Abstract
An antibody-based analytical method for the detection of a chemical flame retardant using antibody fragments isolated from an alpaca has been developed. One specific chemical flame retardant congener, 2,2′,4,4′-tetrabrominated diphenyl ether (BDE-47), is often the major poly-BDE (PBDE) congener present in human and environmental samples and that which is the most frequently detected. An alpaca was immunized with a surrogate of BDE-47 covalently attached to a carrier protein. The resulting mRNA coding for the variable domain of heavy-chain antibodies (VHH) were isolated, transcribed to cDNA, and cloned into a phagemid vector for phage display library construction. Selection of VHHs recognizing BDE-47 was achieved by panning under carefully modified conditions. The assay sensitivity for detecting BDE-47 was down to the part-per-billion (microgram per liter) level. Cross-reactivity analyses confirmed that this method was highly selective for BDE-47 and selected hydroxylated metabolites. When exposed to elevated temperatures, the camelid VHH antibodies retained more reactivity than a polyclonal antibody developed to the same target analyte. The use of this VHH antibody reagent immobilized onto a Au electrode for impedance biosensing demonstrates the increased versatility of VHH antibodies.
Polybrominated diphenyl ethers (PBDEs) are a class of compounds that have been used as flame retardant additives since the 1970s. They have been widely used in electronics, furniture foam, and plastics. Since PBDEs are used as additive chemicals, they possess a greater potential to leach from the original product during their lifetime.1 In 2004, the United States phased out the manufacture and import of two of the three formulations (penta-BDE and octa-BDE): http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/pbde.html. The third formulation (deca-BDE) was phased out at the end of 2013: http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html. Despite the bans, the continued release of PBDEs from already existing products is expected for many years to come.2
Although the use of PBDEs has declined, environmental and human monitoring for PBDEs levels has begun and will continue, due to historical high production volumes and the persistence of PBDEs in the environment.1 PBDEs are presently on the designated chemicals list for the California Biomonitoring Program and are targets for the Centers for Disease Control and Prevention’s National Report on Human Exposure to Environmental Chemicals. PBDEs have been found extensively in human breast milk,3 food products,4,5 and house dust.5,6 Currently, it is suspected that prenatal exposure to PBDEs results in neurodevelopmental deficiencies7,8 and reproductive effects9,10 due to its structural similarity to thyroid hormones.
Human and environmental monitoring programs are often limited by the cost and complexity of sample testing. From previous monitoring work, one specific congener, 2,2′,4,4′-tetra-BDE (BDE-47) is often the PBDE congener present at the highest concentrations and that which is the most frequently detected. BDE-47 was selected as the representative congener to monitor because, when it is present, the other PBDE congeners are as well. Because of this, and based on our previous work developing successful polyclonal antibodies (pAbs) that selectively recognize BDE-47,11 we aimed to develop a more stable and sustainable source of antibodies highly selective for BDE-47.
Immunoassays traditionally have relied on either pAbs from a wide range of animals (e.g., goats, rabbits, mice) or monoclonal antibodies (mAbs) from mice. pAbs can differ considerably between individuals and over time within an individual. This variability detracts from their utility as a standard and precise analytical tool. From a technical standpoint, pAbs are less expensive, faster to produce and often more sensitive than mAbs. However, in the long term, the possession of a single, highly selective antibody in virtually unlimited supply can be very attractive. The development of mAbs, led by Kohler and Milstein12 in the 1970s, has eliminated the variability in molecular recognition that plagued analyses using pAbs. Therefore, mAbs have become the preferred biological recognition molecule of immunoassays intended for regulatory purposes.
Recently, a new type of antibody molecule has been discovered in camelids13 (Figure 1). These antibodies are devoid of the light chain and still exhibit antigen-binding exclusively on the variable domain of the heavy chain (VHH). The single domain nature of VHHs makes them highly amenable to genetic manipulation and easy to express in various expression systems.14,15 With the discovery of the natural existence of VHH and advances in molecular engineering, the ability to express VHHs in prokaryotic cultures opens new opportunities for developing antibodies that allow for high-throughput screening, exhibit monoclonality properties and have the ability to perpetuate in culture.
Figure 1.

Schematic representation of the peptide domains for camelid antibodies. (Image adapted from ref (16).) The molecular weight of a conventional Ab is 150–160 kDa, a camelid HCAb is 90–100 kDa, and a nanobody is 12–15 kDa.
In this work, we aimed to develop a more stable and sustainable source of antibodies selective for BDE-47. An alpaca was immunized with a surrogate molecule of BDE-47 covalently attached to a carrier protein. The resulting mRNA coding for the heavy chain antibodies was isolated, transcribed to cDNA, and cloned into a phagemid vector for phage display library construction. Selection of VHHs recognizing free BDE-47 was achieved by panning under carefully modified conditions. Sensitivity down to part-per-billion level was achieved, which is relevant for biomonitoring work. Furthermore, we have demonstrated the ease with which these reagents could be incorporated into a well-established electrochemical impedance biosensor format that typically employs conventional antibodies. These VHH-based assays could be used for routine biomonitoring efforts to perform screening analyses of samples before more costly follow-up methods are completed. Thus, the development of rapid methods for chemical detection has great potential in the area of environmental chemistry and human health biomonitoring.
