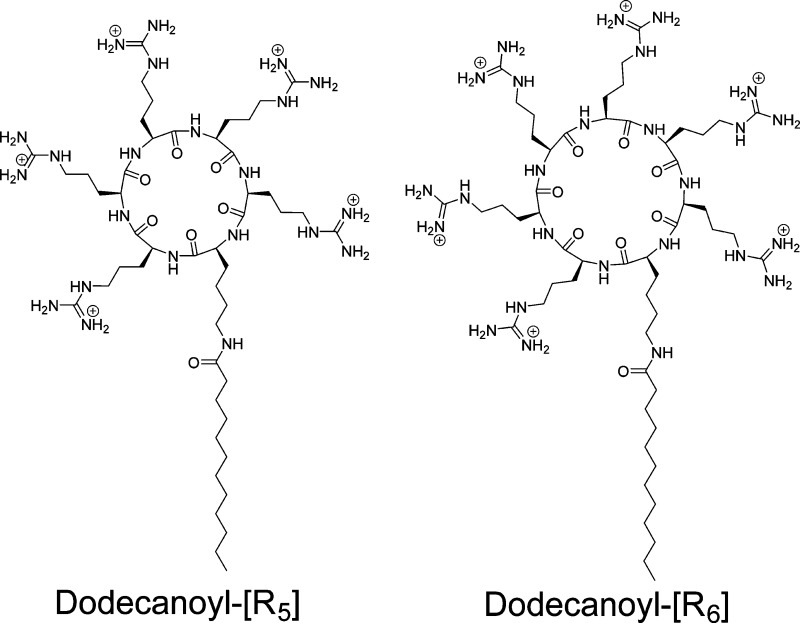
Abstract
Many of the reported arginine-rich cell-penetrating peptides (CPPs) for the enhanced delivery of drugs are linear peptides composed of more than seven arginine residues to retain the cell penetration properties. Herein, we synthesized a class of nine polyarginine peptides containing 5 and 6 arginines, namely, R5 and R6. We further explored the effect of acylation with long chain fatty acids (i.e., octanoic acid, dodecanoic acid, and hexadecanoic acid) and cyclization on the cell penetrating properties of the peptides. The fluorescence-labeled acylated cyclic peptide dodecanoyl-[R5] and linear peptide dodecanoyl-(R5) showed approximately 13.7- and 10.2-fold higher cellular uptake than that of control 5,6-carboxyfluorescein, respectively. The mechanism of the peptide internalization into cells was found to be energy-dependent endocytosis. Dodecanoyl-[R5] and dodecanoyl-[R6] enhanced the intracellular uptake of a fluorescence-labeled cell-impermeable negatively charged phosphopeptide (F′-GpYEEI) in human ovarian cancer cells (SK-OV-3) by 3.4-fold and 5.5-fold, respectively, as shown by flow cytometry. The cellular uptake of F′-GpYEEI in the presence of hexadecanoyl-[R5] was 9.3- and 6.0-fold higher than that in the presence of octanoyl-[R5] and dodecanoyl-[R5], respectively. Dodecanoyl-[R5] enhanced the cellular uptake of the phosphopeptide by 1.4–2.5-fold higher than the corresponding linear peptide dodecanoyl-(R5) and those of representative CPPs, such as hepta-arginine (CR7) and TAT peptide. These results showed that a combination of acylation by long chain fatty acids and cyclization on short arginine-containing peptides can improve their cell-penetrating property, possibly through efficient interaction of rigid positively charged R and hydrophobic dodecanoyl moiety with the corresponding residues in the cell membrane phospholipids.
Keywords: acylation, cyclic peptide, cyclization, drug delivery, polyarginine
Introduction
Polyarginine peptides are known as one of the widely used classes of cell-penetrating peptides (CPPs) and cellular delivery tools.1 It has been reported that the presence of the guanidine group in the side chain of arginine plays a key role for improved ability of arginine-rich peptides to cross the cell membrane.2,3 Various systematic structural investigations have been performed to determine the required number of arginine residues and the length of the peptide for the optimization of cellular uptake.2,4 Short polyarginine peptides containing less than six arginine residues did not exhibit significant cellular uptake in several previously reported investigations.2,4,5 Thus, the presence of more than six arginine residues in the structure of polyarginine peptides is critical for their efficient cell-penetrating functions.
However, several investigations were conducted to increase the cellular uptake of polyarginines by attaching the fatty acid to the N-terminal of the peptide. It has been previously reported that the acylation of the N-terminal by fatty acids can facilitate the intracellular uptake of polyarginines.6−8 For instance, Katayama et al. synthesized acylated octa-arginines and discovered that the introduction of hydrophobic fatty acid enhanced the intracellular uptake of octa-arginine peptide and its conjugated ubiquitin.8 Furthermore, Lee et al. designed a class of lipopeptides carrying 7–15 arginine residues. Among them, myristoylated-hendeca-arginine (C14R11) was found to be the most efficient cell-penetrating peptide.7 However, the fatty acylated polyarginine peptides that contain 7–15 arginine residues can potentially cause toxicity, and they can be easily degraded by proteases.
Moreover, linear peptides carrying l-form are not stable in serum and therefore have a limited application for in vivo studies (Figure S2, Supporting Information).9 Replacing l-form amino acids with d-form to improve the peptide stability leads to high cost production. However, cyclic peptides show more proteolytic stability than linear counterparts. Thus, the synthesis and development of cyclic CPPs containing short amino acid sequence with less toxicity is desired.
Herein, we designed acylated cyclic polyarginine peptides (ACPPs) containing five arginine residues and investigated their ability as cell-penetrating peptides. We compared ACPPs with a corresponding acylated linear polyarginine peptide (ALPP) and a nonacylated cyclic polyarginine as controls. We hypothesize that the combination of acylation and cyclization of short polyarginine peptides having less than six arginine residues will increase the intracellular uptake and can generate peptides with molecular transporter properties. For convenience, square brackets [ ] and parentheses ( ) were used to represent cyclic and linear peptides, respectively. Phosphopeptide pTyr-Glu-Glu-Ile (pYEEI) is an optimal peptide ligand for binding to the Src tyrosine kinase SH2 domain. In this study, we used negatively charged fluorescein-labeled phosphopeptide F′-GpYEEI as a model cell-impermeable compound.
Experimental Section
Peptide Synthesis
All peptides were synthesized by Fmoc/tBu solid-phase peptide synthesis strategy either manually or using Rainin PS3 synthesizer (Protein Technologies Inc.). Manual reactions were carried out in a glass reaction vessel with a sintered glass frit by mixing under nitrogen bubbling at room temperature. Fmoc-l-amino acid building blocks, fatty acids, and preloaded H-Arg(pbf)-2-chlorotrityl resin were used as starting materials. 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU), hydroxybenzotriazole (HOBT), and N,N-diisopropylethylamine (DIPEA) in N,N-dimethylformamide (DMF) were used as coupling and activating reagents, respectively, for manual synthesis. In the case of using peptide synthesizer, 0.4 M N-methylmorpholine in DMF was used instead of DIPEA. Piperidine in DMF (20%) was employed to deprotect Fmoc group at each step.
