Abstract
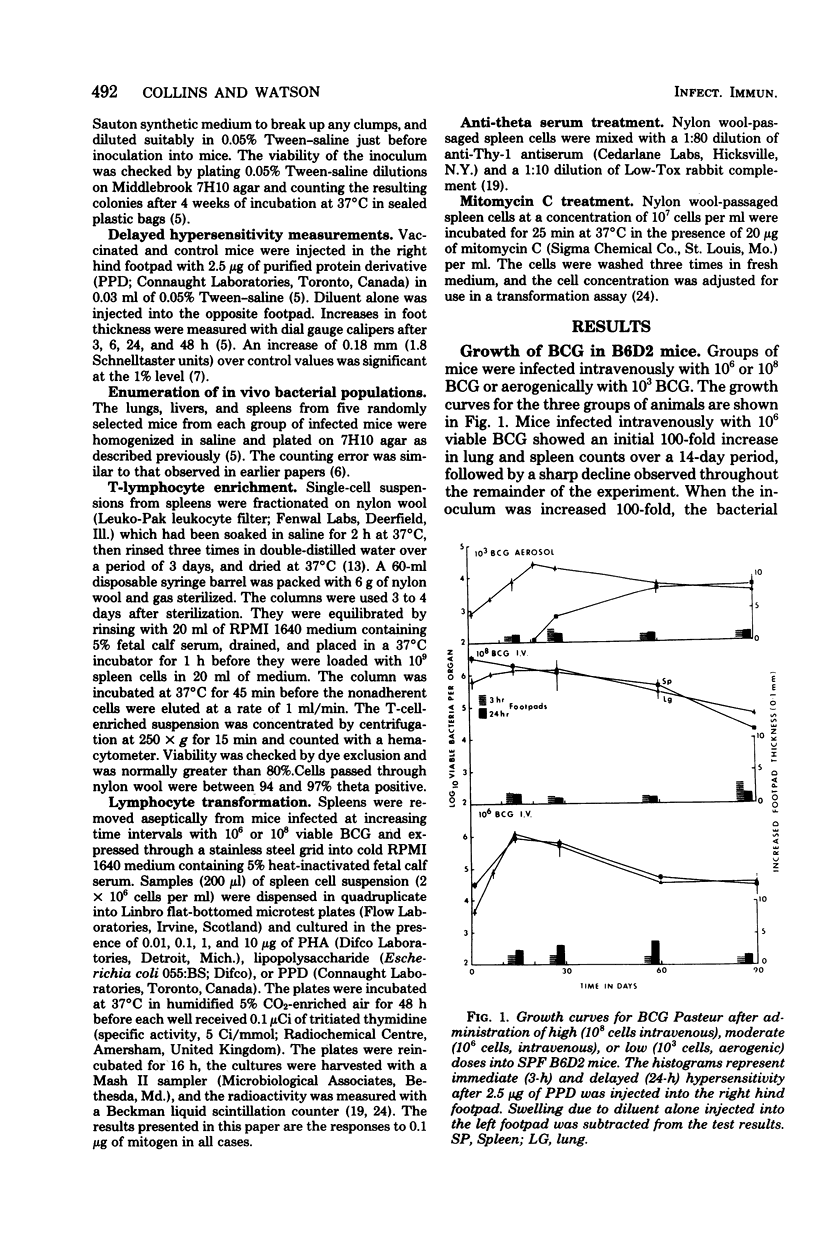
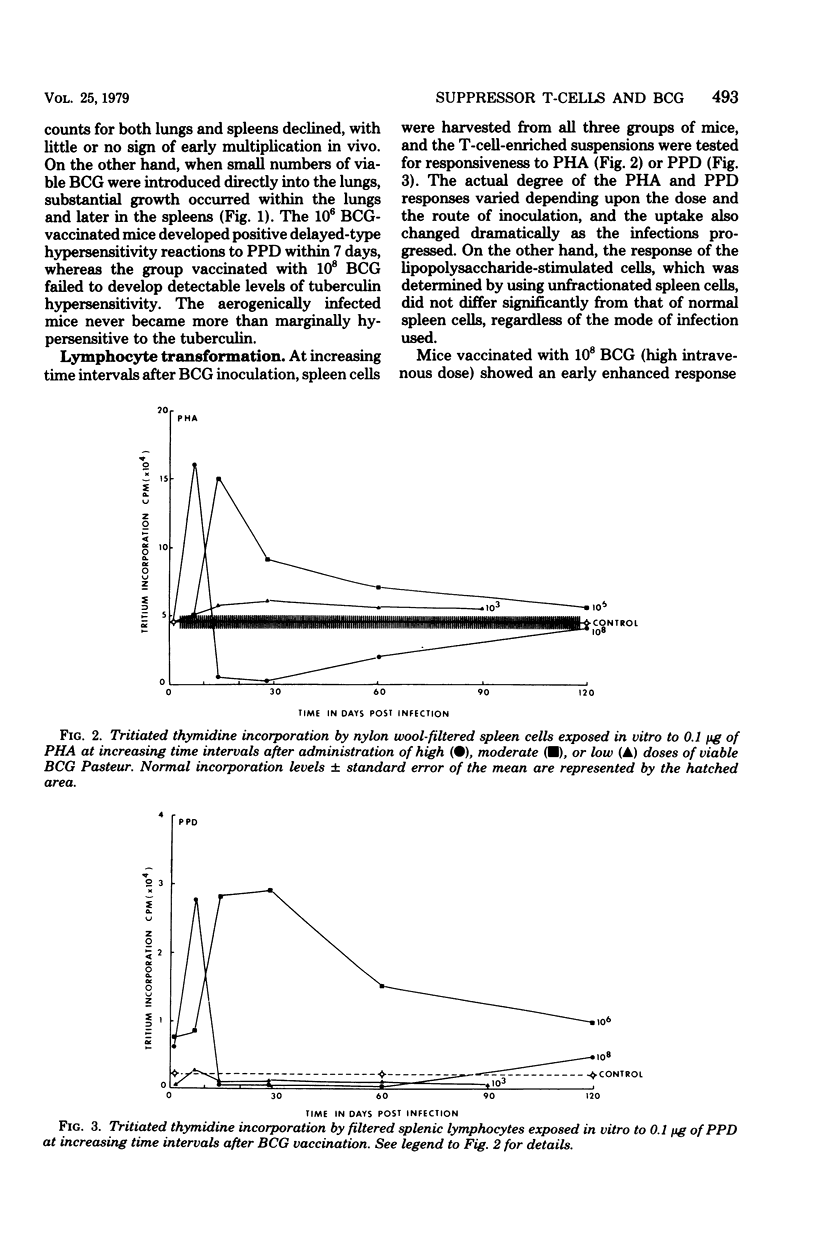
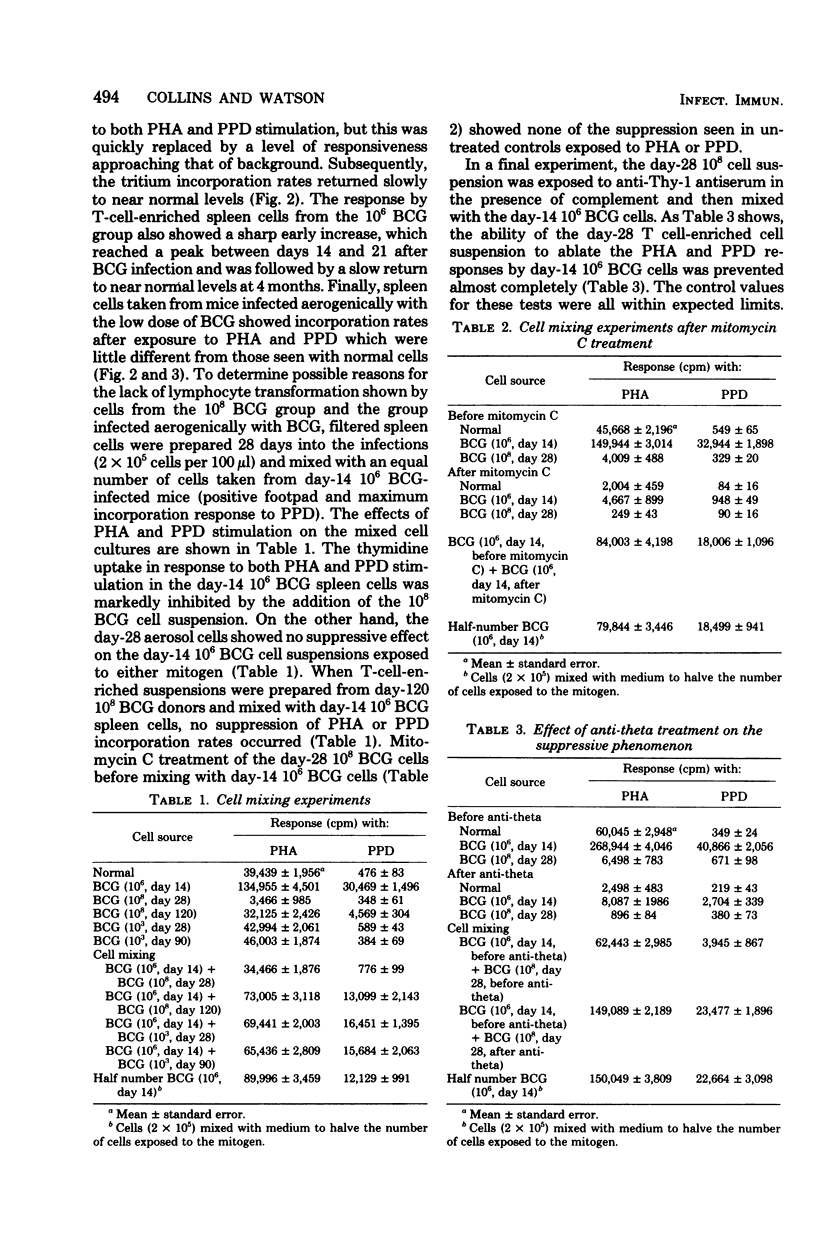
Specific pathogen-free B6D2 hybrid mice were infected with high (10(8) cells, intravenous), moderate (10(6) cells, intravenous), and low 10(3) cells, aerogenic) doses of viable BCG Pasteur. The growth of the BCG in the lungs and spleens of the three groups was followed over a 90-day period and correlated with the level of tuberculin hypersensitivity. Spleen cells were harvested from the three groups of mice at increasing time intervals and filtered through nylon wool to remove adherent cells, and the level of blast transformation after exposure to phytohemagglutinin and purified protein derivative was determined. Early in the BCG infection both the high- and the intermediate-dose groups showed enhanced thymidine incorporation by the spleen cell cultures, followed by a profound depression late in the infection. At this time, both groups of mice were anergic to purified protein derivative injected into footpads. Cell mixing studies demonstrated the presence of a population of suppressor cells in the spleens of the anergic