Experimental Section
All regents and consumables were purchased from Fisher/Thermo Scientific or Sigma, unless stated otherwise. Pure chemical standards (i.e., PBDEs, tetrabromobisphenol-A, triclosan, etc.) were handled in a fume hood and after being used were disposed of as hazardous waste. Due to the use of bacteriophage and bacterial cultures, all items that were in contact with these reagents were immersed in a 10% bleach solution for at least 1 h before they were discarded or autoclaved.
Immunization Protocol
All animal studies were conducted in accordance with the guidelines established by the internal institutional animal care and use committee. A 4 year old castrated male alpaca received a subcutaneous injection of a 2 mL emulsion of 10 mM phosphate-buffered saline (PBS) and incomplete Freund’s adjuvant (1:1 by volume) containing 200 μg of hapten C1-keyhole limpet hemocyanin (KLH).11 Subsequent immunizations were completed with the same dose at 2 week intervals. Serum samples were collected prior to the first immunization and 4 days prior to the third, fourth, and fifth immunizations.
Phage Displayed VHH Library Construction
The library construction followed the protocol previously described.17 Briefly, lymphocytes were isolated from 10 mL of alpaca blood following the fourth immunization. Total RNA was extracted, used to synthesize cDNA, and amplified using VHH IgG specific primers. The generated VHH cDNAs were cloned into the pComb3x phagemid vector (generous gift from Dr. Carlos Barbas III, Scripps Research Institute, La Jolla, CA) and expressed as pIII fusion protein on the surface of M13 filamentous phage. The resulting phage display VHH library was amplified in a suppressor strain of Escherichia coli, cultured, and harvested by poly(ethylene glycol) (PEG) precipitation before panning. Briefly, the culture was centrifuged (8000g for 15 min at 4 °C) and the supernatant was transferred to a new centrifuge bottle. A solution of PEG/NaCl (5×, PEG-8000 20% w/v, NaCl 2.5 M) was added to the supernatant and incubated on ice for 2 h. The solution was again centrifuged, and the white phage pellet was resuspended in 10 mL of suspension buffer (PBS containing 1× protease inhibitor cocktail, 0.02% NaN3, 0.5% bovine serum albumin (BSA)), and filtered through a 0.22 μm membrane.
BDE-47 Selective VHH Phage Clone Selection
Phage clones binding specifically BDE-47 were selected by competitive elution with minor modifications to the previously described method.18 Four rounds of panning were conducted with increasingly stringent conditions (Table 1). Hapten C1 (see Table 2 for molecular structure) was employed as both the immunizing and the coating hapten because it presents the entirety of the BDE-47 structure and possesses a rigid handle. Two wells of a microtiter plate (Nunc MaxiSorp) were coated with 100 μL of the coating antigen, BDE-C1 conjugated to BSA, in carbonate/bicarbonate coating buffer (CCB; 100 mM, pH 9.8), while eight wells were coated with 100 μL of BSA (30 μg/mL, in PBS) for postabsorption, at room temperature (RT). All 10 wells were blocked with 3% skim milk in PBS. For each round of panning, 150 μL of the amplified phage library (about 1012 PFU) and 10 μL of methanol were first added to the 2 BDE–C1–BSA-coated wells. For the first round of panning, the phages were incubated with constant shaking for 2 h. These wells were washed with five rinses of 200 μL per well of PBS–Tween (PBST, 0.05% Tween) with 5 min incubations between rinses. The bound phages were eluted by the addition of 100 μL of BDE-47 analyte in 5% methanol with shaking for 1 h. Then, 25 μL of the eluted phages was dispersed into each of the BSA-coated wells to allow for binding of any nonspecific phages. After a 30 min incubation at RT with shaking, the phages in the supernatant were collected and pooled. ER2738 E. coli were infected with the eluted phages and titered on LB–ampicillin (amp) agar plates (recorded as “output” titers), and 150 μL was used for reamplification. The reamplified phages were titered again on LB–amp agar plates (recorded as “input” titers), and 200 μL of the amplified phages was employed again in the next round of panning. For the second, third, and fourth rounds, the same procedure was used, except for the following two changes: the first incubation in the antigen-coated wells was completed in 1 h instead of 2 h, and the number of rinses was increased to 10 times from 5. After each round of panning, the phage pool activity toward the coating antigen and by competition with the BDE-47 analyte was confirmed by enzyme-linked immunosorbent assay (ELISA) and competitive inhibition ELISA (cELISA), respectively.
Table 1. Summary of the Panning Conditions.
| round of panning | no. of washes | coating antigen concn (μg/mL) | BDE-47 elution concn (μg/L) |
|---|---|---|---|
| 1 | 5 | 10 | 1000 |
| 2 | 10 | 5 | 500 |
| 3 | 10 | 1 | 50 |
| 4 | 10 | 1 | 10 |
Table 2. Structures of Coating Haptens and Influence on Sensitivity of the Anti-BDE-47 VHH Assay: The Row in Bold Identifies the Hapten That Resulted in the Most Sensitive Assay and Was Used for the Remainder of the Experiments.

