Abstract
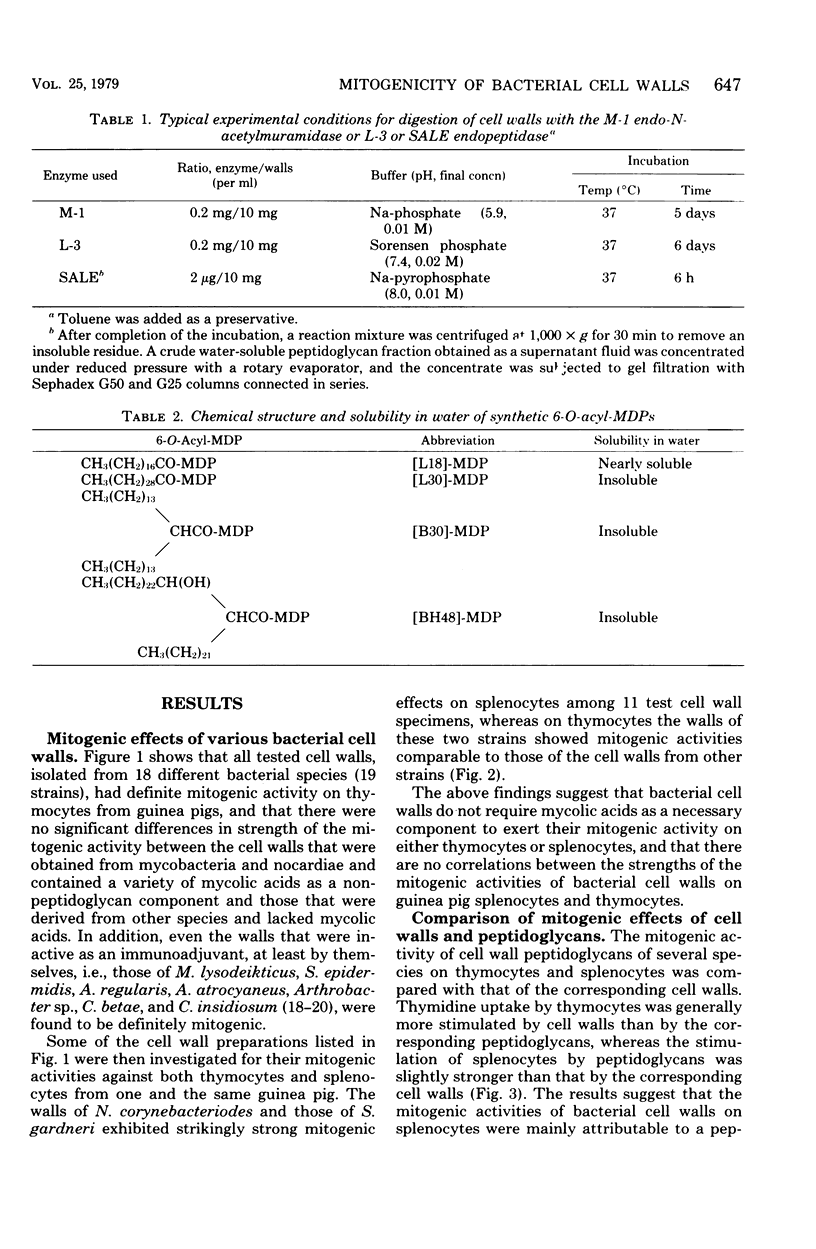
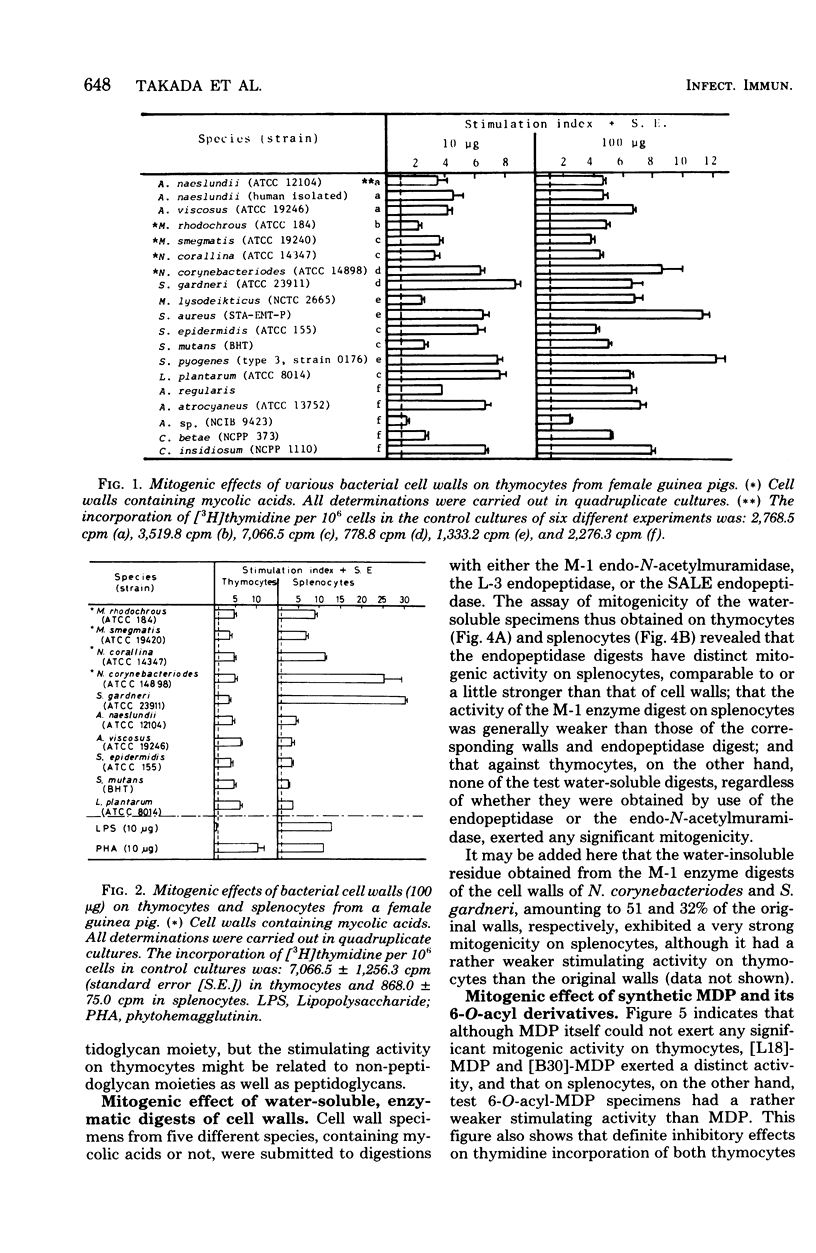
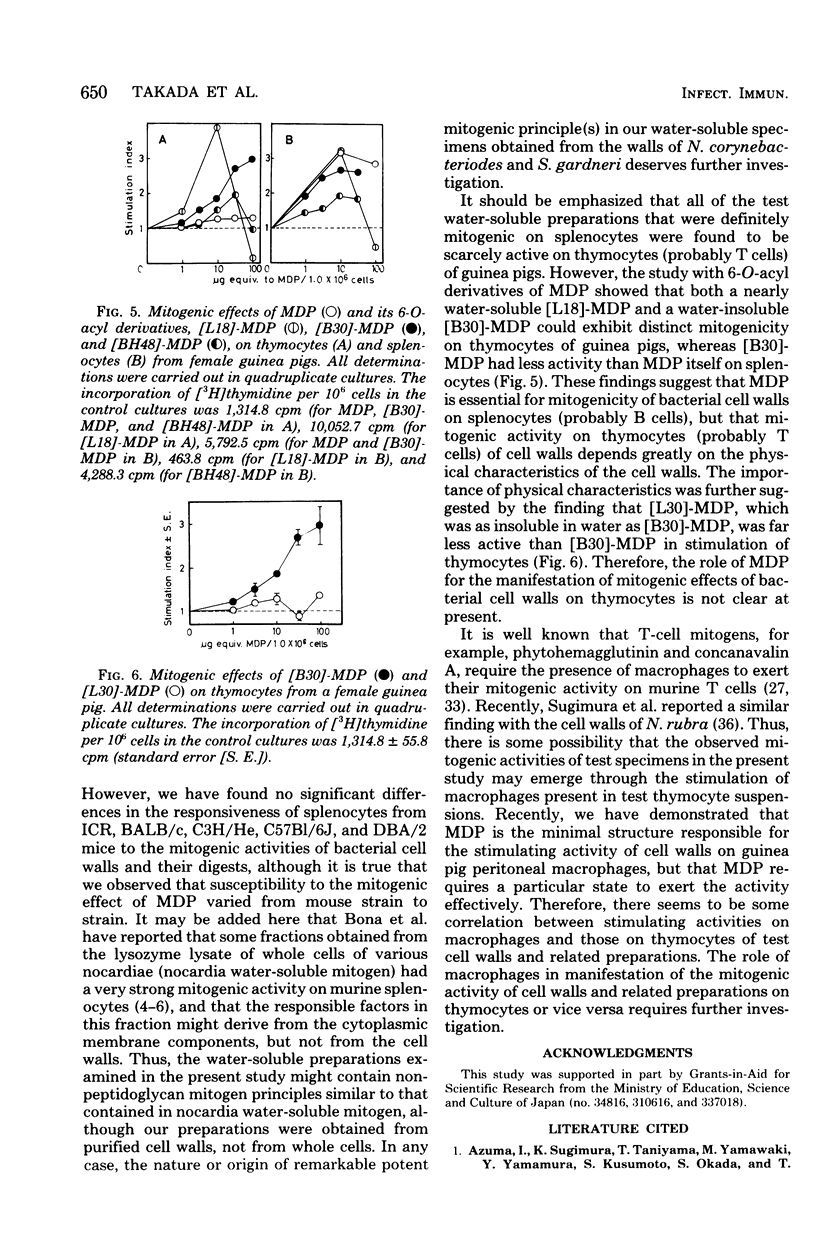
Stimulation of [3H]thymidine incorporation of thymocytes and splenocytes from guinea pigs by various bacterial cell walls and their peptidoglycans, by enzymatic digests, and by synthetic muramyl dipeptides was studied as an indication of mitogenic activity. Cell wall and peptidoglycan preparations, isolated from 19 strains belonging to 18 different species, definitely increased [3H]thymidine incorporation of thymocytes as well as splenocytes, regardless of mycolic acid contents as a non-peptidoglycan component. Both the cell walls from Nocardia corynebacteriodes (containing mycolic acids) and those from Streptomyces gardneri (lacking mycolic acids) showed far stronger mitogenic activities on splenocytes than other cell walls (stimulation index, 25 to 30). Furthermore, water-soluble enzymatic digests, notably the endopeptidase digests, which generally were greater in degree of polymerization of peptidoglycan subunits than the glycosidase digests obtained from representative cell walls, were found to have as distinct a stimulating activity on splenocytes as the original cell walls. In contrast, solubilization of the cell walls by enzymes, irrespective of endopeptidases or glycosidases, was accompanied by disappearance of the mitogenic activity on thymocytes. On the other hand, studies with synthetic 6-O-acyl-MurNAc-l-Ala-d-isoGln preparations (6-O-acyl-MDPs) revealed that 6-O-stearoyl-MDP and 6-O-(2-tetradecylhexadecanoyl)-MDP, unlike MDP, had distinct mitogenic activity on thymocytes, whereas their activity on splenocytes was rather weaker than MDP itself. The findings presented here suggest that