Summary
Peroxisomal ABC transporters have been studied extensively in Arabidopsis but not in monocotyledonous species. Using barley, it is shown that their biochemical functions are conserved in flowering plants.
Key words: Aleurone, gene duplication, germination, indole butyric acid, oil body, seed size.
Abstract
In oilseed plants, peroxisomal β-oxidation functions not only in lipid catabolism but also in jasmonate biosynthesis and metabolism of pro-auxins. Subfamily D ATP-binding cassette (ABC) transporters mediate import of β-oxidation substrates into the peroxisome, and the Arabidopsis ABCD protein, COMATOSE (CTS), is essential for this function. Here, the roles of peroxisomal ABCD transporters were investigated in barley, where the main storage compound is starch. Barley has two CTS homologues, designated HvABCD1 and HvABCD2, which are widely expressed and present in embryo and aleurone tissues during germination. Suppression of both genes in barley RNA interference (RNAi) lines indicated roles in metabolism of 2,4-dichlorophenoxybutyrate (2,4-DB) and indole butyric acid (IBA), jasmonate biosynthesis, and determination of grain size. Transformation of the Arabidopsis cts-1 null mutant with HvABCD1 and HvABCD2 confirmed these findings. HvABCD2 partially or completely complemented all tested phenotypes of cts-1. In contrast, HvABCD1 failed to complement the germination and establishment phenotypes of cts-1 but increased the sensitivity of hypocotyls to 100 μM IBA and partially complemented the seed size phenotype. HvABCD1 also partially complemented the yeast pxa1/pxa2Δ mutant for fatty acid β-oxidation. It is concluded that the core biochemical functions of peroxisomal ABC transporters are largely conserved between oilseeds and cereals but that their physiological roles and importance may differ.
Introduction
The peroxisome is the sole site of β-oxidation of fatty acids and related molecules in plants and fungi, and is required for very long chain fatty acid metabolism and signalling in mammals (Baker et al., 2006; Poirier et al., 2006; Wanders and Waterham, 2006). In plants, β-oxidation is important not only during germination, seedling establishment, fertilization, and dark-induced senescence, but also in a number of additional key roles (reviewed in Theodoulou and Eastmond, 2012; Linka and Theodoulou, 2013). These include biosynthesis of the hormones jasmonic acid (JA) and indole acetic acid (IAA), production of volatile benzenoids and benzoyloxyalkylglucosinolates, and also salicylic acid biosynthesis (Reumann et al., 2004; Kliebenstein et al., 2007; Gfeller et al., 2010; Strader et al., 2011; Klempien et al., 2012; Lee et al., 2012; Qualley et al., 2012; Bussell et al., 2014).
Transport of β-oxidation substrates into the peroxisome is mediated by ATP-binding cassette (ABC) proteins belonging to subfamily D (Theodoulou et al., 2006). In mammals and fungi, peroxisomal ABCD transporters are expressed as ‘half-size’ proteins, each containing a transmembrane domain (TMD) and a nucleotide-binding domain (NBD), which homo- or heterodimerize to form a functional transporter (Theodoulou et al., 2006). Plants are unusual in having ‘full-size’ peroxisomal ABCD transporters, in which two dissimilar domains are expressed as a single polypeptide with the structure TMD–NBD–TMD–NBD, which is considered to represent a fused heterodimer (Verrier et al., 2008). The prototypical plant member of the ABCD subfamily is the Arabidopsis thaliana protein, COMATOSE (CTS; also known as AtPXA1, PED3, ACN2, AtABCD1; Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002; Hooks et al., 2007). Although the transporter is functional when the two halves are artificially expressed as separate proteins, both are required for function (Nyathi et al., 2012). Biochemical and genetic evidence suggests that CTS accepts fatty-acyl CoAs as substrates and cleaves off the coenzyme A (CoA) moiety during the transport cycle (Footitt et al., 2002; Nyathi et al., 2010; De Marcos Lousa et al., 2013). Acyl-activating enzymes then re-esterify the fatty acids to CoA in the peroxisome lumen, which is a prerequisite for entry into β-oxidation (Fulda et al., 2004; De Marcos Lousa et al., 2013). This unusual transport mechanism appears to be common to plant, yeast, and mammalian ABCD proteins (van Roermund et al., 2012).
Alleles of cts have been identified in several forward genetic screens in Arabidopsis, providing important clues to the physiological functions of plant peroxisomal ABC transporters and their underlying biochemical bases (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002; Hooks et al., 2007). The cts-1 mutant was identified in a screen for genes which control germination and is also impaired in seedling establishment (Russell et al., 1998; Footitt et al., 2002). Whilst the establishment phenotype reflects the inability to mobilize storage lipid and can be rescued with exogenous sucrose (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002), fatty acid mobilization is not required for germination (Pinfield-Wells et al., 2005; Footitt et al., 2006; Kelly et al., 2011). The germination defect of cts mutants was recently shown to be associated with accumulation of the JA precursor 12-oxophytodienoic acid (OPDA) which triggers up-regulation of the ABSCISIC ACID-INSENSITIVE 5 (ABI5) transcription factor, leading to inhibition of seed coat rupture (Kanai et al., 2010; Dave et al., 2011). CTS is thought to mediate OPDA transport into the peroxisome, since null mutants are JA deficient, but a second, minor import pathway also exists, as cts mutants are not male sterile (Theodoulou et al., 2005). It is possible that OPDA could enter the peroxisome by passive diffusion and be subject to vectorial acylation involving peroxisomal OPDA:CoA ligase (Kienow et al., 2008), though an as yet unidentified transporter cannot be ruled out.
Alleles of cts have been isolated in screens for resistance to the natural auxin, indole-3-butyric acid (IBA) and the artificial pro-auxin, 2,4-dichlorophenoxybutyric acid (2,4-DB) (pxa1-1, Zolman et al., 2001; ped3 series, Hayashi et al., 1998, 2002). These compounds are metabolized by one round of β-oxidation to generate IAA and 2,4-dichlorophenoxyacetic acid (2,4-D), respectively, which inhibit root and hypocotyl growth (Hayashi et al., 1998; Zolman et al., 2001; Rashotte et al., 2003). This implies that CTS can transport these aromatic compounds or their CoA esters, although this has not been tested experimentally. Finally, a cts allele (acn2) was shown to be resistant to fluoroacetate, implying a role in acetate metabolism (Hooks et al., 2007). Thus, it appears that plants have apparently evolved a broad specificity peroxisomal ABC transporter to mediate the import of the wide range of substrates that they need to process by β-oxidation (Baker et al., 2006). This is in contrast to mammalian and yeast peroxisomal ABC transporters which exhibit restricted substrate selectivities as judged by cross-kingdom complementation experiments and characterization in yeast and in vivo (van Roermund et al., 2008, 2011, 2014; Zhang et al., 2011).
With the exception of a study implicating a CTS homologue in the control of seed size in tomato (Orsi and Tanksley, 2009), ABCD proteins have not been investigated in plant species other than Arabidopsis, and it remains to be determined to what extent the functions identified thus far are shared with other taxa and whether their relative importance differs. Arabidopsis and other oilseeds utilize oil stored primarily in cotyledons as the main source of energy during seedling establishment. In contrast, in cereals such as barley, starch stored in the endosperm fulfils this role, although the embryo, scutellum, and aleurone contain significant amounts of stored lipid (Jones and Jacobsen, 1991). Tissue-specific transcriptome analysis has shown that genes of the β-oxidation pathway are induced in both endosperm/aleurone and embryo from 24h after imbibition of barley grains (Sreenivasulu et al., 2008), and enzymes of β-oxidation are present in both tissues. Gibberellic acid (GA) stimulates the breakdown of oil reserves and their conversion to sugar by gluconeogenesis in cereal aleurone (Doig et al, 1975; Newman and Briggs, 1976; Fernandez and Staehelin, 1987), although sensitivity to GA is cultivar dependent (Eastmond and Jones, 2005). It has been proposed that mobilization of stored triacylglycerol (TAG) from aleurone tissue provides energy and carbon skeletons for the synthesis of α-amylase that is needed to mobilize starch. An alternative hypothesis is that sucrose provided by gluconeogenesis in the aleurone could be transferred to the growing embryo via the scutellum, to support embryo growth (Eastmond and Jones, 2005). Although the embryo and scutellum are rich in oil, these tissues lack glyoxylate cycle enzyme activities (Holtman et al., 1994), and thus may respire fatty acids, rather than use them for gluconeogenesis (Eastmond and Jones, 2005). In conclusion, β-oxidation might play key roles in barley germination, an important trait in agriculture and malting.
In this study, the roles of peroxisomal ABC transporters in a model cereal, barley (Hordeum vulgare L.), were investigated and experiments were carried out to test whether they perform similar functions to those in oilseeds such as Arabidopsis. The isolation of two barley CTS homologues, HvABCD1 and HvABCD2, is reported and their collective roles are analysed using RNA interference (RNAi). In parallel, the individual functions of HvABCD1 and HvABCD2 were investigated by testing their ability to complement the Arabidopsis cts-1 mutant and the yeast pxa1/pxa2Δ mutant which lacks the homologous transporter. Together, these approaches enabled the physiological roles of barley peroxisomal ABC transporters to be probed and the contributions of the two different genes to different known biochemical functions to be assessed. It is concluded that the general capabilities of peroxisomal ABC transporters of Arabidopsis and barley are similar but that the two paralogues in barley may play distinct roles.
Materials and methods
Plant material and growth conditions
Barley, Hordeum vulgare L. var. Golden Promise, was grown under controlled conditions of 15 °C/12 °C (day/night) and a 16h photoperiod [80% relative humidity, 500 μmol m–2 s–1 metal halide lamps (HQI) supplemented with tungsten bulbs]. Seeds were sown in 5 litre pots containing Levington’s C2 compost (http://www.everris.com/uk/Home/Ornamental-Horticulture/Products/Product.aspx/Professional-Growing-Media/Potting-and-Pot-Plant-Compost/_/_/MCP, last accessed 21 May 2014). Heads were harvested at maturity, dried for 7 d, and threshed by hand to prevent damage to the husk and embryo.