To cleave the linear peptide from the resin, a mixture of trifluoroacetic acid (TFA)/triisoproylsilane (TIS)/water (92.5/5/2.5, v/v/v) was used. However, in the synthesis of cyclic peptides, the side chain protected linear peptides were first cleaved from the resin by using a cleavage cocktail, containing 2,2,2-trifluoroethanol (TFE)/acetic acid/dichloromethane (DCM) (2:1:7, v/v/v). Cyclization reaction was performed by employing a mixture of 1-hydroxy-7-azabenzotriazole (HOAT) and N,N′-diisopropylcarbodiimide (DIC) in anhydrous DMF/DCM for 12 h. After solvent evaporation, the peptide was deprotected and cleaved from the resin by using a cleavage cocktail reagent “R”, containing TFA/thioanisole/1,2-ethanedithiol (EDT)/anisole (90:5:3:2, v/v/v/v) for 2–3 h. The crude peptides were precipitated and washed with cold diethyl ether. To purify the crude peptides, we used a reversed-phase high pressure liquid chromatography (RP-HPLC) system using Shimadzu LC-8A preparative liquid chromatography on a Phenomenex Gemini C18 column (10 μm, 250 × 21.2 mm) with a gradient 0–100% of acetonitrile (CH3CN) containing 0.1% TFA (v/v) and water containing 0.1% TFA (v/v) for 1 h with a flow rate at 15.0 mL/min at the wavelength of 214 nm.
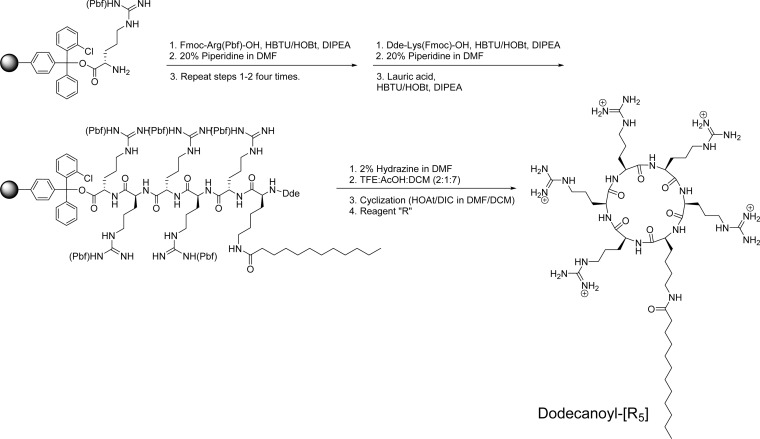
As a representative example, the synthesis of dodecanoyl-[R5] is described here. H-Arg(pbf)-2-chlorotrityl resin (660 mg, 0.35 mmol, 0.53 mmol/g) was swelled in DMF for 40 min by N2. Fmoc-Arg(pbf)-OH (681 mg, 1.05 mmol, 3 equiv) was coupled to the N-terminal of the resin, using HBTU (398 mg, 1.05 mmol, 3 equiv), HOBT (142 mg, 1.05 mmol, 3 equiv), and DIPEA (366 μL, 2.1 mmol, 6 equiv) in DMF (15 mL) by agitating the resin for 1 h using N2. After the coupling, the resin was washed with DMF, followed by Fmoc-deprotection with piperidine in DMF (20%). The subsequent three Fmoc-Arg(pbf)-OH couplings and one Dde-Lys(Fmoc)-OH (559 mg, 1.05 mmol, 3 equiv; Dde =1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl) coupling was carried out in the same manner, respectively. After removing the Fmoc group in side chain of lysine, dodecanoic acid (210 mg, 1.05 mmol, 3 equiv) was coupled using HBTU, HOBT, and DIPEA. Then Dde protection group at N-terminal of peptide was removed by 2% hydrazine in DMF, followed by washing with DMF and DCM. The side chain protected linear peptides were cleaved from the resin by using a cleavage cocktail, TFE/acetic acid/DCM (2:1:7, v/v/v), for 1 h. The filtrate was evaporated, and the residue was dried overnight in a vacuum. The cyclization was conducted under a dilute condition with anhydrous DMF/DCM (5:3, v/v, 250 mL), using HOAT (190 mg, 1.4 mmol, 4 equiv) and DIC (240 μL, 1.54 mmol, 4.4 equiv), and stirred for 12 h under nitrogen atmosphere. After cyclization, the solvent was evaporated, and the side chain deprotection was carried out by the addition of reagent “R” for 2 h. The crude dodecanoyl-[R5] was precipitated and washed with cold diethyl ether and purified by preparative RP-HPLC system as described above.
Fluorescein-labeled peptides were synthesized with the same protocol before the attachment of fatty acid. We used Fmoc-12-aminododecanoic acid instead of dodecanoic acid, and after removing Fmoc group, 5(6)-carboxyfluorescein diisobutyrate (CFDI) was attached using 7-azabenzotriazol-1-yl-oxytris(pyrrolidino)phosphonium hexafluorophosphate (PyAOP), HOAT, and DIPEA. As an example, we started fluorescein-labeled peptide synthesis in smaller scale with H-Arg(pbf)-2-chlorotrityl resin (208 mg, 0.11 mmol, 0.53 mmol/g). Fmoc-Arg(pbf)-OH (214 mg, 0.33 mmol, 3 equiv), Dde-Lys(Fmoc)-OH (176 mg, 0.33 mmol, 3 equiv), and Fmoc-12-aminododecanoic acid (144 mg, 0.33 mmol, 3 equiv) were used to couple each building block to the resin using HBTU (125 mg, 0.33 mmol, 3 equiv), HOBT (45 mg, 0.33 mmol, 3 equiv), and DIPEA (115 μL, 0.66 mmol, 6 equiv) in DMF. CFDI (170 mg, 0.33 mmol, 3 equiv) was coupled with amino group of 12-aminododecaonic chain using PyAOP (172 mg, 0.33 mmol, 3 equiv), HOAT (45 mg, 0.33 mmol, 3 equiv), and DIPEA. The reaction mixture was agitated for 3 h using N2. After removal of Dde protecting group with 2% hydrazine in DMF and washing with DMF and DCM, side chain protected fluorescein linear peptides were cleaved from resin using TFE/acetic acid/DCM (2:1:7, v/v/v). The following cyclization and purification steps were same as above. The molecular weights of final products were confirmed by an AXIMA performance matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometer (Shimadzu Corporation).
Dodecanoyl-[R5]: MALDI-TOF (m/z) C48H94N22O7 calcd. 1090.7676; found 1091.7576 [M + H]+. Dodecanoyl-[R6]: MALDI-TOF (m/z) C54H106N26O8 calcd. 1246.8687; found 1247.7397 [M + H]+. Dodecanoyl-(R6): MALDI-TOF (m/z) C48H96N22O8 calcd. 1108.7781; found 1109.7308 [M + H]+. [R5]: MALDI-TOF (m/z) C36H72N22O6 calcd. 908.6005; found 909.6772 [M + H]+. F′-Dodecanoyl-[R5]: MALDI-TOF (m/z) C69H105N23O13 calcd. 1463.8262; found 1464.6556 [M + H]+. F′-Dodecanoyl-(R5): MALDI-TOF (m/z) C69H107N23O14 calcd. 1481.8368; found 1482.7586 [M + H]+. W-Dodecanoyl-[R5]: MALDI-TOF (m/z) C61H107N25O9 calcd. 1333.8684; found 1334.8713 [M + H]+. W4-[R5]: MALDI-TOF (m/z) C82H114N30O11 calcd. 1694.9283; found 1696.3111 [M + H]+. Octanoyl-[R5]: MALDI-TOF (m/z) C44H86N22O7 calcd. 1034.7050; found 1035.7084 [M + H]+. Hexadecanoyl-[R5]: MALDI-TOF (m/z) C52H102N22O7 calcd. 1146.8302; found 1147.8404 [M + H]+.