Units of antibody activity per milliliter determined at the EC50 point.
“– –” = not determinable given the low activity.
BDE-47 Selective VHH Expression and Purification
From the agar plate containing the fourth elution output titer, 16 individual clones were randomly selected and grown individually in overnight cultures. Two 2 mL cultures were prepared for each clone: one for DNA extraction and one for VHH–pIII protein extraction. Both cultures for each clone were spun down at 3000g for 10 min. For the DNA extracted cultures, the Qiagen Mini Prep kit was employed and the sequences were submitted to the UC Davis DNA Sequencing facility. For the protein extraction, the bacterial protein extraction reagent kit (BPER) was employed and the obtained protein was further characterized by ELISA and cELISA.
From the cELISA results, the clone with the highest sensitivity to BDE-47 [i.e., lowest half-maximal inhibitory concentration (IC50 value)] was selected. In order to express this selected VHH protein free from the pIII peptide, 50 ng of DNA was transformed into 20 μL of Top10F′ cells (Life Technologies) by heat shock following manufacturer’s instructions. Individual colonies were amplified overnight, and an aliquot was grown in a 200 mL culture to OD (600 nm) ∼0.8 and induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After overnight growth, the cells were collected by centrifugation at 3000g for 10 min and the protein was extracted using the BPER method. The extracted VHH protein, which contains a 6xHis tag [as well as a hemagglutinin (HA)], was passed through a 0.22 μm filter and then applied directly to a HisPur resin for purification. After washing with 40 mM imidazole/PBS, the protein was eluted with 6 mL of 250 mM imidazole/PBS. The purity was assessed by running a 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE), stained with Sypro Ruby gel stain (Life Technologies), and visualized on a UV gel document reader. The eluted protein was dialyzed against three 1 L changes of PBS, and the protein concentration was determined using a NanoDrop Lite (Thermo Scientific). The VHH protein was stored at 1 mg/mL at −15 °C prior to analysis.
BDE-47 VHH ELISA Characterization
Coating Hapten Influence
A suite of previously prepared coating antigens was employed to determine the optimal coating antigen that would result in the most sensitive assay. Wells of high-binding microtiter plates were coated with various antigens (structures and names provided in Table 2) at 1 μg/mL in CCB for 1 h at RT with gentle rocking. Wells were blocked with 0.5% BSA in PBST. The plates were incubated with duplicate serial dilutions of VHH (100 μL/well) in PBS for 1 h at RT with gentle rocking. Plates were then washed with five washes of PBST. Secondary antibody (anti-HA-horse radish peroxidase (HRP; Abcam, Cambridge, MA) was used at manufacturer’s recommended protocol. Following another wash with PBST five times, HRP activity was determined by colorimetric tetramethylbenzidine substrate solution addition and stopped with 1 M sulfuric acid. Well absorbance was read at 450 nm with a plate reader. The amount of antibody activity toward the hapten was determined by calculating the units of antibody activity at the EC50.
To determine the influence of coating hapten on sensitivity toward BDE-47, cELISAs were conducted. The plates were coated as before with each hapten in duplicate at 1 μg/mL. The concentration of VHH used for each hapten was 2 times the EC50 value as determined by ELISA and added equally to all wells. BDE-47 was serially diluted and added to the wells. VHH antibody (50 μL/well) in PBS and BDE-47 (50 μL/well) in PBS containing 40% dimethyl sulfoxide (DMSO) were incubated for 1 h at RT with gentle rocking and developed as described above.
Temperature Stability
Stability of the anti-BDE-47 VHH (clone no. 7), as well as the pAb, was assessed by heating aliquots of each to temperatures of 55, 75, and 95 °C for 10 and for 60 min in PCR tubes in a DNA engine thermal cycler (BioRad, Hercules, CA). After cooling to RT, the antibody samples were assayed for performance by ELISA, as described above.
Cross-Reactivity
The selectivity of the assay was determined by cELISA to examine the activity of the anti-BDE-47 VHH toward structurally related BDE congeners and other contaminants. The analytes included BDE-99 (98% purity, AccuStandard, New Haven, CT), BDE-100 (98% purity, AccuStandard), BDE-143 (98% purity, AccuStandard), BDE-153 (98% purity, AccuStandard), BDE-183 (98% purity, AccuStandard), 5′-OH-BDE-47 (98% purity, AccuStandard), 6′-OH-BDE-47 (98% purity, AccuStandard), tetrabromobisphenol A (TBBPA; 97% purity), bisphenol A (99% purity), triclosan (97% purity), and triclocarban (99% purity). All of these analytes were employed at the highest soluble concentration in PBS buffer containing 40% DMSO. Serial dilutions were performed in this same buffer. Plates were coated at 1 μg/mL of C2-BSA and blocked with 0.5% BSA in PBST, as described above. The final VHH antibody concentration in each well was 0.5 μg/mL. Cross-reactivity (CR) values were defined as
| 1 |
Electrochemical Impedance Spectroscopy (EIS) Measurements
To evaluate if these VHH antibodies were amenable for use in biosensor formats commonly utilizing conventional IgG antibodies, an electrochemical impedance biosensor format was employed (Figure 2). The Au working electrode (WE) was fixed by an O-ring onto an electrochemical cell constructed from virgin Teflon with an electrode area of 0.19 cm2 and a cell volume of 6 mL. The Au electrode was cleaned and then modified by immersion for 17 h into 1.0 mM 11-mercaptanoic acid (11-MUA) and 50 mM PBS (pH = 10) to form a self-assembled monolayer with carboxylate termination. The terminal carboxylate groups were then activated for 1 h in 75 mM 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide hydrochloride (EDC) and 15 mM N-hydroxysulfosuccinimide (sulfo-NHS) in 50 mM PBS solution (pH = 7.3). The VHH-coated electrode is then created by immersion for 1 h into a solution containing 50 μg/mL VHH and 50 mM PBS at pH 7.3, which forms amide bonds to the amine groups on the VHH surface. This is followed by immersion in 0.1% BSA for 1 h to reduce the nonspecific adsorption.