Identification and cloning of HvABCD1 and HvABCD2
Analysis of the rice genome and barley expressed sequence tag (EST) sequences revealed two CTS homologues in grasses. The barley genes were designated HvABCD1 and HvABCD2, according to nomenclature conventions prescribed in Verrier et al. (2008). HvABCD1 was amplified from barley cDNA using primer pair CTS-a startF/Hv14973R2 (Supplementary Table S1 at JXB online) and blunt-end cloned into pBluescript, to give pCTS1/BS. Plasmids for the expression of HvABCD1 and HvABCD2 under the control of the native CTS promoter were constructed as follows: first, the promoter region of AtCTS was amplified from pG0229-T ProGFP (Footitt et al., 2007a) with primer pair AtCTSp5′Nco/AtCTSp3′Kpn, excised with NcoI/KpnI, and cloned in the corresponding sites of pENTR11 (Invitrogen), to give pE-AtCTSp. The open reading frame (ORF) of HvACBD1 was amplified from pCTS1/BS using primer pair HvCTSa Kpn5′/HvCTSa Not3′ and cloned into the KpnI/NotI sites of pE-AtCTSp. Following confirmation of the insert by sequencing, the entry clone was recombined with pKanGWFS7 (Karimi et al., 2002) using Gateway technology (Invitrogen), to give pAtCTS:HvABCD1. HvABCD2 was amplified in a nested PCR strategy. The first 670bp of HvABCD2 cDNA was amplified from barley cDNA with primers CTSb Kpn5′/CTSb NotI-BglII R and cloned in the KpnI/NotI sites of pE-AtCTSp to create pE-CTSb5′. The remaining cDNA was amplified from 24h imbibed barley embryo cDNA, using primer pair CTSb Bgl IIF/CTSb Not3′ and cloned into the BglII/NotI sites of pE-CTSb5′. The inserts were confirmed by sequencing and the two entry clones were recombined into pGWB7 (Nakagawa et al., 2007) to give pAtCTS:HvABCD2.
Genetic mapping
The cDNA sequences of HvABCD1 and HvABCD2 were BLASTed against the recently released sequence assembly of the barley cv. Morex genome (IBSC, 2012) identifying corresponding genomic sequence contigs (HvABCD1=morex_contig_47855, HvABCD2=morex_contig_368523). Both contigs have been integrated into a genetically ordered sequence context using POPSEQ (Mascher et al., 2013) in two biparental segregating populations: the reference Morex×Barke recombinant inbred line (RIL) population and OWB doubled haploid population (IBSC 2012). Map positions are related to the positions of the barley iSelect markers as reported in Comadran et al (2012).
Reverse transcription–PCR (RT–PCR)
For the experiment presented in Fig. 1A, embryos and aleurone were isolated from barley grains and incubated in water for different periods (0, 4, 12, 24, 48, and 72h). RNA was extracted from embryos using a modified TRIZOL extraction protocol, and aleurone tissue RNA was extracted according to Singh et al. (2003). Poly(dT) cDNA was prepared from 2 μg of total RNA with SuperScript III reverse transcriptase (Invitrogen). Amplification was conducted using the primer pairs HvABCD1 F/R and HvABCD2 F/R; cycle conditions: 96 °C 2min; 30 cycles of (94 °C 30 s, 55 °C 30 s, 72 °C 30 s); 72 °C 7min. Constitutively expressed α-tubulin was used as a loading control (Nicot et al., 2005; Jarošová and Kundu, 2010) and PM19 was used as a developmental control (Ranford et al., 2002). Amplifications were conducted using the primer pairs α TUB F/R [cycle conditions: 96 °C 2min; 25 cycles of (94 °C 30 s, 58 °C 30 s, 72 °C 30 s); 72 °C 7 min] and PM19 F/R [cycle conditions: 96 °C 2min; 30 cycles of (94 °C 30s, 55 °C 30 s, 72 °C 30 s) ×30; 72 °C 7 min], respectively.
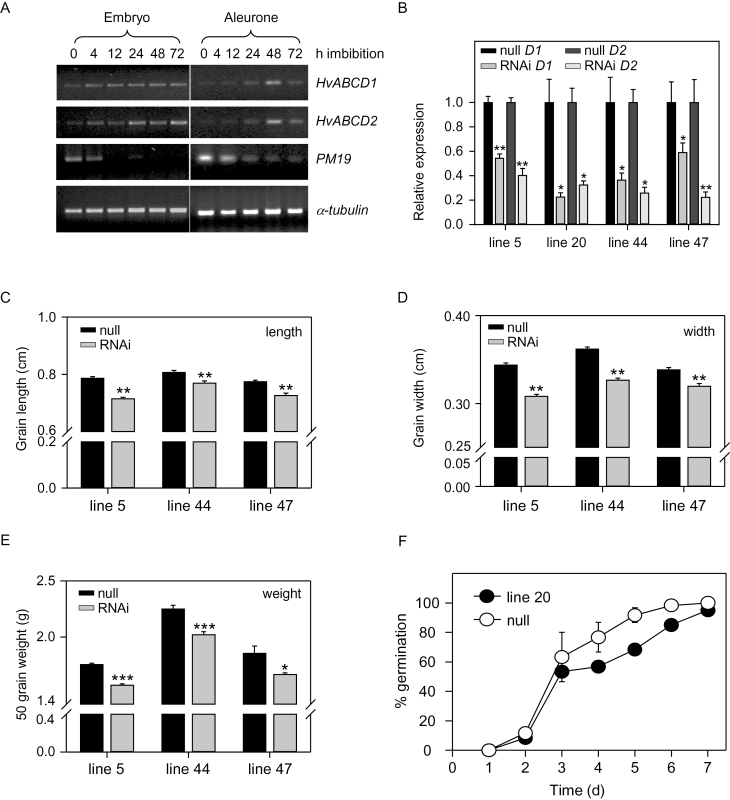
Fig. 1.
Seed phenotypes of HvABCDi lines. (A) RT–PCR analysis of HvABCD1 and HvABCD2 expression in embryo and endosperm during germination. (B) Quantitation of HvABCD1 and HvABCD2 transcripts in RNAi lines, using Q-PCR. Transcript abundance in each RNAi line is expressed relative to abundance in the respective null segregant. Values are means ±SE (n=3); *P<0.05, **P<0.01. (C–E) Grain length, width, and weight in RNAi lines, relative to the respective null segregants. Values are means ±SE (n=5, 50 seeds per replicate); *P<0.05, **P<0.01, ***P<0.001. (D) Germination of RNAi line 20 and the corresponding null segregant over 7 d. Values are means ±SE (n=3).
Generation of RNAi lines
A 497bp fragment of HvABCD2 with high sequence identity to HvABCD1 (Supplementary Fig. S1 at JXB online) was amplified from cDNA using primers HvABCDRNAiF and HvABCDRNAiR, and recombined sequentially into pGWC (kind gift of Dr Ranjan Swarup, University of Nottinhgam) and pBract207, via Gateway cloning (Invitrogen). Transgenic barley plants (var. Golden Promise) were generated by the BRACT transformation facility (John Innes Centre, Norwich, UK), using Agrobacterium-mediated transformation of immature embryos, according to the method of Harwood et al. (2009). Copy number and zygosity determination were carried out by IDna GENETICS (Norwich, UK).
Analysis of RNAi lines
Quantitative real-time PCR (Q-PCR)
RNA was extracted from seedling leaf material (Zadoks code 15, which corresponds to five visible leaves), using a Nucleospin RNA plant mini-kit (Macherey-Nagel, Germany). Leaf material (70–100mg) was ground to a powder in liquid N2 and resuspended in 400 μl of RA1 extraction buffer, containing 1% (w/v) PVP-40. Following clarification by centrifugation, the supernatant was processed according to the manufacturer’s instructions. Poly(dT) cDNA was prepared from 1mg of total RNA with SuperScript III reverse transcriptase (Invitrogen), using primers as specified in Supplementary Table S1 at JXB online. Quantitative PCR was performed using SYBR-green Sensimix (Bioline), in 384-well optical reaction plates with a Roche LightCycler 480 apparatus (Roche/Applied Biosystems). PCR conditions and quantification were as specified by the manufacturer (Roche). The relative number of copies obtained for each transcript was normalized against HvELF1 and HvTubulin (Nicot et al., 2005; Jarošová and Kundu, 2010) or AtCTL1 and AtCTL3 (De Rybel et al., 2010) transcript values for each sample as an internal reference.
Seed size measurement
Five samples of 50 grains from homozygous HvABCD1/2 RNAi lines and their respective null segregants were weighed. Seeds were photographed using a camera attached to a light microscope. Length and width were determined from micrographs, following correction for magnification.
Germination assays
Fifty grains were placed in Petri dishes containing two layers of Whatman No. 2 filter paper and 4ml of water. The dishes were sealed with Micropore tape and incubated at 22 °C under continuous white light (150 μmol m–2 s–1). Germinated caryopses, defined by the emergence of coleorhizae beyond the seed coats, were scored every 24h over 7 d and removed from the dishes. Assays were performed in triplicate.
Root growth assays
Grains from homozygous HvABCD1/2 RNAi lines and their respective null segregants were plated on 0.5× Murashige and Skoog (MS) medium with 0.5% (w/v) sucrose containing the indicated concentrations of 2,4-DB or IBA, or no supplements. Experiments were carried out in triplicate with four grains per replicate.
JA treatment
Seedlings of HvABCD1/2 RNAi lines were grown in a growth chamber (20 °C day/15 °C night, 70% relative humidity, 16h light). Ten-day-old plants were sprayed with 2ml of methyl jasmonate (Sigma-Aldrich, Germany) at 2mg ml–1 in water. After 48h, the treatment was repeated and leaf material was sampled 72h after the first treatment. RNA extraction and Q-PCR were performed as described above.
Scanning electron microscopy
Intact grains were imbibed in sterile distilled water (SDW) for 2–5 d, on moistened filter paper sealed in Petri dishes and placed in the dark at room temperature. Following imbibition, root and shoot were removed if present and the remaining tissue was mounted onto cryo electron microscope stubs using OCT compound (Agar Scientific UK) and plunge-frozen in pre-slushed liquid N2. They were then transferred under vacuum to the Alto 2500 (Gatan UK) cryo chamber stage which was pre-cooled to –180 °C. Here they were fractured using the cold blade mounted in the chamber and gentle etching was performed through sublimation by raising the temperature of the stage to –95 °C for 2min. The stage temperature was returned to –140 °C and the samples were sputter-coated with AuPd for 60 s to a thickness of ~10nm. Samples were transferred to the JEOL JSM 6700 scanning electron microscope (SEM) chamber and mounted on the stage cooled to –150 °C, for examination. This temperature was maintained during examination of the fractured surface, and images were recorded using the on-board system and software.