MDP is the minimal structure for the mitogenic activities of bacterial cell walls on guinea pig splenocytes, but that MDP, though distinctively active by itself, requires a polymerized form to exert effectively its inherent stimulating activities on splenocytes. On the other hand, on thymocytes, MDP, unless it takes a particular form or has appropriate additive groups, cannot exert its mitogenic activities.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Sugimura K., Yamawaki M., Uemiya M., Yamamura Y. Mitogenic activity of cell-wall components in mouse spleen cells. Microbiol Immunol. 1977;21(2):111–115. doi: 10.1111/j.1348-0421.1977.tb02813.x. [DOI] [PubMed] [Google Scholar]
- Azuma I., Taniyama T., Sugimura K., Aladin A., Yamamura Y. Mitogenic activity of the cell walls of mycobacteria, nocardia, corynebacteria and anaerobic coryneforms. Jpn J Microbiol. 1976 Aug;20(4):263–271. doi: 10.1111/j.1348-0421.1976.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Bona C., Damais C., Chedid L. Blastic transformtion of mouse spleen lymphocytes by a water-soluble mitogen extracted from Nocardia. Proc Natl Acad Sci U S A. 1974 May;71(5):1602–1606. doi: 10.1073/pnas.71.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Damais C., Dimitriu A., Chedid L., Ciorbaru R., Adam A., Petit J. F., Lederer E., Rosselet J. P. Mitogenic effect of a water-soluble extract of Nocardia opaca: a comparative study with some bacterial adjuvants on spleen and peripheral lymphocytes of four mammalian species. J Immunol. 1974 Jun;112(6):2028–2035. [PubMed] [Google Scholar]