Figure 2.
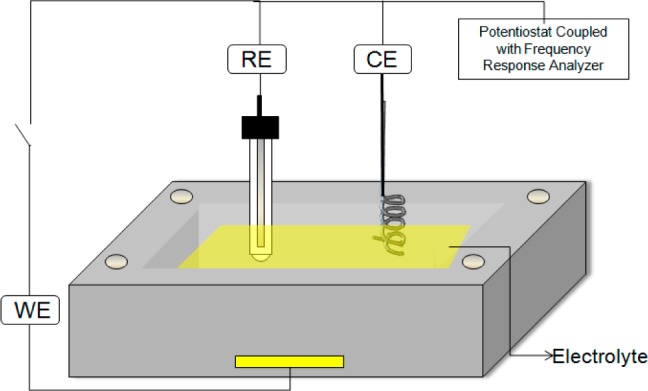
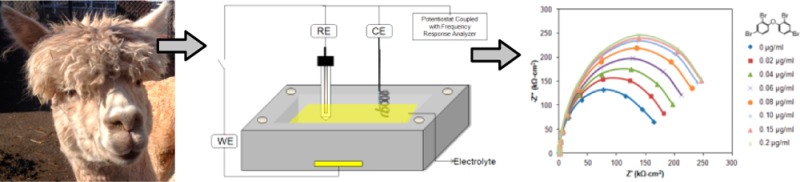
Schematic representation of an electrochemical cell setup employed. Au working electrode (WE) is used to immobilize protein reagents: reference electrode (RE), counter electrode (CE).
All electrochemical impedance spectroscopy measurements were performed with a three-electrode configuration using a Pt spiral counter electrode (CE) and a Ag/AgCl (saturated KCl) reference electrode (RE) (Figure 2). The background test solution contained 50 mM PBS and 5.0 mM K3Fe(CN)6/K4Fe(CN)6 at pH 7.3, with varying concentrations of BDE-47. Impedance measurements were performed using a Gamry Reference 600 potentiostat (Gamry Instruments, Warminster, PA) over the frequency range from 0.05 Hz to 15 kHz with an ac probe amplitude of 5 mV. Each impedance spectrum takes about 2.8 min to acquire.
Results and Discussion
BDE-47 Selective VHH Phage Clone Selection
After four rounds of panning, of the 16 clones selected, 15 grew sufficiently in an overnight culture to provide suitable DNA for sequencing. Of these 15 clones that were successfully sequenced, there were only four unique peptide sequences (defined as more than two amino acid changes out of the total 140 amino acids for the VHH portion of the peptide), but these varied from each other by more than 30 amino acids (Figure 3). The highest density of amino acid changes occur in the complementarity determining regions (CDRs) (1, 2, and 3), as expected, while framework region (FR) 3 contains only five to six changes. The most abundant pattern (with seven homologous clones) was annotated as group A. There were two clones in group B and three clones in both groups C and D. From the culture media, the secreted VHH–pIII peptide products were assessed for initial sensitivity toward BDE-47.
Figure 3.
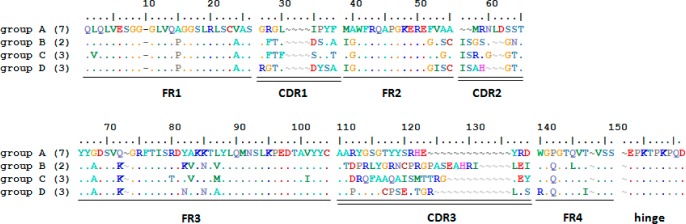
Sequences corresponding to the four VHH groups were aligned, numbered, and divided into framework (FR) and complementarity determining regions (CDR) using previously published llama (ref (19)) and alpaca (ref (20)) sequence analyses based on standard immunoglobulin numbering (ref (21)). “∼” = gap to produce alignment with standard immunoglobulin structure; “.” = same amino acid residue as group A in that position. The number in parentheses indicates the number of times that sequence was repeated within the 15 clones selected.