Confocal microscopy
Grains were de-husked and de-embryonated using a sterile scalpel before sterilizing by immersion in 20% (v/v) sodium hypochlorite for 10min. The samples were thoroughly washed for 1min under a flowing stream of SDW. Each grain was placed in a 1.5ml Eppendorf tube and covered with ~1ml of 1 μM GA3, and left to imbibe at room temperature for 8–96h. At the required time points, grains were washed in SDW and dried on filter paper to remove excess water. Grains were individually mounted onto cryostat holders using Tissue-Tek, OCT compound (Agar Scientific UK) and were quickly plunged into liquid N2. When bubbling ceased, the frozen samples were transferred to the chamber of the Leica 1850 Cryostat (Leica Microsystems UK) and left for 30min to allow the temperature to equilibrate to the chamber temperature of –20 °C before sectioning. Sections were removed from the sample and discarded until the mid-region was reached ~1.6mm from the tip. The following 10 sections, each 16 μm thick, were collected on glass slides for staining. Sections were stained using Nile Red at 1 μl ml–1 for 1min, followed by washing with SDW and sealing with a cover slip. Sections were imaged using a Zeiss LSM 780 system, with laser 514nm selected and emission collected between 539nm and 753nm.
Arabidopsis complementation
Wild-type (Ler) and cts-1 plants were transformed with pAtCTS:HvABCD1 and pAtCTS:HvABCD2 by floral dip. Lines expressing HvABCD2 in the cts-1 background were obtained by selecting cts-1 transformants which germinated on 0.5× MS medium and confirming genotype by selection and PCR analysis. As HvABCD1 did not complement the germination phenotype of cts-1 (see the Results), transgenic lines were obtained in the Ler background, crossed to cts-1 and a homozygous line identified by PCR genotyping. It was not possible to produce multiple independent homozygous lines for HvABCD1. Assays for germination, establishment, and hypocotyl growth were carried out as described in Dietrich et al. (2009). Seed size was determined as described above for barley (30 seeds per line). The effect of OPDA on root growth was determined as described in Zhang and Turner (2008).
Yeast complementation
HvABCD1 was amplified with primer pair FT206/FT207, restricted with KpnI/PstI, and cloned into the corresponding sites of pEL30 and pIJL30. The inserts were sequenced to confirm the absence of PCR-generated mutations. Yeast transformation and oleate growth tests were carried out as described in Nyathi et al. (2010). β-Oxidation measurements were carried out as described in van Roermund et al. (1995); in brief, 1-14C-labelled fatty acids were supplied to intact cells followed by quantification of 14CO2 and 14C-labelled β-oxidation products by liquid scintillation counting.
Results
Barley has two CTS homologues which are expressed in embryo and endosperm
Comparative analysis of sequenced plant genomes has revealed that cereals contain two CTS homologues, consistent with a gene duplication occurring after divergence of the Gramineae family (Nyathi et al., 2012). Full-length cDNAs corresponding to the barley CTS homologues were isolated by RT–PCR and designated HvABCD1 and HvABCD2, according to the naming convention outlined in Verrier et al. (2008). HvABCD1 and HvABCD2 were located on the barley genome by BLASTing the cDNA sequence against a sequence assembly of the Morex genome that had been genetically ordered using the POPSEQ approach (Mascher et al., 2013). HvABCD1 was located at 148.4 cM on chromosome 3H on the Morex×Barke map. HvABCD2 was located at 8.9 cM on chromosome 1H on the reference Morex×Barke RIL population map and 11.2 cM on 1H on the Oregon Wolfe Barley doubled haploid map (International Barley Sequencing Consortium, 2012). The orthologous genes in rice mapped to syntenic positions on the corresponding chromosomes.
Examination of HvABCD1 and HvABCD2 expression profiles using the Bio-Array Resource barley eFP browser (Patel et al., 2012) revealed that both genes were widely expressed in different tissues, with HvABCD2 transcripts being generally more abundant (Supplementary Fig. S2 at JXB online). The expression of HvABCD1 and HvABCD2 was then investigated in more detail in germinating barley grains over 3 d imbibition, using RT–PCR. PM19, which encodes a putative plasma membrane transporter, was used as a developmental control. Consistent with previous reports (Ranford et al., 2002), transcripts were present in unimbibed seeds but declined upon germination (Fig. 1A). This confirmed the developmental status of the grains, since dormant embryos have been shown to retain high levels of PM19 message for up to 72h of imbibition (Ranford et al., 2002). HvABCD1 transcripts were barely detectable in dry grains but were present in embryos at a similar level from 4h to 72h imbibition. HvABCD2 expression in embryos increased steadily over this period (Fig. 1A). Both genes were expressed transiently in aleurone tissue at 48h imbibition, corresponding to a time point around germination. This suggests that β-oxidation could be important for embryo and aleurone function during germination and seedling establishment in barley.
Suppression of HvABCD1 and HvABCD2 affects grain size but not germination
In order to understand the function of CTS homologues in barley growth and development, expression of both HvABCD genes was reduced simultaneously using an RNAi approach. Barley (cv. Golden Promise) was transformed with a construct designed to suppress both HvABCD transcripts (Supplementary Fig. S1 at JXB online), driven by the constitutive Ubi1 promoter (Harwood et al., 2009). Several HvABCD1/2 RNAi lines (hereafter referred to as HvABCD1/2i lines) were obtained and, for each one, a null segregant line was also selected for use as a control. Expression levels were analysed by Q-PCR analysis of leaf material. Abundance of HvABCD1 and HvABCD2 transcripts varied from 22% to 59% and 22% to 40% of levels measured in corresponding null segregant lines, respectively (Fig. 1B).
Since natural variation in seed size has been associated with ABCD transporter genes in tomato and Arabidopsis, and cts alleles have small seeds (Russell, 1998; Alonso-Blanco et al., 1999; Orsi and Tanksley, 2009), it was investigated whether suppression of HvABCD1 and HvABCD2 affected grain size in barley. Grains of three different RNAi lines exhibited significantly reduced length, width, and weight, compared with null segregants (Fig. 1C–E). The germination of seeds from several HvABCD1/2i lines and their respective nulls was also measured over 7 d. Although the percentage germination of null lines at 7 d was somewhat variable, in none of the RNAi lines tested was germination markedly different from that of the respective null segregant (Fig. 1F; Supplementary Fig. S3 at JXB online). This is in agreement with results from tomato, where ABCD function does not appear to affect the rate of germination (Orsi and Tanksley, 2009), but in contrast to Arabidopsis, in which cts mutants are arrested late in phase II of germination (Russell et al., 2000; Footitt et al., 2002, 2006; Carrera et al., 2007).
Suppression of HvABCD1 and HvABCD2 does not markedly affect seedling establishment and lipid mobilization
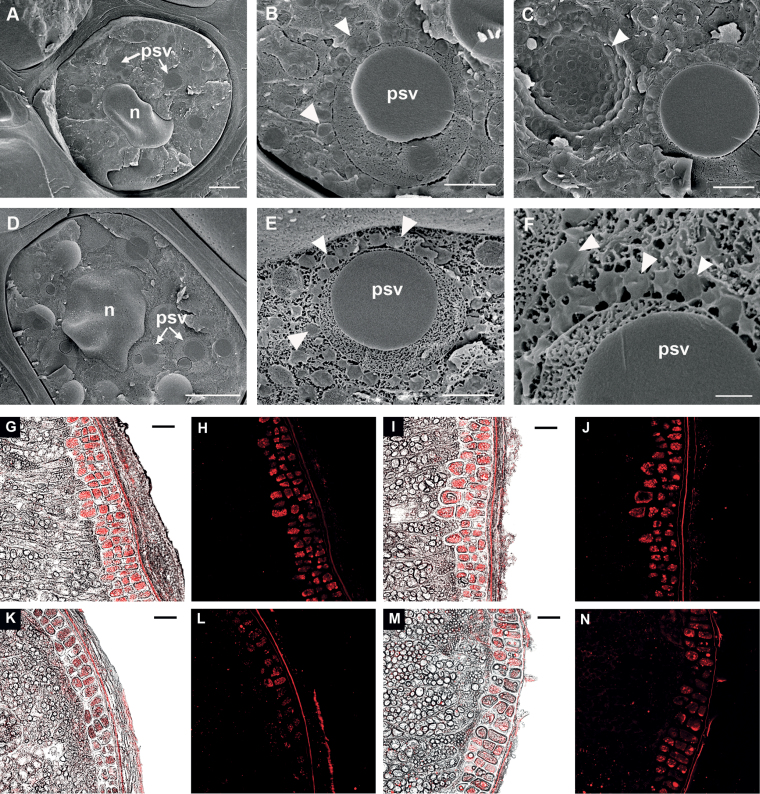
In Arabidopsis, peroxisomal β-oxidation plays a critical role in mobilization of storage reserves during seedling establishment (Theodoulou and Eastmond, 2012). However, seedling establishment was not visibly affected in HvABCD1/2i lines, which is perhaps unsurprising, given that barley contains abundant reserves of starch in the endosperm. Nevertheless, since mobilization of TAG stored in the cereal aleurone has been suggested to facilitate the exploitation of endosperm starch and/or to support embryo growth (Eastmond and Jones, 2005), oil body morphology was examined over 5 d imbibition. After 2 d imbibition, scanning electron microscopy revealed that aleurone cells of wild-type cells contain numerous protein storage vacuoles (PSVs), surrounded by oil bodies (Fig. 2A–C). The oil body membrane is continuous with the PSV membrane, as previously reported (Fernandez and Staehelin, 1987); this is particularly evident where freeze-fracture has generated images showing extracellular (E-)faces with ‘scars’ where oil bodies have been removed along with PSVs (Fig. 2C). After 5 d, numerous oil bodies were still visible, though they appeared less spherical (Fig. 2D–F). It was also possible to visualize aleurone oil bodies by confocal microscopy using Nile Red staining of TAG in unfixed cryo-sections of de-embyronated grains treated with GA (Fig 2G–N). After 5 d, the abundance of oil bodies had declined (Fig. 2K–N), in agreement with reports that GA stimulates lipid mobilization in this tissue (Fernandez and Staehelin, 1987; Eastmond and Jones, 2005). Since it was not possible to derive quantitative data from these images, fatty acids were quantified in de-embryonated, GA-treated barley grains, but were not found to be significantly different in RNAi and null segregant lines (data not shown).
Fig. 2.