Cell Culture
Human ovarian adenocarcinoma (SK-OV-3), leukemia (CCRF-CEM), and embryonic kidney (HEK 293T) cells were purchased from American Type Culture Collection. The SK-OV-3 and HEK 293T cells were grown in Eagle’s minimum essential medium (EMEM), and RPMI-1640 medium (ATCC, Manassas, VA) was used for CCRF-CEM cells in a humidified atmosphere of 5% CO2 at 37 °C. Both media were supplemented with fetal bovine serum (FBS, 10%) and penicillin–streptomycin solution (1%, 10,000 units of penicillin and 10 mg of streptomycin in 0.9% NaCl).
Cytotoxicity Assay
Cytotoxicity of peptides was examined by MTS proliferation assay in two human cancer cell lines (SK-OV-3 and CCRF-CEM) and one human normal cell line (HEK 293T). Cells were seeded into 96-well plates (SK-OV-3 (5 × 103 cells/well), CCRF-CEM (1 × 105 cells/well), and HEK 293T (1 × 104 cells/well)). Then, the cells were incubated with complete media (100 μL) overnight. Different concentrations (0–600 μM) of peptide solution (10 μL) were added to cells and incubated at 37 °C with 5% CO2 for 24 h. Then, CellTiter 96 aqueous solution (20 μL) was added to each well and incubated for 1–4 h. The absorbance was measured at 490 nm using microplate reader. The cells without any peptide were used as the control.
Flow Cytometry
Cellular Uptake of Fluorescein-Labeled Peptide
SK-OV-3 cells were grown in 6-well plates (2 × 105 cells/well) with complete EMEM media 24 h prior to the experiment. The fluorescein-labeled peptide stock solution (1 mM) was prepared in water and diluted in Gibco Opti-MEM I reduced serum medium to obtain the final concentration of 5 μM. The media were removed, and the mixture containing fluorescein-labeled peptide solution (5 μM) was added. After 1 h incubation, trypsin–EDTA solution was added to detach cells from the plate’s surface and remove cell surface binding peptides. After 5 min, the complete media (2 mL) were added to neutralize the trypsin. The cells were collected and centrifuged at 2500 rpm for 5 min. Then they were washed with PBS (without calcium and magnesium) twice. Samples were prepared in FACS buffer for analysis. Finally, the cells were analyzed by BD FACSCalibur or FACSVerse flow cytometer using FITC channel. The data collection was based on the mean fluorescence signal for 10,000 cells. All assays were carried out in triplicate. 5(6)-Carboxyfluorescein (FAM) was used as a negative control.
Mechanism Study of Cellular Uptake by Removing Energy Sources
For examination of the cellular uptake mechanism of F′-dodecanoyl-[R5] at low temperature, the assay was carried out at 4 °C to inhibit the energy-dependent cellular uptake pathways. The SK-OV-3 cells were preincubated at 4 °C for 15 min and incubated with the fluorescein-labeled peptide for 1 h at 4 °C. Cells were collected and analyzed using flow cytometry with the previously described protocol above. The data collected at 37 °C were used as the control. To perform the ATP-depletion assay, cells were incubated with sodium azide (10 mM) and 2-deoxy-d-glucose (50 mM) for 1 h before adding the fluorescein-labeled peptide. During the incubation time (1 h), the fluorescein-labeled bicyclic peptide (5 μM) was prepared in the Opti-MEM I reduced serum medium in the presence of sodium azide (10 mM) and 2-deoxy-d-glucose (50 mM). Then, the cells were incubated with this solution for 1 h. The following sample preparation and flow cytometry analysis protocol was the same as described above.
Confocal Laser Scanning Microscopy (CLSM)
SK-OV-3 cells were seeded with complete EMEM on coverslips in 6-well plate (1 × 105 cells/well) and kept until 50% confluency. The media were removed, and cells were incubated with F′-dodecanoyl-[R5] (10 μM) and F′-dodecanoyl-(R5) (10 μM) in Gibco Opti-MEM I reduced serum medium (Life Technologies, Grand Island, NY) for 1 h at 37 °C. Then cells were washed with 1× phosphate buffered saline with calcium and magnesium (PBS+) for three times. The coverslips were mounted on microscope slides, and images were obtained using Carl Zeiss LSM 700 system with a 488 nm argon ion laser excitation and a BP 505–530 nm band-pass filter.
Intracellular Uptake of a Phosphopeptide, F′-GpYEEI
SK-OV-3 and CCRF-CEM cells were seeded in 6-well plates (2 × 105 cells/well for SK-OV-3 and 1 × 106cells/well for CCRF-CEM) and grown with complete EMEM media (RPMI-1640 for CCRF-CEM) overnight. A mixture of fluorescein-labeled phosphopeptides F′-GpYEEI (5 μM) and peptides (10 μM) were prepared in OPTI-MEM I reduced serum medium at room temperature and incubated for 15 min. Then the cells were incubated with the premixed solution at 37 °C with 5% CO2 for 1 h. The sample preparation for FACS analysis was carried out by previously mentioned protocol described before. In this assay, DMSO and F′-GpYEEI were used as the negative controls.
Results and Discussion
Chemistry
The acylated cyclic polyarginine peptides were synthesized by Fmoc/tBu solid-phase peptide synthesis method. Fmoc-l-Arg(pbf)-OH was coupled on H-Arg(pbf)-loaded 2-chlorotrityl resin in the presence of HBTU, HOBT, and DIPEA in DMF. Then Dde-Lys(Fmoc)-OH was attached, and a fatty acid was coupled to the side chain of lysine. Dde protecting group was removed by 2% hydrazine in DMF, and a cleavage cocktail containing TFE/acetic acid/DCM (2:1:7 v/v/v) was used for 1 h to cleave the side-chain protected linear peptides from the resin. Cyclization of linear peptides was carried out in the presence of a mixture of HOAT and DIC in anhydrous DMF/DCM for 6–24 h. The side-chain deprotection of cyclic peptide was carried out by a cleavage cocktail reagent “R” for 2 h. The crude peptides were precipitated and purified with RP-HPLC as described above. As a representative example, the synthesis of dodecanoyl-[R5] is shown in Scheme 1.
Scheme 1. Synthesis of Dodecanoyl-[R5] as a Representative Example.