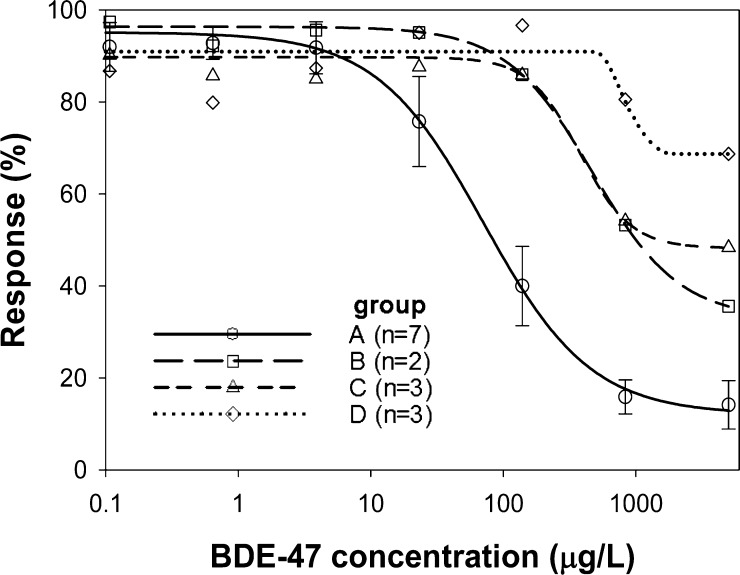
A cELISA was completed employing the supernatant containing secreted VHH–pIII protein from the individual clone’s overnight cultures. The competing analyte was BDE-47 (Figure 4). The lines on the graph indicate the means (± standard deviation) for all of the clones with unique peptide sequences. Group A clones exhibited the highest sensitivity (IC50 = 68 ± 9.3 μg/L), followed by groups B and C (IC50 = 400 μg/L), and group D (IC50 = not determinable). From the seven clones in group A, one clone (no. 7) was selected for cell transformation, VHH protein amplification, and further assay characterization.
Figure 4.
Competitive inhibition profile of the phage colony groups against their target BDE-47. Each group represents pooled values for the clones and each point is a mean. Standard deviation bars are only shown for group A.
Coating Antigen Selection
The selected anti-BDE-47 VHH (clone no. 7) from group A was transformed into a nonsuppressor cell line Top10F′ with an amber stop codon allowing amplification of the VHH gene without pIII, but still possessing the 6xHis and HA tags for purification and detection, respectively. The purified VHH protein was then screened for its activity toward a variety of heterologous haptens previously described11 (Table 2). Screening was completed by titering the VHH protein against the hapten–BSA conjugates coated at 1 μg/mL. The VHH exhibited no activity toward those haptens possessing only one ring (type D) or those substituted in the 2′ position (type A). Those haptens substituted in either the 4′ (type B) or 5′ (type C) positions yield higher activity. Of these haptens, the highest sensitivity toward BDE-47, as determined by the lowest IC50 value, was obtained using the C2 hapten. The other haptens (C1, the immunizing hapten, and B2) possessing three carbon linking arms also produced comparably sensitive assays. This is in contrast to the pAb developed previously in this laboratory where the heterologous hapten assay format greatly improved the assay sensitivity.11 Hapten C2 was the selected hapten used for the rest of the study and for development of the standard curve employing the purified VHH (Figure 5).
Figure 5.
BDE-47 competitive inhibition ELISA standard curve, employing hapten C2-BSA and HIS-purified clone no. 7 from group A. Each point indicates the mean ± standard deviation of triplicate samples. The IC50 value was determined using a four-parameter logistic plot equation and is reported as value ± standard error.
Analyte Cross-Reactivity
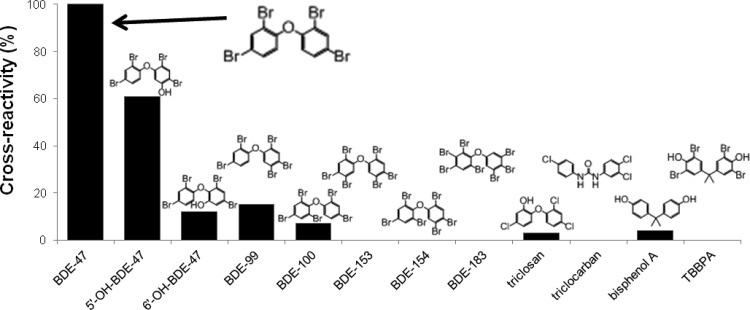
The selectivity of the assay was examined by conducting CR analyses with a variety of structurally similar compounds (Figure 6). The antibody is highly selective for the hydroxylated metabolite 5′-OH-BDE-47 (60% CR) and less so for the 6′-OH-BDE-47 (∼10% CR). The antibody also demonstrates CR with the BDE-99 (15%) and BDE-100 (7%) congeners, but no CR with BDE-153, -154, and -183 congeners. Little (<5%) or no CR was observed for triclosan, triclocarban, bisphenol A, and TBBPA. The selectivity of this assay makes it valuable for human biomonitoring where both the parent and the metabolites can serve as indicators of exposure. In addition, the assay will not detect other structurally similar compounds (i.e., triclosan) or chemicals used for the same purpose (i.e., TBBPA used as a fire retardant) that are also currently being detected in human biomonitoring samples.22−24
Figure 6.