Oil body morphology in barley grains. (A–F) SEM cryo-fractured images of wild-type (var. ‘Golden Promise’) barley aleurone cells. (A–C) Two day imbibed grains; (D, E), 5 d imbibed grains. (A, D) Aleurone cells, showing nucleus (n) and numerous protein storage vacuoles (psv). (B, E) PSV, surrounded by oil bodies (OBs), indicated by arrowheads. (C) PSV fractured across, surrounded by OBs; the arrowhead indicates the extracellular face (‘E-face’) where PSV and OBs have been removed during fracture. (F) Close-up of (E), showing connections between OBs and PSV. OBs are indicated by arrowheads. (G–N) Confocal images of unfixed barely grain sections showing Nile Red staining of OBs in aleurone cells. De-embryonated grains were imbibed for 24h (G–J) or 96h (K–N) in the presence of 1 μM GA. (G, H, K, L) HvABCD1/2 RNAi line 5; (I, J, M, N) null segregant. In G, I, K and M, the confocal image is overlaid on the bright field image. Scale bars: A=5 μm, B, C, E=1 μm; D=4 μm; F=400nm; G–N=50 μm.
HvABCD proteins play roles in auxin and jasmonate metabolism
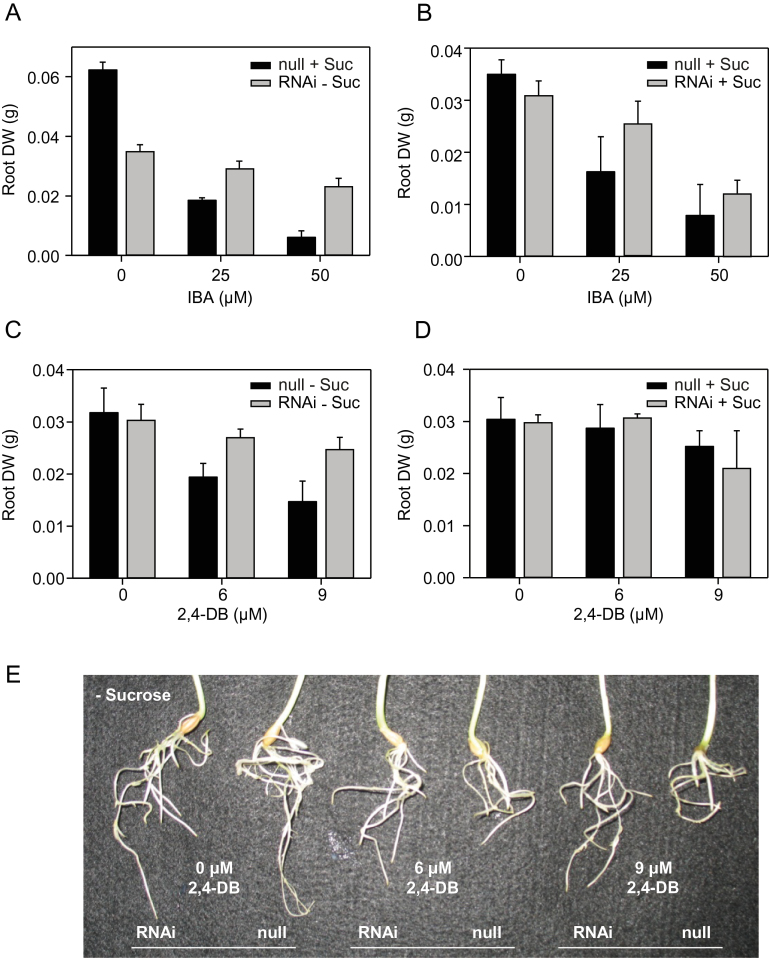
It was next investigated whether barley ABCD transporters are required for β-oxidation of auxins, as has been shown for CTS (Zolman et al., 2001; Hayashi et al., 2002). Both IBA and 2,4-DB treatment inhibited root growth, as judged by the reduced root dry weight of null segregant lines (Fig. 3). The response was dose dependent, although higher levels of the hormones were required to inhibit growth, compared with Arabidopsis (Dietrich et al., 2009). In the absence of sucrose, HvABCD1/2i lines exhibited resistance to IBA and 2,4-DB (Fig. 3A, C, E), suggesting that one or both of the barley transporters mediate import of these compounds into the peroxisome for β-oxidation. Interestingly, the inhibitory effect of IBA and 2,4-DB on barley root growth was reduced in the presence of sucrose (Fig. 3B, D), unlike in Arabidopsis where sucrose potentiates the effect of these compounds (Dietrich et al., 2009). Because exogenous sucrose inhibits lipid mobilization in Arabidopsis seedlings (Martin et al., 2002; Fulda et al., 2004), the effect of sucrose on pro-auxin toxicity has been interpreted to imply a direct competition between fatty acids and IBA or 2,4-DB for ABCD-mediated transport (Dietrich et al., 2009). The lack of such an effect in barley roots may indicate that the rate of fatty acid β-oxidation is not very high in this system or, alternatively, that sucrose promotes root growth and that this effect outweighs the inhibitory effect of auxins.
Fig. 3.
Root responses of HvABCDi lines to auxin precursors. (A) Dry weight of RNAi line 5 roots grown in different concentrations of indole-3-butyric acid (IBA). (B) As A, in the presence of 0.5% sucrose. (C) Dry weight of RNAi line 20 roots grown in different concentrations of 2,4-dichlorophenoxybutyric acid (2,4-DB). (D) As C, in the presence of 0.5% sucrose. Values are means ±SE (n=4). (E) Images of roots of RNAi line 5 and the corresponding null segregant, grown in medium lacking sucrose, containing different concentrations of 2,4-DB. Data are representative of experiments with different lines. (This figure is available in colour at JXB online.)
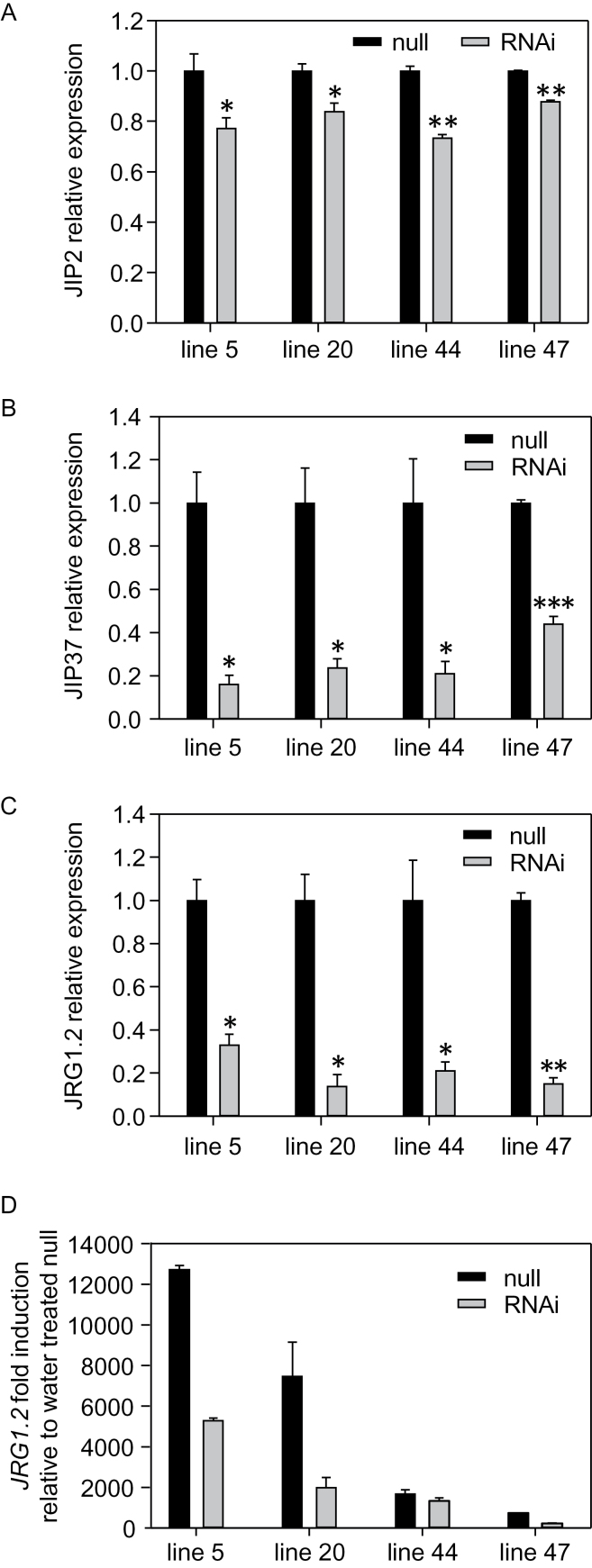
ABCD transporters have also been implicated in import of OPDA for JA biosynthesis in Arabidopsis: cts mutants exhibit reduced levels of JA and lower expression of the JA-responsive gene, VSP2 (Theodoulou et al., 2005). Therefore, the expression of three barley genes which respond to endogenous JA production, JIP2, JIP37, and JRG1.2 (Kramell et al., 2000; Walia et al., 2007), was tested. Although the level of expression varied for different genes tested, in each case, expression was reduced in leaves of HvABCD1/2i lines, compared with null segregants (Fig. 4A–C). This result is consistent with a role for either or both of these genes in JA biosynthesis. Spraying with methyl jasmonate confirmed that JRG1.2 expression was induced by exogenous hormone treatment in both controls and RNAi lines, though to a lesser extent in the latter (Fig. 4D).
Fig. 4.
Expression of JA-responsive genes in HvABCDi lines. (A–C) Quantitation of JIP2, JIP37, and JRG1.2 transcripts in RNAi lines, using Q-PCR. Transcript abundances are expressed relative to the respective null segregant lines. Values are means ±SE (n=3); data are representative of two independent experiments. *P<0.05, **P<0.01, ***P<0.001. (D) Effect of exogenously applied methyl jasmonate (2mg ml–1) on expression of JRG1.2 in RNAi lines and the respective null segregants. Bars show transcript abundance relative to water-treated nulls. Values are means ±SD (n=3).