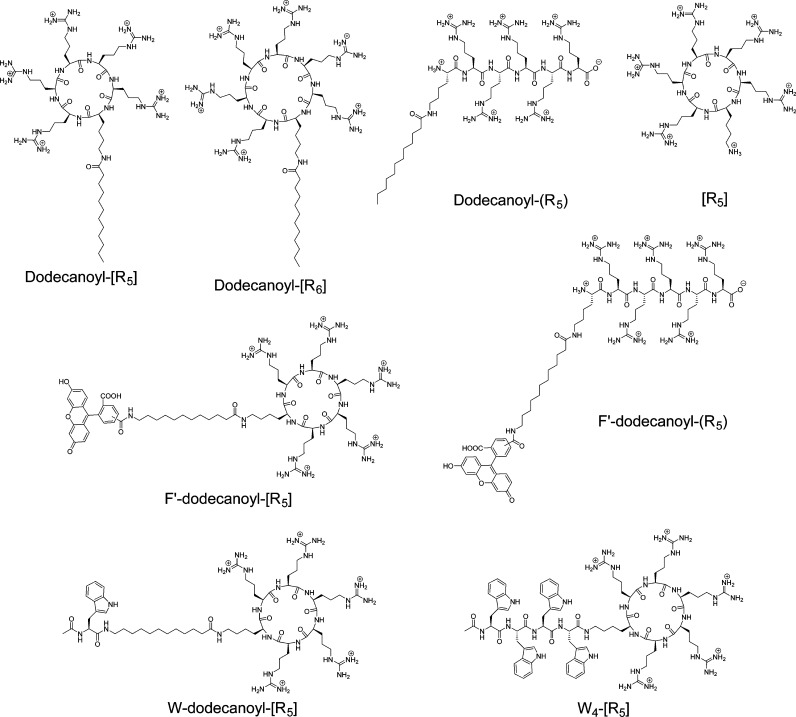
A corresponding acylated linear polyarginine peptide (ALPP) was synthesized for comparative studies with the cyclic peptide (ACPP). Moreover, a cyclic polyarginine without fatty acid [R5] was also synthesized to investigate the effect of the fatty acid on cyclic peptide and its effect on molecular transporting efficiency. To investigate whether the peptide alone can enter into cells, fluorescein-labeled F′-dodecanoyl-[R5] and F′-dodecanoyl-(R5), where F′ = fluorescein, were synthesized for FACS and microscopy investigations. All synthesized peptides are shown in Figure 1.
Figure 1.
Chemical structures of synthetic peptides used in this study (F′, fluorescein-labeled; [ ], cyclic peptide; ( ), linear peptide).
Cytotoxicity Assay of Synthetic Peptides
The cytotoxicity of all peptides were tested in two different cancer cell lines, adherent (SK-OV-3) and nonadherent (CCRF-CEM) cells, and a normal cell line (HEK 293T) using MTS assay (Figure 2). Cyclic polyarginine [R5] without fatty acid was used to explore the effect of N-terminal acylation on cytotoxicity and cellular uptake. ALPP (dodecanoyl-(R5)) and [R5] peptides showed consistently less cytotoxicity in all three cells compared to ACPPs (dodecanoyl-[R5] and dodecanoyl-[R6]). After 24 h incubation, ACPPs showed approximately 20% toxicity in cells at a concentration of 25 μM in CCRF-CEM cells. However, dodecanoyl-linear (R5) and [R5] did not exhibit significant cytotoxicity at 25 μM and showed less than 20% toxicity at the concentration of 100 μM. In SK-OV-3 cells, dodecanoyl-(R5) and [R5] showed more than 80% cell viability at the concentration of 25 μM. In normal cells (HEK 293T), all peptides exhibited less than 5% toxicity at 25 μM. This differential behavior of the peptides in normal, and cancer cells can be possibly rationalized through the interaction between polyarginine peptides and cell membranes. The membrane of cancer cells holds more negative charges compared to that in normal cells because of the presence of anionic lipids such as phosphatidylserine.10 Therefore, polyarginine peptides can be interacted with cancer cells more effectively compared to normal cells. Consequently, higher cell viability was observed in normal cells. These data indicated that ACPPs are more toxic than ALPP and a nonacylated cyclic peptide [R5], especially at concentration of ≥25 μM. Thus, a noncytotoxic concentration of 5–10 μM was used in cell-based assays.
Figure 2.
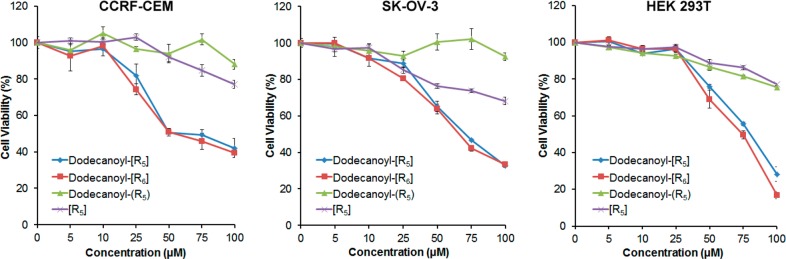
Comparison of cytotoxicity between cyclic and linear acylated polyarginine peptides and nonacylated cyclic peptide [R5] at various concentrations against CCRF-CEM, SK-OV-3, and HEK 293T after 24 h.
Cellular Uptake of Fluorescein-Labeled Acylated Cyclic and Linear PP
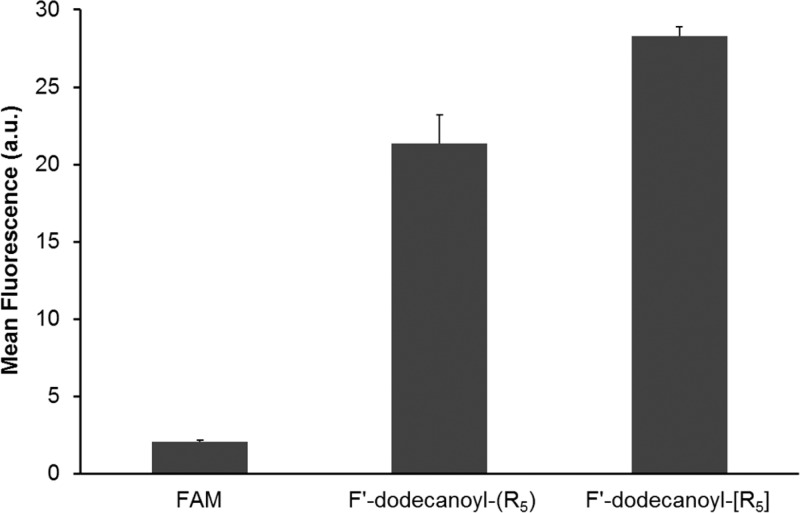
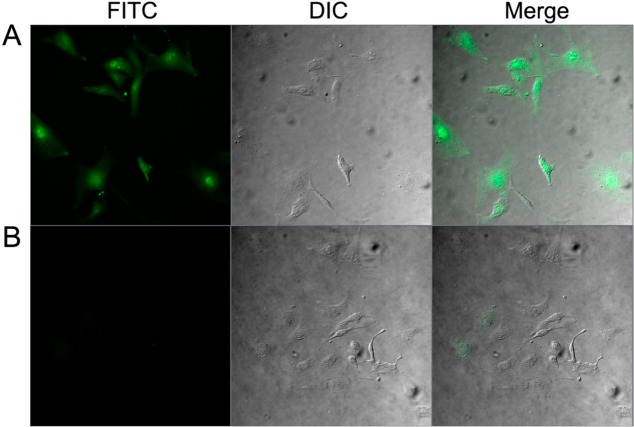
The intracellular uptake studies of fluorescein-labeled acylated cyclic and linear PP, F′-dodecanoyl-[R5] and F′-dodecanoyl-(R5), was carried out in SK-OV-3 cells by using flow cytometry and confocal laser scanning microscopy (CLSM) methods. Fluorescein (FAM, F′) alone was selected as a negative control. As it is shown in Figure 3, the F′-dodecanoyl-[R5] and F′-dodecanoyl-(R5) showed approximately 13.7- and 10.3-fold higher cellular uptake than that of control 5,6-carboxyfluorescein (FAM), respectively, in SK-OV-3 cells. F′-dodecanoyl-[R5] showed 1.3-fold higher cellular uptake compared to that of F′-dodecanoyl-(R5). The cellular uptake of F′-dodecanoyl-[R5] was confirmed by CLSM images (Figure 4). F′-Dodecanoyl-[R5] showed higher fluorescence intensity compared to that of F′-dodecanoyl-(R5) in SK-OV-3 cells. Therefore, ACPP F′-dodecanoyl-[R5] was found to be a more efficient cell-penetrating peptide compared to its linear counterpart. As shown in Figure 4, the fluorescence signal is extended through the whole cells, suggesting that F′-dodecanoyl-[R5] can get localized in the nucleus as well as cytoplasm.