Cross-reactivity profile of the purified anti-BDE-47 VHH antibody as compared to other selected contaminants. Chemical structures for each analyte are provided and are aligned over its respective bar. In the case where no IC50 value could be determined for the analyte, no resulting CR% could be determined. Each analyte was assessed in duplicate.
Temperature Stability
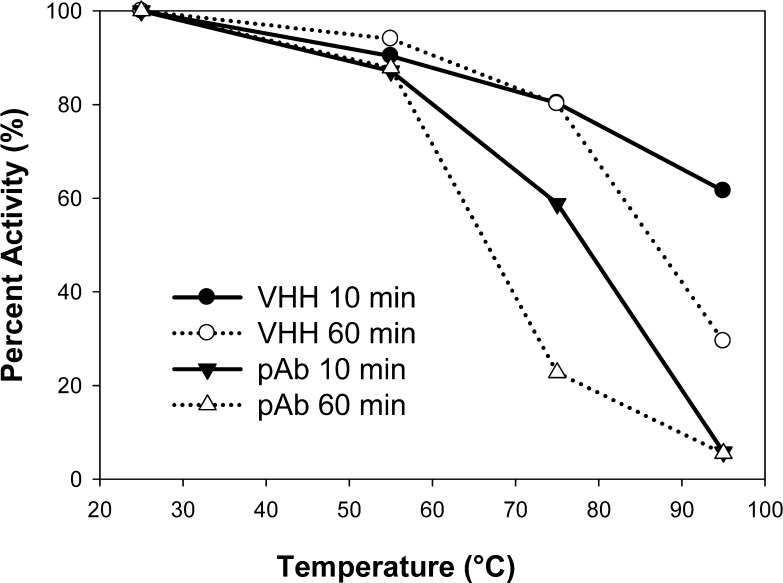
Many studies have presented results suggesting that some VHH peptides display improved thermal stability as compared to conventional IgGs.25 Often these studies demonstrated that a fraction of the VHHs tested are completely temperature-tolerant (no reduced activity when heated), while some VHHs do lose a varying amount of activity. Therefore, in an effort to characterize how the anti-BDE-47 VHH antibody performs compared to its rabbit pAb counterpart, these antibodies were heated to various temperatures for two different durations. A standard antigen selective ELISA was performed to assess retention of binding activity after temperature incubation. As shown in Figure 7, for both the VHH and the pAb, the activity drops with increasing temperature and with increased duration at that temperature. However, the VHH antibody retains greater than 50% of its activity when exposed to 95 °C for 10 min and more than 25% of its activity when exposed to 95 °C for 60 min, while the pAb’s activity is below 6% after either 10 or 60 min of incubation at 95 °C. These results are similar to studies that also compared the thermal stability of VHHs and conventional antibodies (rabbit or mouse IgG) toward their respective antigens.25−28
Figure 7.
Thermal stability of the anti-BDE-47 VHH (clone no. 7) and pAb antibodies. Antibodies were incubated at increasing temperatures for 10 and 60 min, and activity was determined by ELISA. Each temperature was assessed in triplicate.
Biomolecular Recognition with Detection by Electrochemical Impedance Spectroscopy
Biosensors typically utilize biomolecules such as antibodies, receptor proteins, or DNA immobilized onto a solid support. VHH may be easier and less expensive to produce for use in solid-state biosensors. Electrochemical impedance spectroscopy was used to demonstrate detection of BDE-47 using a VHH immobilized onto a Au electrode (Figure 2). Impedance methods have been widely studied for biosensor signal transduction.29−31 During impedance biosensing, ac interrogation of the interface allows indirect monitoring of the change in polymer/protein film thickness at an electrode onto which a recognition biomolecule is immobilized. Figure 8 is a Nyquist plot of the impedance spectrum as a function of increasing BDE-47 concentration at a Au electrode onto which the anti-BDE-47 VHH is immobilized.
Figure 8.
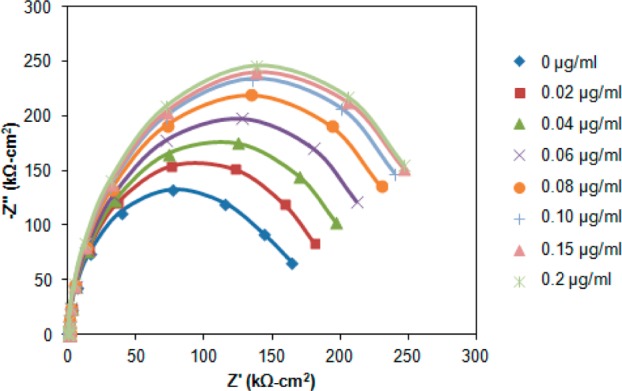
Nyquist plot of the impedance response for increasing concentrations of BDE-47 at a Au electrode onto which the BDE-47 VHH is immobilized.