HvABCD1 and HvABCD2 differ in their ability to complement different phenotypes of Arabidopsis cts-1
Whilst analysis of the barley HvABCD1/2i lines permitted assignment of several different physiological roles to peroxisomal β-oxidation (Figs 1–4), this approach does not provide information about the relative contributions of HvABCD1 and HvABCD2 to these processes. In order to probe their individual functions, HvABCD1 and HvABCD2 were expressed in the Arabidopsis cts-1 null mutant under the control of the native CTS promoter, as has been carried out previously for site-directed cts mutants (Dietrich et al., 2009). Two homozygous lines (D2.1 and D2.2) expressing HvABCD2 were obtained following transformation of the cts-1 mutant. However, it was not possible to recover lines for HvABCD1 by this method, as seeds were unable to germinate. Therefore, a line was established in the wild-type background (Ler) and crossed to cts-1. A homozygous cts-1 line expressing HvABCD1 (D1.1) was recovered by mechanically disrupting the seed coat and culturing on sucrose-containing medium. All lines produced transcripts, as measured by Q-PCR analysis of seedlings; however, expression of HvABCD1 was low relative to the expression level of endogenous CTS (Supplementary Fig. S4 at JXB online).
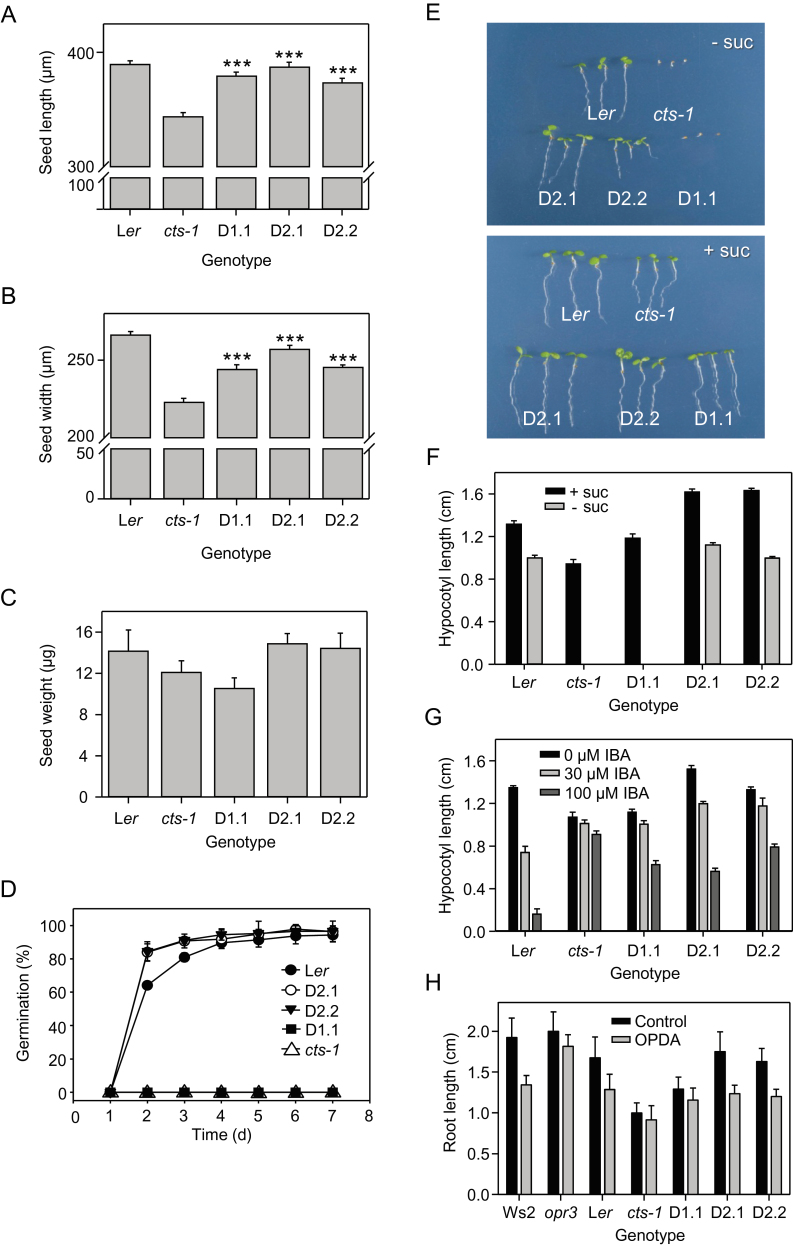
Seed size was first measured in different genotypes. cts-1 seeds were smaller than those of Ler plants produced under identical conditions, being on average 88% of the length and 84% of the width of the wild type (Fig. 5A, B). This was accompanied by a reduction in average seed weight to 85% of the wild type, although this difference was not statistically significantly different (Fig. 5C). In cts-1 lines expressing HvABCD2, seed length and weight were restored to wild-type values, and seed width was significantly increased relative to cts-1 but still significantly different from Ler. Complementation was less complete in the line expressing HvABCD1 (Fig. 5A–C). Germination kinetics of lines D2.1 and D2.2 were indistinguishable from those of the wild type, whereas line D1.1 did not germinate, in agreement with the inability to recover transgenic lines via direct transformation of the cts-1 mutant (Fig. 5D). Thus HvABCD2 but not HvABCD1 is able to complement cts-1 germination. Since CTS performs distinct biochemical functions in germination and seedling establishment, the ability of transgenic lines to establish in the absence of sucrose was then tested. Sterilized seeds were plated on 0.5× MS medium, chilled for 2 d, and transferred to a growth chamber. Lines D2.1 and D2.2 produced green cotyledons after 5 d in constant light and were able to extend hypocotyls in the dark (Fig. 5E, F), although line D2.2 did not grow as well as the wild type (Fig. 5E). Seeds of line D1.1 were induced to germinate as described above and then transferred to medium lacking sucrose, but were unable to mobilize their storage lipid, as indicated by their inability to expand cotyledons and hypocotyls. However, development appeared normal in the presence of sucrose, indicating that there were no obvious deleterious effects of the transgenes (Fig. 5E, F).
Fig. 5.
Complementation of cts-1 by HvABCD1 and HvABCD2. (A, B) Length and width of seeds of cts-1, complemented lines, and the wild type (Ler). Values are means ±SE (n=3; 30 seeds per replicate). t-test indicates significant difference of transgenic lines from cts-1 (***P<0.001). (C) Seed weight. Values are means ±SE (n=5; 80 seeds per replicate). (D) Germination over 7 d on water agarose. Values are means ±SE (n=3; 50 seeds per replicate). (E) Seedling establishment on 0.5× MS with or without 0.5% sucrose. In the upper panel, seeds of cts-1 and cts-1 AtCTS:HvABCD1.1 were induced to germinate by mechanically rupturing the seed coat and plating on sucrose medium for 2 d before transfer to medium lacking sucrose (–suc). Seedlings were rearranged on a fresh plate for photography. (F) Elongation of hypocotyls in the dark on 0.5× MS with or without 0.5% sucrose. (G) Elongation of hypocotyls in the dark on 0.5× MS containing 0.5% sucrose and different concentrations of IBA. Values are means ±SE (n=3; 20 hypocotyls per replicate) in F and G. (H) Effect of oxophytodienoic acid (OPDA) on root growth. Medium is 0.5× MS containing 0.5% sucrose; values are means ±SE (n=25). The opr3 mutant lacks oxophytodienoate reductase and is unable to convert OPDA to JA. (This figure is available in colour at JXB online.)
Responses of the transgenic lines to hormones were also investigated. Seeds were induced to germinate and the effect of IBA on hypocotyl length was tested in sucrose-containing medium. As hypocotyls are less sensitive to IBA than roots (Rashotte et al., 2003), this assay is less influenced by transporter expression levels (discussed in Dietrich et al., 2009). Under these conditions, Ler exhibited a dose-dependent inhibition of hypocotyl elongation, whereas cts-1 was largely resistant up to 100 μM IBA (Fig. 5G). Partial sensitivity to 100 μM IBA was restored in cts-1 lines expressing either HvABCD1 or HvABCD2, indicating that both transporters can contribute to auxin metabolism, as suggested by the barley RNAi lines. The effect of the jasmonate precursor OPDA on root elongation was also tested (following rescue of germination for cts-1 and cts-1 pAtCTS:HvABCD1 seeds, as described above). OPDA inhibited elongation of wild-type (Ler) roots, but the opr3 mutant was resistant since it lacks 12-oxophytodienoic acid reductase and is unable to convert OPDA to JA (Stintzi and Browse, 2000; Zhang and Turner, 2008; Fig. 5H). cts-1 roots were shorter than those of Ler under control conditions, but were also resistant to OPDA, consistent with the fact that this mutant has been shown to be impaired in JA production (Theodoulou et al., 2005). OPDA sensitivity was restored in roots of cts-1 lines expressing HvABCD2 but not in the line expressing HvABCD1.
HvABCD1 partially complements the yeast pxa1/pxa2Δ mutant for fatty acid β-oxidation
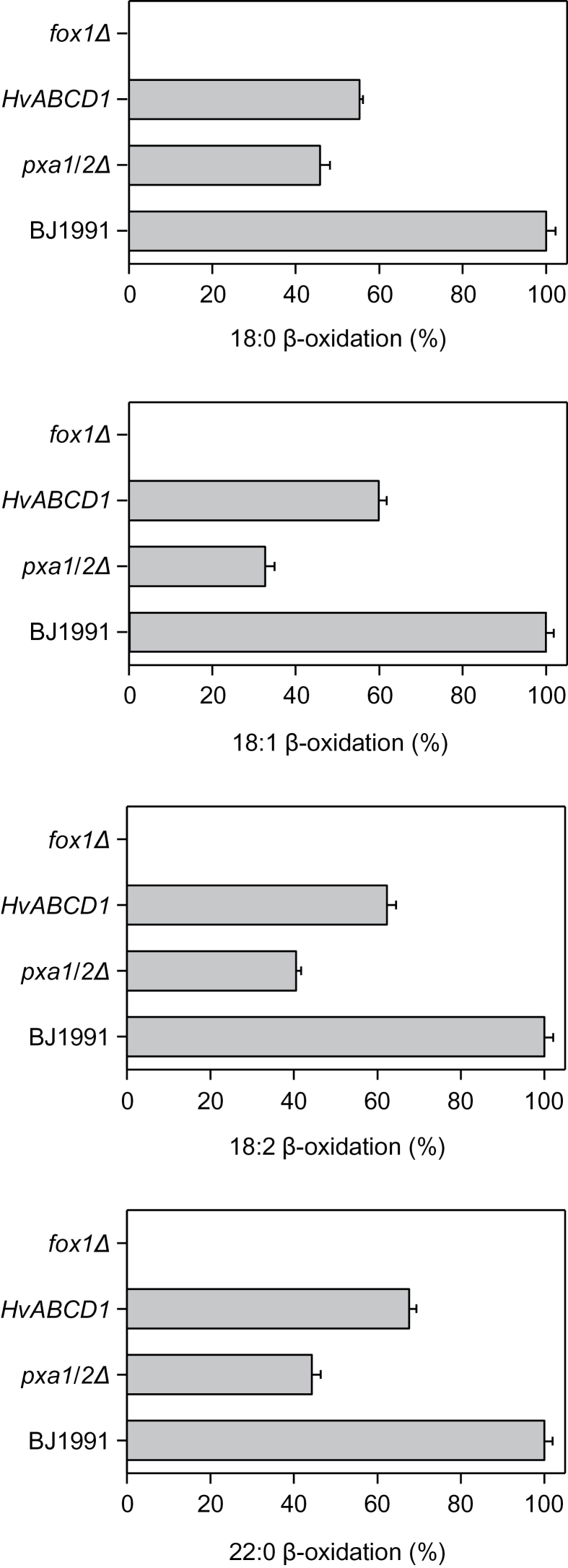
Since HvABCD1 did not complement the seedling establishment phenotype of cts-1, a role for this transporter in fatty acid metabolism was investigated further by expression in a Saccharomyces cerevisiae mutant which lacks Pxa1p/Pxa2p, the yeast heterodimeric transporter which is homologous to CTS. pxa1/pxa2Δ is defective in growth on oleate (18:1) as a sole carbon source, but expression of HvABCD1 under the control of the yeast catalase promoter restored growth to near wild-type levels (data not shown). pxa1/pxa2Δ cells transformed with HvACBD1 exhibited increased β-oxidation of several different long chain fatty acids compared with cells transformed with vector lacking an insert, indicating that the barley transporter can accept saturated and unsaturated long chain fatty acyl-CoAs in this heterologous system (Fig. 6). Expression of HvABCD2 was also attempted using a similar strategy, but no complementation of the pxa1/2Δ mutant was observed (data not shown).