Figure 3.
Comparative cellular uptake of F′-dodecanoyl-[R5] and F′-dodecanoyl-(R5) (5 μM) in SK-OV-3 cells (1 h).
Figure 4.
Confocal laser scanning microscope image of (A) F′-dodecanoyl-[R5] and (B) F′-dodecanoyl-(R5). The peptides were incubated for 1 h in SK-OV-3 cells at 10 μM concentration.
Cellular Uptake Mechanistic Study of F′-Dodecanoyl-[R5]
The mechanism of the cellular internalization of F′-dodecanoyl-[R5] was investigated by a temperature control assay at 4 °C along with ATP depletion assay. These two assays have been widely used to examine the energy-dependent endocytosis.11 FACS results showed that the intracellular uptake of F′-dodecanoyl-[R5] was significantly reduced at 4 °C, indicating that the mechanism of internalization was mainly dependent on the endocytosis pathways (Figure 5).12 Furthermore, ATP depletion assay was performed to investigate receptor-mediated endocytosis.13 To induce ATP depletion, SK-OV-3 cells were preincubated with sodium azide (10 mM) and 2-deoxy-d-glucose (50 mM) for 1 h prior to the experiment, and a similar concentration was maintained during the incubation (1 h) with F′-dodecanoyl-[R5]. The results showed that the cellular uptake of F′-ACPP was inhibited in the presence of sodium azide and 2-deoxy-d-glucose, suggesting that receptor-mediated endocytosis is involved for the cellular uptake of F′-ACPP. In ATP depletion assay, the basic cellular uptake of FAM was higher compared to that in temperature control assay. However, this is evident that the intracellular uptake of F′-dodecanoyl-[R5] was inhibited, and there was no significant difference between the cells treated with FAM compared to those treated with F′-dodecanoyl-[R5] in temperature control and ATP depletion assays, suggesting that endocytosis is the major pathway for the cellular uptake of F′-dodecanoyl-[R5].14
Figure 5.
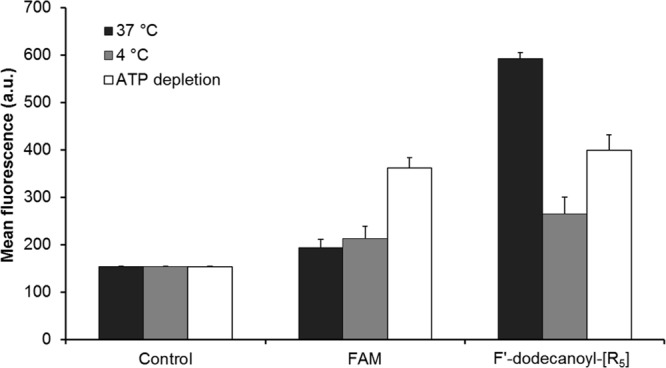
Cellular uptake of F′-dodecanoyl-[R5] (5 μM) in SK-OV-3 cells in temperature control assay at 37 and 4 °C, and ATP depletion assay with NaN3 (10 mM) and 2-deoxy-d-glucose (50 mM) analyzed by flow cytometry. Cells with no treatment were used as control.
Molecular Transporter Property of Peptides
The ability of ACPPs as a molecular transporter was evaluated and compared by selecting a fluorescein-labeled phosphopeptide, F′-GpYEEI, as a molecular cargo. Phosphopeptide, pYEEI (pTyr-Glu-Glu-Ile) is an optimal peptide template for the SH2 domain of Src tyrosine kinase. Several analogues of this peptide have been synthesized as potent ligands for this target.15−17 Because of the presence of the negatively charged amino acid residues in the structure of the peptide including phosphorylated tyrosine, it does not cross the cell membrane easily. Moreover, the internalization of the negatively charged phosphopeptide in cancer cells by diffusion is more difficult because cancer cell membranes are composed of more negatively charged lipids. Thus, cellular delivery of cell-impermeable negatively charged phosphopeptides is significantly challenging. We have previously reported different peptide-based carriers for the intracellular delivery of negatively charged phosphopeptides as model cell-impermeable drugs in several cell lines.18
In this study, the intracellular uptake of F′-GpYEEI was monitored in the presence and absence of synthetic peptides after 1 h incubation by flow cytometry. As it is exhibited in Figure 6, the ACPPs (dodecanoyl-[R5] and dodecanoyl-[R6]) delivered the phosphopeptide more efficiently compared to ALPPs, dodecanoyl-(R5) and -[R5]. The intracellular uptake of F′-GpYEEI in the presence of dodecanoyl-[R5] and dodecanoyl-[R6] was enhanced by 3.4- and 5.5-fold higher than the uptake in the absence of ACPPs. However, dodecanoyl-(R5) and -[R5] only improved 1.3- and 1.4-fold intracellular uptake, respectively. The results showed that acylated and cyclized polyarginine peptides can deliver the phosphopeptide effectively. However, the intracellular uptake of the phosphopeptide did not improve significantly in the presence of either acylated linear polyarginine peptide or cyclic [R5]. These data suggest that a combination of acylation and cyclization would improve the molecular transporting efficiency of the polyarginine-based peptide (containing less than six arginines) for the intracellular delivery of a cell-impermeable phosphopeptide. This has been previously reported that the acylated linear octa-arginine increased the cellular uptake of molecular cargoes by just adding fatty acid to the N-terminal of octa-arginine.8 However, we discovered that both cyclization and acylation in a short penta-arginine can significantly improve the delivery of a cell-impermeable phosphopeptide in SK-OV-3 cells.
Figure 6.