- Ciorbaru R., Adam A., Petit J. F., Lederer E., Bona C., Chedid L. Isolation of mitogenic and adjuvant active fractions from various species of Nocardiae. Infect Immun. 1975 Feb;11(2):257–264. doi: 10.1128/iai.11.2.257-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorbaru R., Petit J. F., Lederer E., Zissman E., Bona C., Chedid L. Presence and subcellular localization of two distinct mitogenic fractions in the cells of Nocardia rubra and Nocardia opaca: preparation of soluble mitogenic peptidoglycan fractions. Infect Immun. 1976 Apr;13(4):1084–1090. doi: 10.1128/iai.13.4.1084-1090.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damais C., Bona C., Chedid L., Fleck J., Nauciel C., Martin J. P. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975 Jul;115(1):268–271. [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L., Lefrancier P., Choay J. In vitro spleen cell responsiveness to various analogs of MDP (N-acetylmuramyl-L-alanyl-D-isoglutamine), a synthetic immunoadjuvant, in MDP high-responder mice. Cell Immunol. 1978 Jan;35(1):173–179. doi: 10.1016/0008-8749(78)90137-5. [DOI] [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L. Nonspecific activation of murine spleen cells in vitro by a synthetic immunoadjuvant (N-acetyl-muramyl-L-alanyl-D-isoglutamine). Cell Immunol. 1977 Nov;34(1):49–56. doi: 10.1016/0008-8749(77)90228-3. [DOI] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- Katayama T., Matsuda T., Kato K., Kotani S. Isolation and purification of D-alanyl-meso-2, 6-diaminopimelic acid endopeptidase of Streptomyces L-3 enzyme using soluble substrates of known chemical structure from Lactobacillus plantarum cell wall digests. Biken J. 1976 Sep;19(3):75–91. [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Perkins H. R. Immunoadjuvant activities of the enzymatic digests of bacterial cell walls lacking immunoadjuvancy by themselves. Biken J. 1977 Jun;20(2):87–90. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Schleifer K. H. Inabilities as an immunoadjuvant of cell walls of the group B peptidoglycan types and those of arthrobacters. Biken J. 1977 Mar;20(1):1–4. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Mills G., Monticone V., Paetkau The role of macrophages in thymocyte mitogenesis. J Immunol. 1976 Oct;117(4):1325–1330. [PubMed] [Google Scholar]
- Ohkuni H., Kimura Y. Increased capillary permeability in guinea pigs and rats by peptidoglycan fraction extracted from Group A streptococcal cell walls. Exp Cell Biol. 1976;44(2):83–94. doi: 10.1159/000163102. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. 2,6-Diamino-3-hydroxypimelic acid in microbial cell wall mucopeptide. Nature. 1965 Nov 27;208(5013):872–873. doi: 10.1038/208872a0. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. Homoserine and diaminobutyric acid in the mucopeptide-precursor-nucleotides and cell walls of some plant-pathogenic corynebacteria. Biochem J. 1971 Feb;121(3):417–423. doi: 10.1042/bj1210417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The preparation of iodinated vancomycin and its distribution in bacteria treated with the antibiotic. Biochem J. 1970 Jan;116(1):83–92. doi: 10.1042/bj1160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Kotani S., Kusumoto S., Tarumi Y., Ikenaka K. Mitogenic activity of adjuvant-active N-acetylmuramyl-L-alanyl-D-isoglutamine and its analogues. Biken J. 1977 Jun;20(2):81–85. [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami H. [Biological activities of a human dental plaque specimen, and cell walls from Actinomyces viscosus and Actinomyces naeslundii]. Osaka Daigaku Shigaku Zasshi. 1978 Jun;23(1):78–95. [PubMed] [Google Scholar]