Fig. 6.
Complementation of pxa1/pxa2Δ by HvABCD1. β-oxidation of different fatty acids by intact yeast cells. Cells grown in oleate medium were incubated with 1-14C-labelled fatty acids, followed by β-oxidation activity measurements. Strains are: wild type (BJ1991), pxa1/pxa2Δ mutant cells transformed with vector lacking the insert, or HvABCD1. fox1Δ is defective in acyl-CoA oxidase (the first committed step of β-oxidation) and serves as a negative control. Results are presented as a percentage relative to the rate of oxidation by wild-type cells for each fatty acid and are means ±SD of three independent experiments.
Discussion
Using two complementary approaches, evidence has been provided that barley and Arabidopsis peroxisomal ABC transporters share several functions. When interpreting phenotypes of transgenic plants, it is important to consider the level of suppression in barley RNAi lines and the level of HvABCD expression in cts-1 complemented lines (Dietrich et al., 2009). Arabidopsis plants heterozygous for cts germinate and are able to establish in the absence of sucrose, but are resistant to 2,4-DB and IBA (Hayashi et al., 1998; Zolman et al., 2001). Thus the auxin phenotypes are more sensitive to loss of function than germination and lipid mobilization in Arabidopsis. Therefore, a minor reduction in ABCD function in an RNAi line could lead to auxin resistance but might not impact markedly on other phenotypes. By this reasoning, a substantial knockdown of ABCD transporter expression is likely to be required to detect effects on lipid metabolism and perhaps also germination. However, the physiological thresholds which underpin the visible phenotypes may vary between barley and Arabidopsis, and the influence of the expression level on phenotypes of complemented plants is to some extent dependent on the substrate specificity of the heterologous transporters. Unfortunately, an antiserum raised to the relatively well-conserved C-terminus of Arabidopsis CTS, which recognizes both NBDs (Footitt et al., 2002; De Marcos Lousa et al., 2009), did not recognize barley CTS (data not shown); thus it was not possible to determine the level of HvABCD1 or HvABCD2 protein expression in transgenic lines. With these caveats in mind, several conclusions can be reached.
ABCD transporters play a role in seed size determination in diverse species
Both barley RNAi lines and the complemented Arabidopsis cts-1 lines support a role for ABCD transporters in control of seed size (Figs 1C–E, 5 A–C). This is in agreement with a study identifying a tomato orthologue as the cause of Seed weight 4.1 (Sw4.1), a major quantitative trait locus (QTL) controlling seed size in the genus Solanum (Orsi and Tanksley, 2009). Although studies with Arabidopsis indicate that plant peroxisomal ABCD transporters have multiple substrates (Linka and Theodoulou, 2013), their precise biochemical role in seed size determination is unknown. However, reduced seed size is also observed in Arabidopsis kat2 mutants which lack 3-ketoacyl CoA thiolase (Footitt et al., 2007b), implying a general role for β-oxidation in this trait. Embryo development appears to be dependent on a functional β-oxidation pathway, as evidenced by increased ovule abortion in cts and kat2 mutants (Footitt et al., 2007a, b) and embryo lethality of acox3 acox4 double mutants, which lack short chain acyl-CoA oxidase activity (Rylott et al., 2003), although the latter phenotype is accession dependent (Khan et al., 2012). In agreement with this, reciprocal crosses suggest that Sw4.1 controls seed weight through zygotic effects and exerts its largest effect during early seed development (Orsi and Tanksley, 2009). Deposition of starch and lipids into endosperm cells is maximal at this stage, perhaps suggesting that β-oxidation might also be important for seed filling, either via provision of energy and carbon skeletons for endosperm reserve synthesis or possibly by hormonal control of this process. Seed size is a key agronomic trait and has increased during domestication in crops, as a result of selection for yield and harvest efficiency (Harlan et al., 1973). Interestingly, in this context, attempts to engineer increased seed oil content by overexpression of oil biosynthetic genes in Brassicas has led to seeds of increased weight and/or size (Zou et al., 1997; Jako et al., 2001; Vigeolas et al., 2007).
HvABCD2 contributes to OPDA metabolism
Transcript abundances of JA-inducible genes were reduced in leaves of barley RNAi lines, consistent with a role for HvABCD genes in conversion of OPDA to JA (Fig. 4). Whilst HvABCD1 did not complement cts-1 for germination, expression of HvABCD2 restored the germination rate to wild-type levels (Fig. 5D), which is supportive of the proposal that HvABCD2 mediates OPDA transport into the peroxisome. In agreement with this, roots of cts-1 lines expressing HvABCD2 but not HvABCD1 exhibited sensitivity to exogenously applied OPDA similar to that of wild-type controls (Fig. 5H), strongly supporting the notion that HvABCD2 is required for the conversion of OPDA to JA.
Although accumulation of OPDA is associated with inhibition of germination in Arabidopsis (Dave et al., 2011), the influence of different jasmonates on barley germination has not been studied in detail. The effects of jasmonates on seed germination appear to be species and even ecotype specific (Linkies and Leubner-Metzger, 2012); however, a recent transcriptome study demonstrated that jasmonate biosynthetic and putative receptor genes are up-regulated in coleorhiza tissue of imbibed after-ripened seeds, relative to dormant seeds, pointing to the importance of this class of compounds in barley germination (Barrero et al., 2009). Suppression of HvABCD1 and HvABCD2 had little or no effect on germination (Fig. 1F; Supplementary Fig. S3 at JXB online), perhaps suggesting that ABCD transport activity is not essential for this function in barley, as has also been proposed for tomato (Orsi and Tanksley, 2009). However, the level of HvABCD knockdown may have been insufficient to impact on germination in the present experiments, and it is also possible that, as in Arabidopsis, an additional peroxisomal import pathway for OPDA operates in barley seeds (Theodoulou et al., 2005).
HvABCD1 and HvABCD2 are functional in fatty acid β-oxidation
Oil bodies have been shown to occupy >40% of the cell volume in barley aleurone, and embryo-derived GA stimulates oil breakdown during germination (Eastmond and Jones, 2005). The TAG composition is similar in Arabidopsis and barley, with the exception that Arabidopsis contains a high level (~25%) of the very long chain fatty acid, eicosanoic acid (20:1) (Hølmer et al., 1973; De Man and Vervenne, 1988; Lemieux et al., 1990). In barley, genes involved in lipid catabolism and starch mobilization are expressed together with key transcripts of sucrose synthesis as early as 24h after imbibition before radical protrusion, suggesting that lipid reserve mobilization could supply sucrose before the seedling becomes photoautotrophic (Sreenivasulu et al., 2008). Although oil bodies declined in abundance over 5 d of imbibition of intact grains and also in response to GA treatment of de-embryonated seeds (Fig. 2), increased oil body or fatty acid retention was not observed in HvABCD1/2i lines. It might have been predicted that suppressing HvABCD1 and HvABCD2 would result in retention of oil bodies, but it is possible that the RNAi lines are not sufficiently impaired in β-oxidation to give a detectable phenotype, consistent with the observation that Arabidopsis mutants in which lipid breakdown is reduced by 50% do not exhibit a marked growth phenotype (Kelly et al., 2011). However, complementation of the Arabidopsis cts-1 mutant for seedling establishment in the absence of sucrose indicates that HvABCD2 can transport a range of different fatty acid species (Fig. 5E, F). Although HvABCD1 did not complement the seedling establishment phenotype of cts-1, it did partially restore fatty acid β-oxidation to the yeast pxa1/pxa2Δ mutant (Fig. 6). The lack of complementation in planta may reflect the low expression level of HvABCD1 but could also result from differences between the two expression systems, such as the ability of the heterologous transporter to interact with the different endogenous peroxisomal acyl-CoA synthetases (van Roermund et al., 2012; De Marcos Lousa et al., 2013).
HvABCD1 and HvABCD2 contribute to IBA metabolism
Barley RNAi lines (Fig. 3) and the complementation of cts-1 (Fig. 5G) suggest a role for HvABCD proteins in IBA metabolism. IAA is synthesized by several different routes (Mano and Nemoto, 2012), including β-oxidation of IBA. Although IBA is a relatively abundant auxin, this was originally thought to be a minor biosynthetic route. However, the physiological importance of IBA-derived IAA has been demonstrated conclusively in Arabidopsis by elegant genetic analysis (Strader and Bartel, 2011; Strader et al., 2011). IBA has also been proposed to be a hormone in its own right (Tognetti et al., 2010). Very little is known regarding the functions of IBA in barley, but the data presented here demonstrate a role for IBA-derived IAA in root growth.