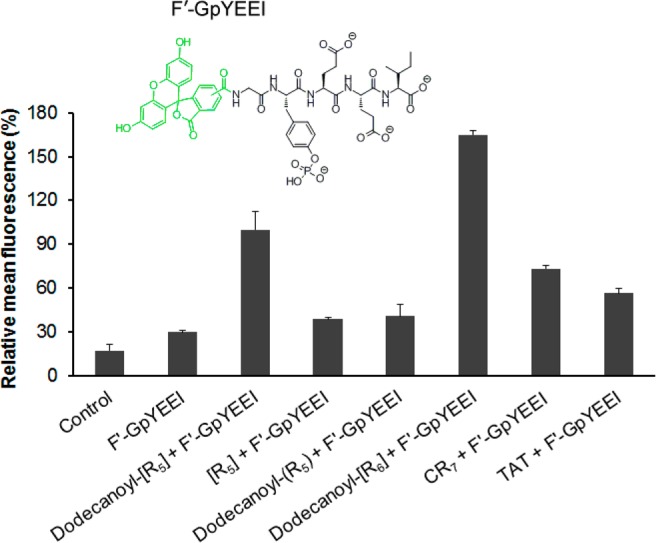
Cellular uptake of F′-GpYEEI (5 μM) in the presence of dodecanoyl-[R5] and -[R5], dosecanoyl-(R5), and dodecanoyl-[R6] (10 μM) in SK-OV-3 cell line. Phosphopeptide delivery efficiency of dodecanoyl-[R5] were compared with known CPPs (R7, CRRRRRRR; TAT, YGRKKRRQRRR). The mean fluorescence of F′-GpYEEI taken by dodecanoyl-[R5] was set as 100%. Cells with no treatment were used as control.
The major driving forces for the intracellular delivery are presumed to be structural rigidity through cyclization of the peptide and the interaction of the fatty acid with the cell membrane. It has been previously reported that the cellular uptake of the peptide can be increased due to the structural rigidity by cyclization of arginine-rich peptides.3 They proposed that the maximal distance between guanidine groups of arginine residue can lead to an efficient transduction of arginine-rich peptides. Our investigations showed that dodecanoyl-[R6] is able to deliver more efficiently by 1.6-fold higher F′-GpYEEI uptake compared to that of dodecanoyl-[R5]. Increasing the number of positively charged arginine residues can enhance the cellular uptake through ionic interactions with the negatively charged phosphopeptide and/or phospholipid in the cell membrane through ionic interactions. However, the higher number of arginine residue is not the only responsible element for the efficient cellular internalization. For example, it has been reported that polyarginine containing 11 amino acids (R11) showed higher cellular uptake compared to the polyarginine containing 13 amino acids (R13). At the same time, R11 was found to be a more potent transporter compared to R9 in prostate cancer cells.19 These investigations showed that an optimal number of arginine residues are required for the highest degree of functionality. However, the greater number of amino acid residues in cyclic peptides can decrease the structural rigidity, which lowers the ability of the peptide to get into cells.
Dodecanoyl-[R5] was also compared with several commonly CPPs, such as CR7 and TAT (YGRKKRRQRRR) peptides. The ACPP improved the cellular uptake of the phosphopeptide by 1.4- and 1.8-fold higher than those of CR7 and TAT, respectively (Figure 6). These results revealed that although other peptides containing arginine can deliver the molecular cargo, ACPP dodecanoyl-[R5] with a shorter peptide sequence than CR7 and TAT can work as a molecular transporter with higher efficiency.
Effect of Fatty Acid Chain Length on Cellular Uptake of ACPPs
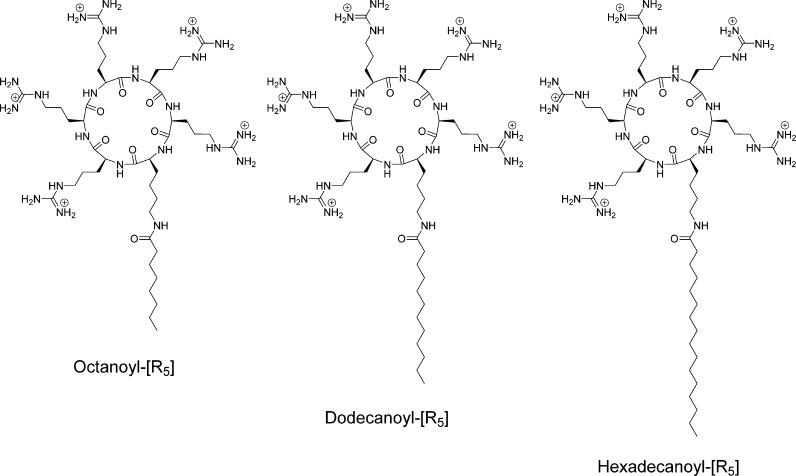
To investigate the effect of the chain length on the cell penetration potency, we synthesized octanoyl-[R5], dodecanoyl-[R5], and hexadecanoyl-[R5] (Figure 7). The cytotoxicity of the peptides was examined in SK-OV-3 cells. ACPPs showed less than 20% toxicity in cells at the concentration of 25 μM (Figure 8A). The in vitro toxicity results showed that increasing the fatty acid chain length caused enhanced toxicity in cells as hexadecanoyl-[R5] was more cytotoxic than dodecanoyl-[R5] and octanoyl-[R5]. These data indicate that the fatty acid chain length could alter the interaction with the cell membrane and disturb the membrane integrity. On the basis of the cytotoxicity data, a concentration of 10 μM was selected for further cell-based assays.
Figure 7.
Chemical structures of ACPPs with different length of fatty acid chains (C8, C12, and C16).
Figure 8.
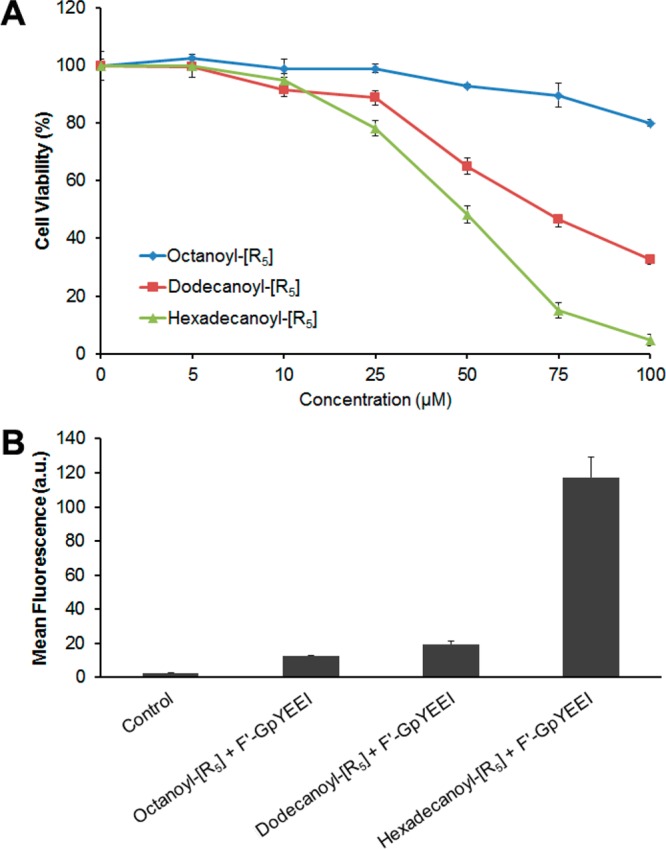
(A) Cytotoxicity assay of cyclic polyarginine peptide-fatty acid conjugates against SK-OV-3 cells (24 h incubation). (B) Cellular uptake of a phosphopeptide, F′-GpYEEI (5 μM), in the presence of peptide-fatty acid conjugates (10 μM) in SK-OV-3 cells. Cells with no treatment were used as control.