The results of Figure 8 can be fit to the Randles equivalent circuit with the differential capacitance (Cd) replaced with a constant phase element (CPE), whose impedance is given by32−34
| 2 |
where T is the CPE magnitude and n is the phase exponent. In the Randles circuit, Rct is the charge-transfer resistance, RS is solution resistance, and ZW is the Warburg impedance, which is not fit here. Table 3 provides the best-fit equivalent circuit parameters for the results shown in Figure 8. From the measurement of Rct in Table 3, the detection limit for BDE-47 is approximately 0.79 μg/L for the Au electrode at which the VHH is immobilized, which is about 39% lower than the detection limit (1.3 μg/L) obtained for a Au electrode at which the pAb is immobilized.35 The improved sensitivity may reflect the lower thickness of the interfacial polymer–protein film associated with the VHH (∼17 kDa) relative to the pAb (∼150 kDa). In other words, the sensitivity and detection limit of electrochemical sensors is improved by sensing through a thinner polymer–protein film. This may be important for impedance detection of small molecule analytes such as endocrine-disrupting chemicals.35
Table 3. Best-Fit Equivalent Circuit Parameters as a Function of BDE-47 Concentration.
| BDE-47 concn (μg/mL) | 0 | 0.02 | 0.04 | 0.06 | 0.08 | 0.1 | 0.15 | 0.2 |
| Rs (Ω·cm2) | 29.98 (0.6) | 28.14 (0.59) | 28.31 (0.63) | 28.49 (0.64) | 28.76 (0.62) | 28.45 (0.62) | 28.28 (0.61) | 28.63 (0.62) |
| CPE-T (μF cm–2 sn–1) | 4.38 (0.09) | 4.42 (0.08) | 4.5 (0.08) | 4.5 (0.09) | 4.47 (0.08) | 4.46 (0.10) | 4.43 (0.08) | 4.42 (0.08) |
| n | 0.95 (0.003) | 0.95 (0.003) | 0.95 (0.003) | 0.95 (0.003) | 0.95 (0.003) | 0.95 (0.003) | 0.95 (0.003) | 0.95 (0.003) |
| Rct (kΩ·cm2) | 158.3 (3.15) | 182.01 (3.51) | 201.77 (3.72) | 221.21 (3.53) | 239.44 (3.81) | 255.75 (3.68) | 266.2 (3.56) | 268.34 (3.54) |
Conclusions
We have demonstrated that a new type of antibody fragment, a VHH from an alpaca, exhibits comparable sensitivity and selectivity to previously developed conventional antibodies yet it also exhibits increased resilience to heat. The BDE-47 hapten used produced not only a substantial response upon immunization but also an array of individual VHHs with diverse sequences and good sensitivity. This again illustrates the value of a rigid conjugated handle on the hapten resulting in a planar molecule which appears to present the analyte mimic well. This work highlights the usefulness of alpaca VHHs as a technology to produce assays for small lipophilic molecules. The success can also be attributed to the fact that the immunizing hapten was previously utilized in rabbits to generate an immune response. This is a recommended strategy for future studies. Several species of camelids have been used to produce VHH for immunoassay development. Our work demonstrates that the alpaca is useful for this approach with their smaller size, lower cost, and gentle disposition offering advantages over many other camelids. Moreover, since the VHH protein is expressed as a single gene, it is easily manipulated with molecular tools, which results in the generation of a large quantity of protein using economical reagents. In addition, binding sensitivities can be improved through in vitro antibody maturation techniques such as site-directed or random mutagenesis. Labels for detection can be incorporated into VHH using simple genetic approaches, such as fusion with enzymes (e.g., AP, luceriferase) or fluorescent proteins (e.g., GFP). Furthermore, our work demonstrates that these antibodies can be rapidly incorporated into assays and sensor platforms that have traditionally been designed for use with conventional antibodies. Because of their smaller size, the VHH can be immobilized at higher densities on surfaces and reactive group incorporation (via genetic means) can improve oriented immobilization.
Acknowledgments
Research reported in this publication was supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health P42ES004699, NIEHS Small Business Innovative Research award 1R43ES017995, and the National Science Foundation award ECCS-1342618. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Darnerud P. O.; Eriksen G. S.; Johannesson T.; Larsen P. B.; Viluksela M. Environ. Health Perspect. 2001, 109, 49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M.; Vorkamp K.; Thomsen M.; Knudsen L. E. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [DOI] [PubMed] [Google Scholar]