HvABCD1 and HvABCD2 may differ in substrate specificity
Since CTS is a broad specificity transporter which plays several distinct roles in growth and development of Arabidopsis, the presence of two transporters in cereals is intriguing. Classically, gene duplication is considered to be a mechanism which increases expression diversity and permits the evolution of tissue or developmental specialization. Duplicate genes also play a role in the establishment of new functions or in subfunctionalization (Li et al., 2005). The ABC transporter superfamily exhibits a particularly high level of gene birth and death, often associated with the acquisition of taxon-specific functions (Annilo et al., 2006; Verrier et al., 2008). HvABCD1 and HvABCD2 display similar expression patterns in embryo and aleurone, suggesting that they may play distinct roles in imbibed grains and indeed throughout the plant, since they are widely expressed in different tissues. This is consistent with their differential ability to complement cts-1. Whilst HvABCD2 complemented all phenotypes tested, HvACBD1 was apparently unable to mediate sufficient fatty acid transport to support seedling establishment when expressed in Arabidopsis and was unable to restore germination to cts-1 seeds (Fig. 5D), suggesting differences in substrate specificity. Although, as discussed above, complementation depends on expression in the correct subcellular location at an appropriate level, it is clear that HvACBD1 was expressed (and presumably targeted correctly) in the present experiments, since it conferred sensitivity of cts-1 hypocotyls to high concentrations of IBA and partially complemented the seed size phenotype (Fig. 5). Transport studies will be required to determine unequivocally the substrate specificities of HvABCD1 and HvABCD2, and further analysis of RNAi lines may reveal additional, perhaps cereal-specific functions for these proteins. In conclusion, monocot and dicot ABCD transporters share a core set of functions, but the retention of two ‘full-length’ HvABCD genes in the grass lineage points to an important functional or regulatory diversification which awaits further investigation.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Alignment of HvABCD1 and HvABCD2 cDNA sequences, showing the region used for the RNA interference construct.
Figure S2. Expression analysis of HvABCD1 and HvABCD2.
Figure S3. Germination of RNAi lines and corresponding nulls over 7 d.
Figure. S4. Q-PCR analysis of gene expression in Arabidopsis transgenic lines and controls.
Table S1. Primers used in this study.
Acknowledgements
This work was supported by BBSRC grant BB/F006934/1 to MJH, which included financial support from SABMiller plc. We thank Dr Wendy Harwood and colleagues at the BRACT transformation facility (John Innes Centre, Norwich) for production of barley transgenic lines. We are grateful to Dr Carine De Marcos Lousa and Professor Alison Baker (Leeds University) for immunoblot analysis of Arabidopsis, Dr Peter Eastmond (Rothamsted Research) for advice on GA treatment, and Kirstie Halsey (Rothamsted Research) for advice regarding confocal microscopy. Rothamsted receives grant-aided support from the BBSRC of the UK. RW and LR acknowledge funding from Scottish Government RESAS Workprogram 5.
Glossary
Abbreviations:
- ABC transporter
ATP-binding cassette transporter
- ABCD
ATP-binding cassette transporter, subclass D
- CTS
COMATOSE
- 2,4-DB
2,4-dichlorophenoxybutyrate
- GA
gibberellic acid
- IAA
indole acetic acid
- IBA
indole butyric acid
- JA
jasmonic acid
- NBD
nucleotide-binding domain
- OPDA
12-oxophytodienoic acid
- TMD
transmembrane domain.
References
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. 1999. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 96, 4710–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annilo T, Chen ZQ, Shulenin S, Costantino J, Thomas L, Lou H, Stefanov S, Dean M. 2006. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics 88, 1–11 [DOI] [PubMed] [Google Scholar]
- Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL. 2006. Chewing the fat: beta-oxidation in signalling and development. Trends in Plant Science 11, 124–132 [DOI] [PubMed] [Google Scholar]
- Barrero JM, Talbot MJ, White RG, Jacobsen JV, Gubler F. 2009. Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Physiology 150, 1006–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussell JD, Reichelt M, Wiszniewski AA, Gershenzon J, Smith SM. 2014. Peroxisomal ATP-binding cassette transporter COMATOSE and the multifunctional protein ABNORMAL INFLORESCENCE MERISTEM are required for the production of benzoylated metabolites in Arabidopsis seeds. Plant Physiology 164, 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Holman T, Medhurst A, Peer W, Schmuths H, Footitt S, Theodoulou FL, Holdsworth MJ. 2007. Gene expression profiling reveals defined functions of the ATP-binding cassette transporter COMATOSE late in phase II of germination. Plant Physiology 143, 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comadran J, Kilian B, Russell J, et al. 2012. A homologue of Antirrhinum CENTRORADIALIS is a component of the quantitative photoperiod and vernalization independent EARLINESS PER SE 2 locus in cultivated barley. Nature Genetics 44, 1388–1392 [DOI] [PubMed] [Google Scholar]
- Dave A, Hernández ML, He Z, Andriotis VM, Vaistij FE, Larson TR, Graham IA. 2011. 12-Oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. The Plant Cell 23, 583–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man W, Vervenne B. 1988. Lipid composition of barley in relation to grain size. Phytochemistry 27, 2037–2039 [Google Scholar]
- De Marcos Lousa C, Dietrich D, Johnson B, Baldwin SA, Holdsworth MJ, Theodoulou FL, Baker A. 2009. The NBDs that wouldn’t die: a cautionary tale of the use of isolated nucleotide binding domains of ABC transporters. Communicative and Integrative Biology 2, 97–99 [PMC free article] [PubMed] [Google Scholar]
- De Marcos Lousa C, van Roermund CW, Postis VLG, Dietrich D, Kerr ID, Wanders RJA, Baldwin SA, Baker A, Theodoulou FL. 2013. Intrinsic acyl-CoA thioesterase activity of a peroxisomal ABC transporter is required for transport and metabolism of fatty acids. Proceedings of the National Academy of Sciences, USA 110, 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, et al. 2010. A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Current Biology 20, 1697–1706 [DOI] [PubMed] [Google Scholar]
- Dietrich D, Schmuths H, De Marcos Lousa C, Baldwin JM, Baldwin SA, Baker A, Theodoulou FL, Holdsworth MJ. 2009. Mutations in the Arabidopsis peroxisomal ABC transporter COMATOSE allow differentiation between multiple functions in planta: insights from an allelic series. Molecular Biology of the Cell 20, 530–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig RI, Colborne AJ, Morris G, Laidman DL. 1975. The induction of glyoxysomal enzyme activities in the aleurone cells of germinating wheat. Journal of Experimental Botany 26, 387–398 [Google Scholar]
- Eastmond PJ, Jones RL. 2005. Hormonal regulation of gluconeogenesis in cereal aleurone is strongly cultivar-dependent and gibberellin action involves SLENDER1 but not GAMYB. The Plant Journal 44, 483–493 [DOI] [PubMed] [Google Scholar]
- Fernandez DE, Staehelin LA. 1987. Does gibberellic acid induce the transfer of lipase from protein bodies to lipid bodies in barley aleurone cells? Plant Physiology 85, 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Cornah JE, Pracharoenwattana I, Bryce JH, Smith SM. 2007b. The Arabidopsis 3-ketoacyl-CoA thiolase-2 (kat2-1) mutant exhibits increased flowering but reduced reproductive success. Journal of Experimental Botany 58, 2959–2968 [DOI] [PubMed] [Google Scholar]
- Footitt S, Dietrich D, Fait A, Fernie AR, Holdsworth MJ, Baker A, Theodoulou FL. 2007a. The COMATOSE ATP-binding cassette transporter is required for full fertility in Arabidopsis. Plant Physiology 144, 1467–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Marquez J, Schmuths H, Baker A, Theodoulou FL, Holdsworth M. 2006. Analysis of the role of COMATOSE and peroxisomal beta-oxidation in the determination of germination potential in Arabidopsis. Journal of Experimental Botany 57, 2805–2814 [DOI] [PubMed] [Google Scholar]
- Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M. 2002. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO Journal 21, 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J. 2004. Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana . The Plant Cell 16, 394–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller A, Dubugnon L, Liechti R, Farmer EE. 2010. Jasmonate biochemical pathway. Science Signaling 3, cm4. [DOI] [PubMed] [Google Scholar]
- Harlan JR, de Wet JMJ, Price EG. 1973. Comparative evolution of cereals. Evolution 27, 311–325 [DOI] [PubMed] [Google Scholar]
- Harwood WA, Bartlett J, Alves S, Perry M, Smedley M, Leyland N, Snape JW. 2009. Barley transformation using Agrobacterium-mediated techniques. Methods in Molecular Biology 478, 137–147 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M. 1998. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. The Plant Cell 10, 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Nito K, Takei-Hoshi R, Yagi M, Kondo M, Suenaga A, Yamaya T, Nishimura M. 2002. Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant and Cell Physiology 43, 1–11 [DOI] [PubMed] [Google Scholar]
- Hølmer G, Ory RL, Høy C-E. 1973. Changes in lipid composition of germinating barley embryo. Lipids 8, 277–283 [DOI] [PubMed] [Google Scholar]
- Holtman WL, Heistek JC, Mattern KA, Bakhuizen R, Douma AC. 1994. β-oxidation of fatty acids is linked to the glyoxylate cycle in the aleurone but not in the embryo of germinating barley. Plant Scence 99, 43–53 [Google Scholar]
- Hooks MA, Turner JE, Murphy EC, Johnston KA, Burr S, Jarosławski S. 2007. The Arabidopsis ALDP protein homologue COMATOSE is instrumental in peroxisomal acetate metabolism. Biochemical Journal 406, 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Barley Genome Sequencing Consortium. 2012. A physical genetic and functional sequence assembly of the barley genome. Nature 491, 711–716 [DOI] [PubMed] [Google Scholar]
- Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC. 2001. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiology 126, 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarošová J, Kundu JK. 2010. Validation of reference genes as internal control for studying viral infections in cereals by quantitative real-time RT–PCR. BMC Plant Biology 10, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Jacobsen JV. 1991. Regulation of the synthesis and transport of secreted protein in cereal aleurone. International Reviews in Cytology 126, 49–88 [DOI] [PubMed] [Google Scholar]