It has been previously reported that there was a relationship between the length of fatty acid in polyarginines and their cellular uptake,7 meaning that the optimal length of fatty acid is required for optimal functionality based on the peptide sequence and cell type. The results exhibited that the cellular uptake of F′-GpYEEI was improved in the order of octanoyl-[R5] < dodecanoyl-[R5] < hexadecanoyl-[R5] (Figure 8B). The cellular uptake of F′-GpYEEI in the presence of hexadecanoyl-[R5] was 9.3- and 6.0-fold higher than that in the presence of octanoyl-[R5] and dodecanoyl-[R5], respectively. These data suggest that the length of the attached fatty acid chain in the structure significantly influences the efficiency of the peptide as a molecular transporter. Sixteen-carbon chain length (C16) was found to be an optimized length for the intracellular delivery of F′-GpYEEI in SK-OV-3 cells.
Effect of Addition of Tryptophan Residues in Molecular Transporter Property
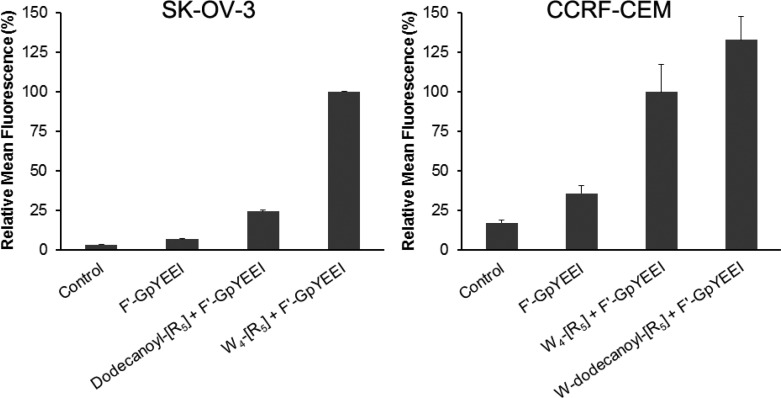
After ACPPs were found to act as CPP and molecular transporter, a systematic investigation was performed to modify the fatty acid to another hydrophobic moiety. Two other conjugates were synthesized. In the first conjugate, W4-[R5], the whole fatty acid chain was replaced with four tryptophan residues. The second conjugate W-dodecanoyl-[R5] had one more tryptophan at the end of the dodecanoyl fatty acid chain. W4-[R5] enhanced intracellular delivery of F′-GpYEEI by 4.1-fold higher compared to that of dodecanoyl-[R5] in SK-OV-3. However, W-dodecanoyl-[R5] improved the uptake of F′-GpYEEI by 1.3-fold higher compared to that of W4-[R5] in CCRF-CEM cells (Figure 9). The cellular uptake is affected by the cell lines. Thus, it is not straightforward to compare the cellular uptakes in SK-OV-3 and CCRF-CEM. However, the results could be assessed indirectly by relative comparison with the cellular uptake by W4-[R5]. These data suggest that the presence of hydrophobic tryptophan moieties could enhance the molecular transporting efficiency. In other words, appropriate modification of hydrophobic moiety in CPPs can increase the drug delivery ability of ACPP. Thus, cyclic nature, short length of polyarginine, and hydrophobic segments were found to be critical elements to generate a peptide with an optimized molecular transporter efficiency.
Figure 9.
Cellular uptake assay of a phosphopeptide, F′-GpYEEI (5 μM), in the presence of W4-[R5] and W-dodecanoyl-[R5] (10 μM) against SK-OV-3 and CCRF-CEM cell lines (1 h incubation). The mean fluorescence of F′-GpYEEI by W4-[R5] was set as 100%. Cells with no treatment were used as control.
Time-Dependent Antiproliferative Assay
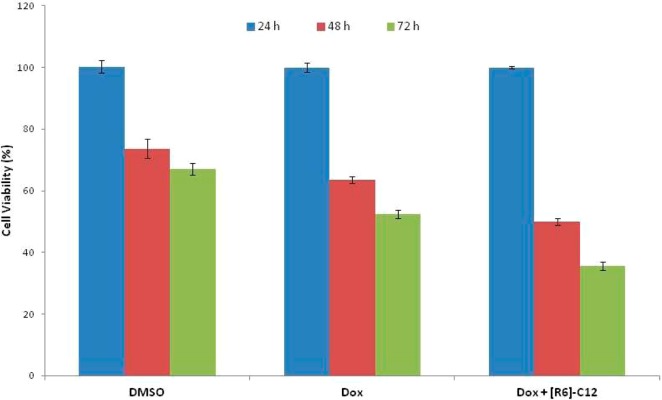
To evaluate whether the presence of peptides can enhance the pharmacological effect of a molecular cargo, the antiproliferative activity of doxorubicin (Dox) was examined in the presence of dodecanoy-[R6] ([R6]-C12) in MCF-7 cells in a time-dependent assay. As it is shown in Figure 10, the antiproliferative potency of Dox (5 μM) was enhanced by 7%, 11%, and 14% in the presence of [R6]-C12 (5 μM) when compared with that of the drug alone after 24, 48, and 72 h incubation, respectively. These results showed that the cell proliferation of MCF-7 cells was inhibited in a time-dependent manner suggesting a sustained drug release by peptide leading to a higher inhibitory effect. The presence of the peptide possibly improved the cellular uptake of Dox and enhanced the antiproliferative activity.
Figure 10.
Time-dependent antiproliferative activity of Dox (5 μM) in the presence and absence of [R6]-C12 (5 μM).
Conclusions
In conclusion, acylated cyclic polyarginine peptides were synthesized and examined as CPPs and potential molecular transporters. ACPPs showed higher potency as molecular transporter compared to the corresponding linear counterpart and cyclic polyarginine without fatty acid. The mechanism of the peptide internalization was found to be energy-dependent endocytosis. Cyclization and acylation reactions on the structure of the peptide enhanced the intracellular uptake of polyarginine peptides although they carry a short length of sequence. This intracellular delivery property of ACPPs can be optimized by modifying the length of fatty acid chain. To the best of our knowledge, this is the first report of cyclic fatty acylated polyarginine peptide as molecular transporter of a cell-impermeable phosphopeptide. This study provided insights about how a combination of the cyclic nature and acylation can improve the cell internalization of polyarginines. Further investigations are undergoing to determine whether conjugation of cell-impermeable hydrophobic drugs to acylated cyclic polyarginines can be an efficient method for designing novel drug delivery systems.