- Solomon G. M.; Weiss P. M. Environ. Health Perspect. 2002, 110, A339–A347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A.; Haffner D.; Colacino J.; Patel K.; Päpke O.; Opel M.; Birnbaum L. Environ. Health Perspect. 2010, 118, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N.; Herrmann T.; Paepke O.; Tickner J.; Hale R.; Harvey E.; La Guardia M.; McClean M. D.; Webster T. F. Environ. Sci. Technol. 2007, 41, 1584–1589. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M.; Kelly S. M.; Allen J. G.; McClean M. D.; Webster T. F. Environ. Sci. Technol. 2008, 42, 3329–3334. [DOI] [PubMed] [Google Scholar]
- Herbstman J. B.; Sjödin A.; Kurzon M.; Lederman S. A.; Jones R. S.; Rauh V.; Needham L. L.; Tang D.; Niedzwiecki M.; Wang R. Y. Environ. Health Perspect. 2010, 118, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R.; Vallero R. O.; Golub M. S.; Suarez J. K.; Ta T. A.; Yasui D. H.; Chi L. H.; Kostyniak P. J.; Pessah I. N.; Berman R. F.; LaSalle J. M. Hum. Mol. Genet. 2012, 21, 2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti P. R. S.; Coburn C. G.; Moser V. C.; MacPhail R. C.; Fenton S. E.; Stoker T. E.; Rayner J. L.; Kannan K.; Birnbaum L. S. Toxicol. Sci. 2010, 116, 297–312. [DOI] [PubMed] [Google Scholar]
- Harley K. G.; Marks A. R.; Chevrier J.; Bradman A.; Sjödin A.; Eskenazi B. Environ. Health Perspect. 2010, 118, 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K. C.; Gee S. J.; Tsai H. J.; Bennett D.; Nishioka M. G.; Blum A.; Fishman E.; Hammock B. D. Environ. Sci. Technol. 2009, 43, 7784–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G.; Milstein C. Nature 1975, 256, 495–497. [DOI] [PubMed] [Google Scholar]
- Hamers-Casterman C.; Atarhouch T.; Muyldermans S.; Robinson G.; Hammers C.; Songa E. B.; Bendahman N.; Hammers R. Nature 1993, 363, 446–448. [DOI] [PubMed] [Google Scholar]
- Arbabi Ghahroudi M.; Desmyter A.; Wyns L.; Hamers R.; Muyldermans S. FEBS Lett. 1997, 414, 521–526. [DOI] [PubMed] [Google Scholar]
- Harmsen M. M.; De Haard H. J. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski J.; Alzogaray V.; Reyelt J.; Unger M.; Juarez K.; Urrutia M.; Cauerhff A.; Danquah W.; Rissiek B.; Scheuplein F.; Schwarz N.; Adriouch S.; Boyer O.; Seman M.; Licea A.; Serreze D. V.; Goldbaum F. A.; Haag F.; Koch-Nolte F. Med. Microbiol. Immunol. 2009, 198, 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; McCoy M. R.; Majkova Z.; Dechant J. E.; Gee S. J.; Tabares-da Rosa S.; González-Sapienza G. G.; Hammock B. D. Anal. Chem. 2012, 84, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabares-da Rosa S.; Rossotti M.; Carleiza C.; Carrión F.; Pritsch O.; Ahn K. C.; Last J. A.; Hammock B. D.; González-Sapienza G. Anal. Chem. 2011, 83, 7213–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen M. M.; Ruuls R. C.; Nijman I. J.; Niewold T. A.; Frenken L. G.; de Geus B. Mol. Immunol. 2000, 37, 579–590. [DOI] [PubMed] [Google Scholar]
- Maass D. R.; Sepulveda J.; Pernthaner A.; Shoemaker C. B. J. Immunol. Methods 2007, 324, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc M. P.; Giudicelli V.; Ginestoux C.; Bodmer J.; Muller W.; Bontrop R.; Lemaitre M.; Malik A.; Barbie V.; Chaume D. Nucleic Acids Res. 1999, 27, 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R.; Antignac J.; Zalko D.; Berrebi A.; Cravedi J.; Maume D.; Marchand P.; Monteau F.; Riu A.; Andre F.; Le bizec B. Chemosphere 2008, 73, 1036–1041. [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B.; Adams D. H.; Kannan K. Chemosphere 2008, 70, 1935–1944. [DOI] [PubMed] [Google Scholar]
- Geens T.; Neels H.; Covaci A. J. Chromatogr., B 2009, 877, 4042–4046. [DOI] [PubMed] [Google Scholar]
- van der Linden R. H. J.; Frenken L. G. J.; de Geus B.; Harmsen M. M.; Ruuls R. C.; Stok W.; de Ron L.; Wilson S.; Davis P.; Verrips C. T. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 1999, 1431, 37–46. [DOI] [PubMed] [Google Scholar]
- Ladenson R. C.; Crimmins D. L.; Landt Y.; Ladenson J. H. Anal. Chem. 2006, 78, 4501–4508. [DOI] [PubMed] [Google Scholar]
- Graef R.; Anderson G.; Doyle K.; Zabetakis D.; Sutton F.; Liu J.; Serrano-Gonzalez J.; Goldman E.; Cooper L. BMC Biotechnol. 2011, 11, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman E. R.; Anderson G. P.; Liu J. L.; Delehanty J. B.; Sherwood L. J.; Osborn L. E.; Cummins L. B.; Hayhurst A. Anal. Chem. 2006, 78, 8245–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E.; Willner I. Electroanalysis 2003, 15, 913–947. [Google Scholar]
- Yang L.; Bashir R. Biotechnol. Adv. 2008, 26, 135–150. [DOI] [PubMed] [Google Scholar]
- Prodromidis M. I. Electrochim. Acta 2010, 55, 4227–4233. [Google Scholar]
- Rammelt U.; Reinhard G. Electrochim. Acta 1990, 35, 1045–1049. [Google Scholar]
- Pajkossy T. J. Electroanal. Chem. 1994, 364, 111–125. [Google Scholar]
- Jüttner K. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar]
- Radhakrishnana R.; Suni I. I.; Bever C. S.; Hammock B. D. ACS Sustainable Chem. Eng. 2014, 2, 1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]