- Kanai M, Nishimura M, Hayashi M. 2010. A peroxisomal ABC transporter promotes seed germination by inducing pectin degradation under the control of ABI5. The Plant Journal 62, 936–947 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. 2002. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science 7, 193–195 [DOI] [PubMed] [Google Scholar]
- Kelly AA, Quettier AL, Shaw E, Eastmond PJ. 2011. Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiology 157, 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan BR, Adham AR, Zolman BK. 2012. Peroxisomal acyl-CoA oxidase 4 activity differs between Arabidopsis accessions. Plant Molecular Biology 78, 45–58 [DOI] [PubMed] [Google Scholar]
- Kienow L, Schneider K, Bartsch M, Stuible HP, Weng H, Miersch O, Wasternack C, Kombrink E. 2008. Jasmonates meet fatty acids: functional analysis of a new acyl-coenzyme A synthetase family from Arabidopsis thaliana . Journal of Experimental Botany 59, 403–419 [DOI] [PubMed] [Google Scholar]
- Klempien A, Kaminaga Y, Qualley A, et al. 2012. Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. The Plant Cell 24, 2015–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, D’Auria JC, Behere AS, Kim JH, Gunderson KL, Breen JN, Lee G, Gershenzon J, Last RL, Jander G. 2007. Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana . The Plant Journal 51, 1062–1076 [DOI] [PubMed] [Google Scholar]
- Kramell R, Miersch O, Atzorn R, Parthier B, Wasternack C. 2000. Octadecanoid-derived alteration of gene expression and the ‘oxylipin signature’ in stressed barley leaves. Implications for different signaling pathways. Plant Physiology 123, 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kaminaga Y, Cooper B, Pichersky E, Dudareva N, Chapple C. 2012. Benzoylation and sinapoylation of glucosinolate R-groups in Arabidopsis. The Plant Journal 72, 411–422 [DOI] [PubMed] [Google Scholar]
- Lemieux B, Miquel M, Somerville C, Browse J. 1990. Mutants of Arabidopsis with alterations in seed fatty-acid composition. Theoretical and Applied Genetics 80, 234–240 [DOI] [PubMed] [Google Scholar]
- Li WH, Yang J, Gu X. 2005. Expression divergence between duplicate genes. Trends in Genetics 21, 602–607 [DOI] [PubMed] [Google Scholar]
- Linka N, Theodoulou FL. 2013. Metabolite transporters of the plant peroxisomal membrane: known and unknown. Subcellular Biochemistry 69, 169–194 [DOI] [PubMed] [Google Scholar]
- Linkies A, Leubner-Metzger G. 2012. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Reports 31, 253–270 [DOI] [PubMed] [Google Scholar]
- Mano Y, Nemoto K. 2012. The pathway of auxin biosynthesis in plants. Journal of Experimental Botany 63, 2853–2872 [DOI] [PubMed] [Google Scholar]
- Martin T, Oswald O, Graham IA. 2002. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiology 128, 472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Muehlbauer GJ, Rokhsar DS, et al. 2013. Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ). The Plant Journal 76, 718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. 2007. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. Journal of Bioscience and Bioengineering 104, 34–41 [DOI] [PubMed] [Google Scholar]
- Newman JC, Briggs DE. 1976. Glyceride metabolism and gluconeogenesis in barley endosperm. Phytochemistry 15, 1453–1458 [Google Scholar]
- Nicot N, Hausman J, Hoffmann L, Evers D. 2005. Housekeeping gene selection for real-time RT–PCR normalization in potato during biotic and abiotic stress. Journal of Experimental Botany 56, 421, 2907–2914 [DOI] [PubMed] [Google Scholar]
- Nyathi Y, De Marcos Lousa C, van Roermund CW, Wanders RJ, Johnson B, Baldwin SA, Theodoulou FL, Baker A. 2010. The Arabidopsis peroxisomal ABC transporter, comatose, complements the Saccharomyces cerevisiae pxa1 pxa2Delta mutant for metabolism of long-chain fatty acids and exhibits fatty acyl-CoA-stimulated ATPase activity. Journal of Biological Chemistry 285, 29892–29902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyathi Y, Zhang X, Baldwin JM, Bernhardt K, Johnson B, Baldwin SA, Theodoulou FL, Baker A. 2012. Pseudo half-molecules of the ABC transporter, COMATOSE, bind Pex19 and target to peroxisomes independently but are both required for activity. FEBS Letters 586, 2280–2286 [DOI] [PubMed] [Google Scholar]
- Orsi CH, Tanksley SD. 2009. Natural variation in an ABC transporter gene associated with seed size evolution in tomato species. PLoS Genetics 5, e1000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RV, Nahal HK, Breit R, Provart NJ. 2012. BAR expressolog identification: expression profile similarity ranking of homologous genes in plant species. The Plant Journal 71, 1038–1050 [DOI] [PubMed] [Google Scholar]
- Pinfield-Wells H, Rylott EL, Gilday AD, Graham S, Job K, Larson TR, Graham IA. 2005. Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. The Plant Journal 43, 861–872 [DOI] [PubMed] [Google Scholar]
- Poirier Y, Antonenkov VD, Glumoff T, Hiltunen JK. 2006. Peroxisomal beta-oxidation—a metabolic pathway with multiple functions. Biochimica et Biophysica Acta 1763, 1413–1426 [DOI] [PubMed] [Google Scholar]
- Qualley AV, Widhalm JR, Adebesin F, Kish CM, Dudareva N. 2012. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proceedings of the National Academy of Sciences, USA 109, 16383–16388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranford JC, Bryce JH, Morris PC. 2002. PM19, a barley (Hordeum vulgare L.) gene encoding a putative plasma membrane protein, is expressed during embryo development and dormancy. Journal of Experimental Botany 53, 147–148 [PubMed] [Google Scholar]
- Rashotte AM, Poupart J, Waddell CS, Muday GK. 2003. Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiology 133, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S, Ma C, Lemke S, Babujee L. 2004. AraPerox. A database of putative Arabidopsis proteins from plant peroxisomes. Plant Physiology 136, 2587–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. 1998. Isolation and analysis of mutants in Arabidopsis thaliana disrupted in the transition between dormancy and germination. PhD thesis, University of Bristol.
- Russell L, Larner V, Kurup S, Bougourd S, Holdsworth M. 2000. The Arabidopsis COMATOSE locus regulates germination potential. Development 127, 3759–3767 [DOI] [PubMed] [Google Scholar]
- Rylott EL, Rogers CA, Gilday AD, Edgell T, Larson TR, Graham IA. 2003. Arabidopsis mutants in short- and medium-chain acyl-CoA oxidase activities accumulate acyl-CoAs and reveal that fatty acid beta-oxidation is essential for embryo development. Journal of Biological Chemistry 278, 21390–21377 [DOI] [PubMed] [Google Scholar]
- Singh G, Kumar S, Singh P. 2003. A quick method to isolate RNA from wheat and other carbohydrate-rich seeds. Plant Molecular Biology Reports 21, 93a–93f [Google Scholar]
- Sreenivasulu N, Usadel B, Winter A, et al. 2008. Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiology 146, 1738–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Browse J. 2000. The Arabidopsis male-sterile mutant, opr3 lacks the 12-oxophytodienoic acid reductase required for jasmonic acid synthesis. Proceedings of the National Academy of Sciences, USA 97, 10625–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Bartel B. 2011. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Molecular Plant 4, 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Wheeler DL, Christensen SE, Berens JC, Cohen JD, Rampey RA, Bartel B. 2011. Multiple facets of Arabidopsis seedling development require indole-3-butyric acid-derived auxin. The Plant Cell 23, 984–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoulou FL, Eastmond PJ. 2012. Seed storage oil catabolism: a story of give and take. Current Opinion in Plant Biology 15, 322–328 [DOI] [PubMed] [Google Scholar]
- Theodoulou FL, Job K, Slocombe SP, Footitt S, Holdsworth M, Baker A, Larson TR, Graham IA. 2005. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiology 137, 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoulou FL, Holdsworth M, Baker A. 2006. Peroxisomal ABC transporters. FEBS Letters 580, 1139–1155 [DOI] [PubMed] [Google Scholar]
- Tognetti VB, Van Aken O, Morreel K, et al. 2010. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. The Plant Cell 22, 2660–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund CWT, Elgersma Y, Singh N, Wanders RJA, Tabak HF. 1995. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO Journal 14, 3480–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund CW, Ijlst L, Majczak W, Waterham HR, Folkerts H, Wanders RJ, Hellingwerf KJ. 2012. Peroxisomal fatty acid uptake mechanism in Saccharomyces cerevisiae . Journal of Biological Chemistry 287, 20144–20153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund CW, Ijlst L, Wagemans T, Wanders RJA, Waterham HR. 2014. A role for the human peroxisomal half-transporter ABCD3 in the oxidation of dicarboxylic acids. Biochimica et Biophysica Acta 1841, 563–568 [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Visser WF, Ijlst L, van Cruchten A, Boek M, Kulik W, Waterham HR, Wanders RJ. 2008. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB Journal 22, 4201–4208 [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Visser WF, Ijlst L, Waterham HR, Wanders RJ. 2011. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid β-oxidation. Biochimica et Biophysica Acta 1811, 148–152 [DOI] [PubMed] [Google Scholar]
- Verrier PJ, Bird D, Burla B, et al. 2008. Plant ABC proteins—a unified nomenclature and updated inventory. Trends in Plant Science 13, 151–159 [DOI] [PubMed] [Google Scholar]
- Vigeolas H, Waldeck P, Zank T, Geigenberger P. 2007. Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotechnology Journal 5, 431–441 [DOI] [PubMed] [Google Scholar]
- Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Close TJ. 2007. Large-scale expression profiling and physiological characterization of jasmonic acid-mediated adaptation of barley to salinity stress. Plant, Cell and Environment 30, 410–421 [DOI] [PubMed] [Google Scholar]
- Wanders RJ, Waterham HR. 2006. Biochemistry of mammalian peroxisomes revisited. Annual Review of Biochemistry 75, 295–332 [DOI] [PubMed] [Google Scholar]
- Zhang X, De Marcos Lousa C, Schutte-Lensink N, Ofman R, Wanders RJ, Baldwin SA, Baker A, Kemp S, Theodoulou FL. 2011. Conservation of targeting but divergence in function and quality control of peroxisomal ABC transporters: an analysis using cross-kingdom expression. Biochemical Journal 436, 547–557 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Turner JG. 2008. Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS One 3, e3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Silva ID, Bartel B. 2001. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiology 127, 1266–1278 [PMC free article] [PubMed] [Google Scholar]
- Zou J, Katavic V, Giblin EM, Barton DL, MacKenzie SL, Keller WA, Hu X, Taylor DC. 1997. Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene. The Plant Cell 9, 909–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.