animals. The suppressive abilities of these cells would be ablated by treatment with anti-Thy-1 antiserum and complement. The aerogenically infected mice were unresponsive to purified protein derivative but showed no evidence of suppressor T-cells. The lack of tuberculin sensitivity in these mice seemed to be due to a lack of sensitized T-cells in the spleen rather than to active immunosuppression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. A., Taub R. N. Immunotherapy of human cancer. Prog Allergy. 1973;17:227–299. doi: 10.1159/000313296. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Bast B. S. Critical review of previously reported animal studies of tumor immunotherapy with nonspecific immunostimulants. Ann N Y Acad Sci. 1976;277(00):60–93. doi: 10.1111/j.1749-6632.1976.tb41692.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. E. Anergy and infection. Adv Intern Med. 1976;21:149–173. [PubMed] [Google Scholar]
- Collins F. M. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7(1):27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Volkman A., McGregor D. D. Transfer of delayed and Arthus sensitivity with blood plasma from x-irradiated guinea-pigs. Immunology. 1970 Sep;19(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Kantor F. S. Regulation of delayed hypersensitivity. Failure to transfer delayed hypersensitivity to desensitized guinea pigs. J Exp Med. 1973 Jan 1;137(1):32–41. doi: 10.1084/jem.137.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin I., Orbach-Arbouys S. Réactivité des cellules spléniques après traitement par le BCG: variation de la réponse selon les tests et les legnées utilisées. Ann Immunol (Paris) 1977 Jan-Mar;128(1-2):271–272. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- Murahata R. I., Mitchell M. S. Modulation of the immune response by BCG: a review. Yale J Biol Med. 1976 Jul;49(3):283–291. [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Orbach-Arbouys S., Poupon M. F. Active suppression of in vitro reactivity of spleen cells after BCG treatment. Immunology. 1978 Mar;34(3):431–437. [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. Development of suppressor T-cells in Mycobacterium habana-infected mice. Infect Immun. 1979 Aug;25(2):497–506. doi: 10.1128/iai.25.2.497-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


