar]
- Roos D., Loos J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. I. Stimulation by phytohaemagglutinin. Biochim Biophys Acta. 1970 Dec 29;222(3):565–582. doi: 10.1016/0304-4165(70)90182-0. [DOI] [PubMed] [Google Scholar]
- Rothberger H., Zimmerman T. S., Spiegelberg H. L., Vaughan J. H. Leukocyte procoagulant activity: enhancement of production in vitro by IgG and antigen-antibody complexes. J Clin Invest. 1977 Mar;59(3):549–557. doi: 10.1172/JCI108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Eulderink F., Lemkes H., van Furth R. Effect of warfarin on the induction and course of experimental endocarditis. Infect Immun. 1976 Dec;14(6):1284–1289. doi: 10.1128/iai.14.6.1284-1289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thörig L., Thompson J., Eulderink F. Effect of warfarin on the induction and course of experimental Staphylococcus epidermidis endocarditis. Infect Immun. 1977 Sep;17(3):504–509. doi: 10.1128/iai.17.3.504-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- van Ginkel C. J., van Aken W. G., Oh J. I., Vreeken J. Stimulation of monocyte procoagulant activity by adherence to different surfaces. Br J Haematol. 1977 Sep;37(1):35–45. [PubMed] [Google Scholar]