Acknowledgments
We acknowledge the American Cancer Society, Grant No. RSG-07-290-01-CDD, for the financial support. We thank National Center for Research Resources, NIH, and Grant Number 8 P20 GM103430-12 for sponsoring the core facility. This material is based upon work conducted at a research facility at the University of Rhode Island supported in part by the National Science Foundation EPSCoR Cooperative Agreement #EPS-1004057.
Glossary
ABBREVIATIONS
- ACPP
acylated cyclic polyarginine peptides
- ALPP
acylated linear polyarginine peptide
- ATP
adenosine triphosphate
- CCRF-CEM
human leukemia carcinoma cell line
- CFDI
5(6)-carboxyfluorescein diisobutyrate
- CLSM
confocal laser scanning microscopy
- CPPs
cell-penetrating peptides
- DCM
dichloromethane
- Dde
1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl
- DIC
N,N′-diisopropylcarbodiimide
- DIPEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- EDT
1,2-ethanedithiol
- EMEM
Eagle’s minimum essential medium
- FAM
5(6)-carboxyfluorescein
- FBS
fetal bovine serum
- Fmoc
9-fluorenylmethoxycarbonyl
- FACS
fluorescence activated cell sorter
- HBTU
2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HEK 293T
human embryonic kidney cell line
- HOAT
1-hydroxy-7-azabenzotriazole
- HOBT
hydroxybenzotriazole
- MALDI-TOF
matrix-assisted laser desorption/ionization-time-of-flight
- MTS
(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)
- NMP
N-methylmorpholine
- PBS
phosphate buffered saline
- PyAOP
7-azabenzotriazol-1-yl-oxytris(pyrrolidino)phosphonium hexafluorophosphate
- RP-HPLC
reversed-phase high pressure liquid chromatography
- SK-OV-3
human ovarian adenocarcinoma cell line
- TFA
trifluoroacetic acid
- TFE
2,2,2-trifluoroethanol
- TIS
triisoproylsilane
Supporting Information Available
Cytotoxicity assays of W4-[R5] and W-dodecanoyl-[R5]; comparison of serum stability of linear and cyclic acylated polyarginine peptides; cell culture; time-dependent antiproliferative assay. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Deshayes S.; Morris M. C.; Divita G.; Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell. Mol. Life Sci. 2005, 62, 1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. J.; Kim D. T.; Steinman L.; Fathman C. G.; Rothbard J. B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [DOI] [PubMed] [Google Scholar]
- Lättig-Tünnemann G.; Prinz M.; Hoffmann D.; Behlke J.; Palm-Apergi C.; Morano I.; Herce H. D.; Cardoso M. C. Backbone rigidity and static presentation of guanidinium groups increases cellular uptake of arginine-rich cell-penetrating peptides. Nat. Commun. 2011, 2, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaki S.; Suzuki T.; Ohashi W.; Yagami T.; Tanaka S.; Ueda K.; Sugiura Y. Arginine-rich peptides: an abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [DOI] [PubMed] [Google Scholar]
- Wender P. A.; Mitchell D. J.; Pattabiraman K.; Pelkey E. T.; Steinman L.; Rothbard J. B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 13003–13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaki S.; Ohashi W.; Suzuki T.; Niwa M.; Tanaka S.; Ueda K.; Harashima H.; Sugiura Y. Stearylated arginine-rich peptides: a new class of transfection systems. Bioconjugate Chem. 2001, 12, 1005–1011. [DOI] [PubMed] [Google Scholar]
- Lee J. S.; Tung C.-H. Lipo-oligoarginines as effective delivery vectors to promote cellular uptake. Mol. BioSyst. 2010, 6, 2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama S.; Hirose H.; Takayama K.; Nakase I.; Futaki S. Acylation of octaarginine: implication to the use of intracellular delivery vectors. J. Controlled Release 2011, 149, 29–35. [DOI] [PubMed] [Google Scholar]
- Youngblood D. S.; Hatlevig S. A.; Hassinger J. N.; Iversen P. L.; Moulton H. M. Stability of cell-penetrating peptide–morpholino oligomer conjugates in human serum and in cells. Bioconjugate Chem. 2007, 18, 50–60. [DOI] [PubMed] [Google Scholar]
- Riedl S.; Rinner B.; Asslaber M.; Schaider H.; Walzer S.; Novak A.; Lohner K.; Zweytick D. In search of a novel target: Phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim. Biophys. Acta 2011, 1808, 2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J. P.; Melikov K.; Vives E.; Ramos C.; Verbeure B.; Gait M. J.; Chernomordik L. V.; Lebleu B. Cell-penetrating peptides: a reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003, 278, 585–590. [DOI] [PubMed] [Google Scholar]
- Saraste J.; Palade G. E.; Farquhar M. G. Temperature-sensitive steps in the transport of secretory proteins through the golgi complex in exocrine pancreatic cells. Proc. Natl. Acad. Sci. U.S.A. 1986, 83, 6425–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATP is required for receptor-mediated endocytosis in intact cells. J. Cell Biol. 1990, 111, 2307–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J. P.; Melikov K.; Vivès E.; Ramos C.; Verbeure B.; Gait M. J.; Chernomordik L. V.; Lebleu B. Cell-penetrating peptides. J. Biol. Chem. 2003, 278, 585–590. [DOI] [PubMed] [Google Scholar]
- Bibbins K. B.; Boeuf H.; Varmus H. E. Binding of the Src SH2 domain to phosphopeptides is determined by residues in both the SH2 domain and the phosphopeptides. Mol. Cell. Biol. 1993, 13, 7278–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G.; Nam N.-H.; Kumar A.; Saleh A.; Shenoy D. B.; Amiji M. M.; Lin X.; Sun G.; Parang K. Synthesis and evaluation of tripodal peptide analogues for cellular delivery of phosphopeptides. J. Med. Chem. 2007, 50, 3604–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G.; Schuler A. D.; Ahmadibeni Y.; Morgan J. R.; Faruqui A.; Huang K.; Sun G.; Zebala J. A.; Parang K. Synthesis and evaluation of phosphopeptides containing iminodiacetate groups as binding ligands of the Src SH2 domain. Bioorg. Chem. 2009, 37, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nasrolahi Shirazi A.; Tiwari R. K.; Oh D.; Banerjee A.; Yadav A.; Parang K. Efficient delivery of cell impermeable phosphopeptides by a cyclic peptide amphiphile containing tryptophan and arginine. Mol. Pharmacol. 2013, 10, 2008–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nasrolahi Shirazi A.; Mandal D.; Tiwari R. K.; Guo L.; Lu W.; Parang K. Mol. Pharmacol. 2013, 10, 500–511. [DOI] [PubMed] [Google Scholar]; c Nasrolahi Shirazi A.; Tiwari R. K.; Oh D.; Sullivan B.; McCaffrey K.; Mandal D.; Parang K. Mol. Pharmacol. 2013, 10, 3137–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Mandal D.; Nasrolahi Shirazi A.; Parang K. Angew. Chem., Int. Ed. 2011, 50, 9633–9637. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Liu W.; Pong R.-C.; Hao G.; Sun X.; Hsieh J.-T. Analysis of oligo-arginine cell-permeable peptides uptake by prostate cells. Amino Acids 2012, 42, 1253–1260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.